Laryngotracheal stenosis: Mechanistic review
Abstract
Background
The purpose of this review article is to summarize the existing literature surrounding wound healing mechanisms in laryngotracheal stenosis.
Methods
A review of general wound healing pathophysiology, followed by a focused review of iatrogenic laryngotracheal stenosis (iLTS) and idiopathic subglottic stenosis (iSGS) as conditions of aberrant wound healing.
Results
iLTS is the scarring of the laryngotracheal complex, coming secondary to injury from prolonged intubation. iSGS is a chronic fibroinflammatory scarring and narrowing of the subglottic airway in the absence of any obvious preceding injury or trauma. They are both thought to result from a prolonged and dysregulated wound healing response that promotes the deposition of pathologic scar in the airway.
Conclusions
Understanding the mechanisms that underlie wound healing will help identify and intervene on the process early in its development and discover future therapies that target individual wound healing mechanisms limiting the incidence of this recalcitrant disease process.
1 INTRODUCTION
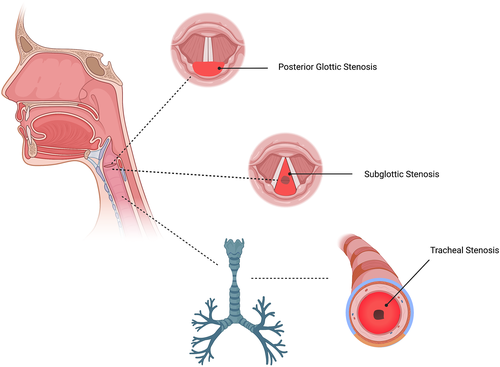
Laryngotracheal stenosis (LTS) comprises a broad set of diagnoses relating to the narrowing of various subsets of the upper airway, including the glottis, supraglottis, subglottis, and trachea due to pathologic scar formation, with severe and potentially fatal respiratory compromise. In addition, this pathologic scar formation leads to impaired phonation. Acquired LTS is most commonly iatrogenic in nature (iLTS) due to prolonged intubation, though it may also develop in response to a previous tracheostomy, external trauma to the neck, chemical inhalation or thermal injury, or inflammatory and autoimmune disease such as sarcoidosis or granulomatosis with polyangiitis. Many cases of subglottic stenosis (SGS) develop in the absence of any preceding injury or underlying illness, termed idiopathic subglottic stenosis (iSGS). This condition, which is a rare and slowly progressive airway disease, results in chronic mucosal inflammation and fibrosis of the subglottic airway and presents almost exclusively in middle-aged white females with no significant underlying medical conditions.1-6
Laryngotracheal stenosis is considered to be the result of aberrant and overzealous wound healing mechanisms, leading to hypertrophic scar formation and airway narrowing. The time-course for the development of mature scar and symptomatic stenosis parallels well-described wound healing physiology. Patients with acute airway injury secondary to a prolonged intubation tend to be initially asymptomatic, with a delayed presentation of difficulty breathing and/or impaired phonation 4–6 weeks after the acute injury. This process is often unrecognized initially if the airway injury is not identified. The importance of wound healing mechanisms in patients with LTS can also be appreciated by the high rate of co-morbid diabetes mellitus, obesity, and vascular disease seen in patients with iatrogenic tracheal and posterior glottic stenosis (PGS).5, 7 Typical anatomic representations of laryngeal stenosis including PGS, SGS and tracheal stenosis are represented in Figure 1.
The purpose of this review article is to summarize the existing literature surrounding wound healing mechanisms in laryngotracheal stenosis from a mechanistic perspective. This will begin with a review of general wound healing pathophysiology, followed by a focused review of iLTS and iSGS as conditions of aberrant wound healing. Current management of laryngotracheal stenosis is primarily surgical, including endoscopic dilation, endoscopic resection, laryngotracheoplasty, and cricotracheal resection. Patients with iLTS have been found to have tracheostomy dependence rates higher than patients with idiopathic or traumatic etiologies by up to 66%.5 This highlights the poor prognosis associated with iatrogenic LTS and the limitations of current therapies. Whereas patients with iSGS have excellent initial responses to treatment, but suffer from high recurrence rates—more than half of patients with iSGS requiring repeated surgical procedures within 12 months of their initial diagnosis.8 The variability in treatment outcomes has complicated patient decision-making as patients try to balance survival, risk of interventions, and quality of life considerations.2 Medical alternatives or adjuncts to the surgical management of LTS are limited by a lack of mechanistic understanding of the pathogenesis of LTS. Currently utilized adjuncts include systemic or local steroid treatments, mitomycin C injections, antibiotics, and other anti-inflammatory medications. However, evidence for each of these approaches is inconsistent, highlighting the need for a deeper understanding of the wound healing mechanisms involved in this process in order to provide targeted and effective treatment options.8
Of note, this paper will not address scarring of the vocal fold and voice disability. For an excellent review of vocal fold wound healing, please reference Branski et al.'s 2006 seminal paper.9
2 WOUND HEALING OVERVIEW
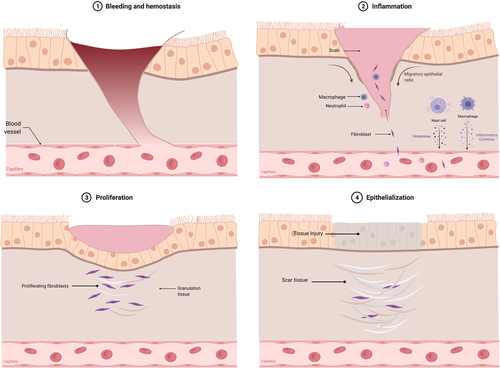
Wound healing is a complex process made up of multiple overlapping stages that begin within seconds and last for up to a year after the initial injury.10 The progression begins with hemostasis, followed by removal of contaminating pathogens, establishment of a temporary scaffold for tissue regrowth, and creation of a fibrous scar that is remodeled onto surrounding tissues. This series of events can be structured in three general phases: inflammation, proliferation, and maturation; with the proliferation stage being comprised of extracellular matrix (ECM) deposition and epithelization.9, 10 The wound healing process is demonstrated in Figure 2.
2.1 Inflammation
The body acts to stop any bleeding via the formation of a fibrin-rich clot within seconds.11 The release of histamine, prostacyclin, and nitric oxide from local cells then leads to increased vascular permeability and vasodilation.11, 12 White blood cells migrate to the area of injury via chemotaxis and begin to sterilize the area.11 Responding macrophages secrete cytokines and multiple growth factors to recruit fibroblasts and other cells to the site of injury.11 The inflammatory phase lasts for minutes to days depending on the severity of the injury and underlying characteristics of the patient. Inflammation contributes to stabilization of the wound, recruiting cells to sterilize the wound and remove non-functional structures.
2.2 Proliferation
The wound healing process then shifts toward repairing damage and replacing lost tissue. The proliferative phase of wound healing may last anywhere from a few days to a few weeks.
2.2.1 Extracellular matrix deposition
Fibroblasts are recruited to the area approximately 2–3 days after the injury and begin to lay the extracellular matrix (ECM) components that serve as a scaffold for tissue ingrowth and eventual scar tissue formation. The early scaffolding is mainly composed of type III collagen, hyaluronic acid, and fibronectin.11, 13
Neo-angiogenesis begins to occur as new capillaries are generated throughout the newly deposited ECM components, forming friable tissue with a granular appearance, termed granulation tissue. As angiogenesis continues, some of the fibroblasts undergo fibroplasia to become myofibroblasts, which have phenotypic characteristics of both fibroblasts and smooth muscle cells. The contractile properties of myofibroblasts are responsible for shrinking the size of the wound. Granulation tissue and myofibroblast contraction result in reversible narrowing of the airway by reducing the size of the wound and resultantly the caliber of the lumen.11, 14
2.2.2 Epithelialization
Re-epithelization may start as early as several hours post-injury and continue throughout the proliferation phase. Keratinocytes from undamaged basal layers at the periphery of the wound begin to proliferate and migrate centrally, until leading epithelial edges meet one another and extinguish the process. This process restores the damaged water-tight barrier by reestablishing a network tight junction proteins, a critical defense to the harsh airway environment.9, 11
2.3 Maturation and scar formation
Myofibroblast contraction works to decrease the overall size of the wound as the temporary ECM is replaced with more permanent tissue. The cells critical for the earlier phases of wound healing, including macrophages, endothelial cells, and myofibroblasts, largely undergo apoptosis, leaving a dense collagen network with interspersed remnant cells. Metalloproteinases (MMPs) produced by these cells work to remodel the collagen along the lines of stress and to replace the previously deposited type III collagen with stronger type I collagen. One theory regarding the localization of upper airway stenosis to the subglottis includes the unique stress points of articulation between the thyroid and cricoid cartilages that could drive altered MMP activity and collagen deposition.15 Architecturally, disorganized bundles of collagen are replaced with stronger parallel arrays. This is the longest step of wound healing, with remodeling occurring for up to a year after the initial injury in concert with the dynamic motion of the airway.11, 14
This highly orchestrated wound healing process often occurs as intended, leaving only minimal scarring and little functional impact; however, comorbid disease and critical illness can create imbalance in any of these steps resulting in aberrant scar formation. Diabetes mellitus, for example, can cause an abnormal inflammatory response and has been identified as a major risk factor for the development of laryngotracheal stenosis.9, 10, 16
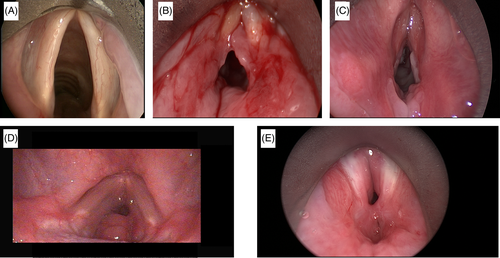
The various phases of wound healing in the airway are demonstrated in the evolution of PGS shown in Figure 3. Despite the distinct clinical profiles that exist between iLTS and iSGS, they are both thought to result from a prolonged and dysregulated wound healing response that promotes the deposition of pathologic scar in the airway. Herein, we review the existing literature surrounding wound healing mechanisms in iLTS and iSGS.
3 IATROGENIC LARYNGOTRACHEAL STENOSIS
Laryngotracheal stenosis may be acquired due to a host of different mechanisms, including prolonged intubation, inhalational thermal injury, external laryngeal trauma, and prior surgical intervention such as tracheostomy. The majority of LTS is the sequelae of prolonged intubation5; therefore, much of the existing literature regarding wound healing and LTS has been garnered from this particular patient population.
Animal models have also proved useful and relevant in this field of study as well, and several different models of laryngeal injury in a few different species, such as rabbits and murine, have been utilized. Given the impracticality of keeping an animal intubated and sedated for days to weeks at a time to simulate a prolonged intubation in humans, most of the described animal models utilize acute mechanical, chemical, and/or thermal mechanisms to induce laryngotracheal injury. Despite this difference in the mechanism of injury, these animals have served as an excellent model for the study of wound healing in laryngotracheal stenosis in response to injurious stimuli.
This review organizes the existing literature into categories of histopathology, inflammatory markers, genetics, and microbiology, including data from human and animal studies.
3.1 Histopathology
Some of the earliest literature on iLTS describes histopathologic findings in patients with acquired laryngeal stenosis. Understanding the histopathologic changes that occur in response to laryngeal trauma is key to understanding the wound healing process.
In 1995, Liu et al. used a whole organ serial section technique to study the sequence of histopathologic changes that occur leading up to mature scar formation in response to intubation trauma. Laryngotracheal complexes from 44 pediatric patients with a history of intubation were obtained at post-mortem examination and serially sectioned. These patients were clinically diverse, with duration of intubation ranging from 1 day to 7 months, with the time from extubation or tracheostomy to the time of death ranging from 1 day to nearly 5 years. This allowed for the unprecedented collection of histopathologic data from different stages of injury and healing.17 In 2002, Duynstee et al. added to this body of literature with a valuable histologic study of 25 surgical specimens obtained from children who underwent cricotracheal resection for iLTS. These studies both demonstrated that ulceration was found to be the earliest histologic change in response to endotracheal intubation. Severity ranged from mild epithelial disruption to full thickness laryngeal ulceration with cartilage penetration, secondary to pressure necrosis and tissue ischemia from direct contact with the endotracheal tube and cuff. While the simple regeneration of epithelium over a superficial ulcer is most often clinically insignificant, the wound healing process of full thickness injury is more complex with potentially severe clinical consequences. In the initial inflammatory response to the injury, the wound bed is prepared with a layer of granulation tissue consisting of capillary blood vessels, inflammatory cells, fibroblasts, and early collagen fibers.17
Epithelial regeneration starts from the periphery of the wound, slowly covering the bed of granulation tissue. Squamous metaplasia is often characteristic of this stage, where the normal ciliated architecture of the subglottic mucosa is lost and replaced with a layer of stratified squamous epithelium. Once a continuous basement membrane forms beneath the epithelium, granulation tissue formation slows and then ceases. Based on the underlying tissue architecture, collagen deposition, and myofibroblast contraction, this new layer of epithelium may be irregular with indentations and furrows as evidence of recent trauma.17, 18
In cases of deep ulceration, exposed cartilage may create a foreign body reaction with the resultant formation of exuberant granulation tissue, at times mimicking the shape of the endotracheal tube or creating exuberant nodular granulation over the vocal processes. In these cases, regenerating epithelium may fail to completely cover exuberant granulation tissue, which then may proliferate beyond the surrounding healing tissue. In many cases, exuberant granulation tissue eventually resolves without significant sequelae, but in some cases it becomes fibrous scar tissue and clinically results in laryngotracheal stenosis with scarring of the posterior glottis or subglottis.17
Fibrosis is the end stage of healing and the pathologic entity primarily responsible for LTS. As the inflammatory and proliferative phases subside, fibroblasts secrete collagen and capillaries are obliterated and resorbed. The previously vessel-rich granulation tissue is slowly replaced with a nearly avascular, stiff scar, with a notable absence of normal subepithelial elastic fibers. Normal submucosal glands and ducts are replaced with irregular cystically dilated structures, and in some cases, small islands of ectopic bone and cartilage are found within the sclerotic tissue. The process of scar formation, maturation, and remodeling proceeds for months. However, the deposition of clinically significant scar occurs over a period of approximately 6–8 weeks after the initial injury, correlating with the development of airway symptoms after a prolonged intubation.17, 18
Airway obstruction may occur due to the deposition of a large amount of collagen-rich scar tissue in the posterior glottis, anterior glottis, subglottis and trachea, thus narrowing the airway lumen. It may also be secondary to just a thin fibrous scar band tethering the vocal processes to one another at the site of resolved exuberant granulation tissue, preventing adequate abduction of the vocal folds. With scar maturation and tissue contraction, the ultrastructure of the larynx may be affected. Distortion of the normally round/ovoid subglottic lumen, dysfunction or fixation of the cricoarytenoid joints leading to vocal fold immobility, and disruption of the triangular glottic aperture may be observed. Taken together, these changes can lead to severe disruptions in respiration, deglutition, and phonation with both life-threatening and life-limiting consequences.17, 18
3.1.1 Histopathology animal
There have been numerous studies published detailing histopathologic findings in animal models of acquired laryngotracheal stenosis. These findings largely correlate with that which has been described in human patients. Several comparative studies have found that superficial mucosal injury often heals without clinical consequence or with only mild soft stenosis. Conversely, cartilaginous exposure and injury was required to produce firm, severe scarring with polymorphic epithelium and bony-cartilaginous islands within the scar.19-22 Of note, adult rabbits appear to be more resistant to cricoid remodeling in response to the scar contraction and maturation, while young animals tend to experience cricoid deformation and collapse.22
There was a relative lack of data on thermal laryngeal injury in the literature until a recent publication by Dion et al., which was the first of its kind to provide a longitudinal analysis of thermal injury and temporal wound healing. In this study, 12 swine underwent tracheostomy and laryngeal thermal injury. These animals were sacrificed at 12 h, 1, 3, 7, 14, and 21 days and their larynges were harvested and microscopically examined, though half of these animals did not survive to their scheduled endpoints. Notable findings included immediate and severe tissue edema and eschar within the first 24 h, most severe at the level of the supraglottis and glottis with relative sparing of the subglottis. Large colonies and mats of filamentous bacteria were present as soon as 12 h after injury, and granulation and epithelial sloughing occurred in the several days following. Injury severity scores were greatest at 24 h, with near resolution of edema by Day 4 and inflammation by Day 14. Early fibrosis was noted as early as Day 7 with mature fibrosis forming by Day 14. By Day 21, the larynges appeared grossly normal.23 While good long term data beyond 3 weeks after this type of injury is lacking, this study filled an important void in the existing LTS literature and confirmed the need for very close monitoring within the first 24–48 h after a burn injury.
3.2 Fibroinflammatory marker profile
Defining the milieu of fibroinflammatory cytokines and molecular markers that drive the pathophysiology of LTS has been the focus of numerous investigations. The goal is not only to better define the condition itself, but to ultimately find a pharmacologic target for modification of the disease course. This section will outline the existing data regarding the fibroinflammatory marker profile of LTS, but it will not address the availability or efficacy of pharmacologic treatments. For an excellent review of this information, please reference Hirshoren and Eliashar's paper from 2009 entitled “Wound-Healing Modulation in Upper Airway Stenosis.”10
One of the earliest studies addressing this topic was published in 1996 by Macauley et al. This study used fibroblast cultures from tissue biopsies from fetal skin, fetal foreskin, and airway scar from a human patient with PGS. They showed that application of TGF-ß1, a key mediator of inflammation and fibrosis, induced maximum levels of mRNA for elastin and procollagen for all three populations. However, expression levels for PGS fibroblasts showed the highest fold increase and were most responsive to the TGF-ß1 signal. In addition, TGF-ß1 induced expression of lysyl oxidase for this cell population, which is responsible for crosslinking elastin and procollagen. Together, these results suggested that PGS fibroblasts may be poised to respond more robustly to the presence of TGF-ß1 than normal fibroblasts via the production of ECM products.24
In 2000, Walner et al. examined the potential role of growth factors in wound healing after laryngotracheal reconstruction (LTR). Histologic sections taken from pediatric patients undergoing LTR were stained for a number of growth factors. These findings were correlated with success after airway surgery, defined as successful decannulation, improvement in airway grade, and need for fewer reconstructive procedures. “Good wound healers” were found to have higher levels of vascular fibronectin, tenascin, and stromal fibronectin while “poor wound healers” (higher airway grade) had higher levels of stromal VEGF. These findings suggest that a differential underlying growth factor environment may explain why two patients suffering the same injury may have very different outcomes. Additionally, an angiogenic response at the cellular level is critical for the wound healing process in the airway.25
In 2017, Yin et al. published findings regarding the effect of hypoxia on laryngotracheal fibroblasts. They demonstrated similar response in the airway to the known transdifferentiation of pulmonary, cardiac, and hepatic fibroblasts into pro-fibrotic and contractile myofibroblasts in response to hypoxia. Using brush biopsies from scarred and nonscarred tracheal mucosa in iLTS patients, the researchers found that iLTS scar specimens displayed increased expression of IL-6 (a fibrosis-related cytokine), αSMA (a myofibroblast marker), and MMP13 (marker of fibrosis) relative to control specimens, confirming a myofibroblast phenotype for the iLTS cells. In vitro cultures of fibroblasts from scarred and normal-appearing portions of the airway were grown under normal and hypoxic conditions, and proliferation rate and expression of IL-6, αSMA, COL1 (type I collagen), and MMP13 were measured. For both groups, fibroblast proliferation rate was significantly greater under hypoxic conditions. For normal/control fibroblasts, expression of IL-6, MMP13, COL1, and αSMA were significantly higher in hypoxic conditions relative to normoxic conditions. Expression levels did not significantly change for iLTS-derived fibroblasts. Taken together, these findings confirm the myofibroblast phenotype of fibroblasts derived from iLTS scar and demonstrate a shift toward the myofibroblast phenotype in response to hypoxia for fibroblasts derived from normal tracheal mucosa. This supports the role of hypoxia in the initial pathogenesis of iLTS, leading to a transdifferentiation of resident fibroblasts into contractile and pro-fibrotic myofibroblasts. Myofibroblasts are critical components of wound healing, responsible for wound contracture and ECM deposition, so their position as effectors of fibrosis and airway narrowing in iLTS is not unexpected.26
In the same year, Motz et al. published on the use of endolaryngeal brush biopsies to assessing gene and protein expression from patients with iLTS and iSGS using quantitative RT-PCR and flow cytometry. Though limited genes and gene products were studied, patients with LTS were found to have significantly increased expression of the fibrosis-related gene Collagen-1 (Coll1) and the TH2-related inflammatory cytokine IL-4 relative to control populations. These findings further support LTS as a disease of inflammation and fibrosis and establish brush biopsy sampling as a relatively noninvasive way of measuring and tracking these markers.27 Also in 2017 Ma et al. studied fibroblasts in culture, harvested from areas of iLTS scar and distal normal appearing trachea in the same patients, thus using internal controls. They found that iLTS fibroblast proliferation rate, cell surface area, and collagen-1 and 3 expression were increased relative to control fibroblasts, though expression of fibronectin, MMP-2, αSMA, and TGF-ß were not significantly different between the groups. Metabolic analysis also showed that iLTS fibroblasts rely primarily on glycolysis and less on oxidative phosphorylation relative to their normal counterparts, which may be at least partially responsible for driving proliferation in these cells, a pattern commonly seen in cancer cells.28
Haft et al. measured RNA expression of 5 mediators of wound healing (TGF-ß1, IL-1, MMP-9, αSMA, and SMAD2) and VEGF (a driver of angiogenesis) and protein expression of 18 cytokines in tissue in 10 patients with LTS (9 with iLTS, 1 with autoimmune etiology). Compared to external control bronchus biopsies, LTS specimens showed marked elevation of TGF-ß1, IL-1, and MMP-9. These results were consistent with findings from their murine model of SGS, where injury was induced by wire brush or acid injury, though the mice in this study also showed elevation of αSMA and SMAD2. Protein array showed marked elevations of TGF-ß1, IL-1ß, and MMP9 in both mouse and human LTS specimens.29, 30 These studies helped to further define the microenvironment of iLTS, while also validating the use of their murine model of iLTS. The fibroinflammatory profile of iatrogenic LTS detailed in these studies is summarized in Table 1.
| Study | Study sample | Fibroinflammatory markers | Conclusion |
|---|---|---|---|
| Macauley et al.24 | Tissue biopsy from fetal skin, fetal foreskin, and airway scar from human patient with PGS | Application of TGF-ß1 increased levels of mRNA for elastin and procollogen Expression levels for PGS fibroblasts had the highest fold increase TGF-ß1 also induced lysyl oxidase, which is responsible for crosslinking elastin and procollagen |
PGS fibroblasts respond more to the presence of TGF-ß1 than normal fibroblasts via the production of ECM products |
| Walner et al.25 | Histologic sections from pediatric patients undergoing LTR | Good wound healers were found to have higher levels of vascular fibronectin, tenascin, and stromal fibronectin Poor wound healers had higher levels of stromal VEGF |
Differential underlying growth factor environment may explain why two patients undergoing the same surgery may have very different outcomes |
| Yin et al.26 | Brush biopsies from scarred and nonscarred tracheal mucosa in iatrogenic LTS patients | Iatrogenic LTS specimens displayed increased expression of IL-6 (a fibrosis-related cytokine), αSMA (a myofibroblast marker), and MMP13 (marker of fibrosis For normal/control fibroblasts, expression of IL-6, MMP13, COL1, and αSMA were significantly higher in hypoxic conditions relative to normoxic conditions |
Myofibroblast phenotype of fibroblasts derived from iLTS scar and demonstrate a shift toward the myofibroblast phenotype in response to hypoxia for fibroblasts derived from normal tracheal mucosa |
| Motz et al.27 | Endolaryngeal brush biopsies | Patients with LTS were found to have significantly increased expression of the fibrosis-related gene Collagen-1 (Coll1) and the TH2-related inflammatory cytokine IL-4 relative to control populations | LTS is a disease of inflammation and fibrosis Brush biopsy sampling is a relatively noninvasive way of measuring and tracking these markers |
| Ma et al.28 | Fibroblasts in culture harvested from areas of iatrogenic LTS scar and distal normal appearing trachea in the same patients | Iatrogenic LTS fibroblast proliferation rate, cell surface area, and collagen-1 and 3 expression were increased relative to control fibroblasts Expression of fibronectin, MMP-2, αSMA, and TGF-ß were not significantly different between the groups |
|
| Haft et al.29 | Bronchus biopsies of 10 patients with LTS | LTS specimens showed marked elevation of TGF-ß1, IL-1, and MMP-9 | Defined the microenvironment of iatrogenic LTS |
3.2.1 Inflammatory markers animal
Animal models from a number of species have proved valuable in investigating mediators of wound healing in LTS as well. In a murine chemomechanical injury model of SGS, Hillel et al. showed that mice with bleomycin brush injury expressed increased levels of collagen-1, TGF-beta, elastin, IL-1ß, and macrophage gene CD11b relative to controls at various time points. They also studied macrophage immunophenotype, and findings suggested that dysregulated M2 macrophages may play a role in abnormal wound healing in LTS.31
Singh et al. studied the migration of airway fibroblasts harvested using a rabbit model of SGS, where a subglottic injury and subsequent stenosis was induced via silver nitrate or CO2 laser cautery injury. Both types of injured SGS fibroblasts migrated faster than normal fibroblasts (though only to a statistically significant degree for the silver nitrate injury group). Interestingly, when the fibroblasts were exposed to prostaglandin E2 (PGE2), a potent anti-inflammatory agent produced during the wound healing process, the SGS-derived specimens were partially refractory to its anti-fibroblastic effects as measured by degree of fibroblast migration and contraction.19 In a similar study using a rabbit model of SGS, Sandaluche et al. found that SGS-derived fibroblasts migrate faster and contract to a greater degree than normal adult counterparts and were again partially refractory to the anti-fibrotic effects of PGE2.32 Taken together, these studies suggest that the dysregulated wound healing response in SGS may be partially due to altered fibroblast responsiveness to the anti-fibroblastic signals produced after an injury such as PGE2.
3.3 Genetics
The genetic background of an individual can impact their wound healing response, and therefore it is reasonable to propose that some patients may be at greater risk of developing LTS after a prolonged intubation than others based on their genetic profile. At this time, little has been published regarding the genetics underlying LTS.
In 2010, Rovo et al. compared the frequencies of four polymorphisms of the TGF-ß1 gene between patients with iLTS secondary to prolonged intubation and patients who were intubated but did not develop iLTS. TGF-ß1 was the target of this investigation given its role as a strong inducer of myofibroblasts and key player in inflammation and fibrosis. They found that the −509 C/T polymorphism was more common in control patients than iLTS patients, suggesting a protective function of this genotype. They also found that the C/C genotype may increase susceptibility to iLTS.33
Anis et al. published two papers in 2014 and 2018 with the aim to identify single nucleotide polymorphisms (SNPs) in wound healing genes that may be associated with iLTS. The first paper investigated SNPs in candidate genes CD14, matrix metalloproteinase-1 (MMP-1), and TGF-ß1 in 40 patients with iLTS and 36 patients with a history of airway injury without development of iLTS. This study showed a significant association between the presence of the MMP-1 SNP with iLTS development. In the 2018 study, however, this association was not found. In this study of 53 iLTS patients and 85 control patients, SNPs in wound healing genes MMP1, MMP3, MMP12, CD14, TGF-ß1, and MCP1 were investigated. In the overall group, no associations were found between the development of iLTS and these particular SNPs, though subgroup analysis did show a possible association between the MMP12 SNP and iLTS development in African Americans.34
Overall, these data provide a starting point for defining the genetics underlying iLTS development; however, a larger sample size and broader investigation into candidate wound healing genes will be required in order to produce actionable data.
3.4 Microbiology
The role of the microbiome in immunity and inflammation has yet to be well defined; however, the importance of the alterations in the microbial environment in the healing of acute and chronic wounds is increasingly being recognized.35 The composition of the laryngeal microbiome in the context of LTS has been the subject of two investigations over the last few years. In 2017, Gelbard et al. utilized culture-independent techniques to profile laryngeal microbial flora. They found that 10 out of 10 iLTS patients showed PCR positivity for Acinetobacter baumannii, which is a known ICU pathogen, compared to only one of 10 iSGS patients.36 In 2019, Hillel et al. used 16S rRNA amplicon sequencing to examine the entire microbial complement among different forms of LTS. In this study, samples were collected from stenotic and normal appearing areas (internal control) of the trachea from iLTS and iSGS patients, and from normal tracheas in healthy patients (external controls). General findings were notable for a decrease in microbial diversity in scar samples compared to nonscar samples. This suggests a role for microbial dysbiosis in LTS pathogenesis, and an inverse correlation between Prevotella, a known commensal bacterium of the respiratory tract, and Streptococcus, a known pathogen with a role in scar deposition elsewhere in the airway, in all samples.37 No unique resident microbes were identified specific to iLTS in this study, which may be secondary to the heterogeneous nature of iLTS with many patients having been exposed to a litany of different antibiotics during a prolonged ICU stay.38
4 IDIOPATHIC SUBGLOTTIC STENOSIS
Idiopathic subglottic stenosis is a chronic, recurrent, and fibroinflammatory condition causing scarring and narrowing of the subglottic airway in the absence of any obvious preceding iatrogenic injury or trauma. It generally presents in otherwise healthy middle-aged white females with symptoms of dyspnea and stridor, unresponsive to empiric treatment for asthma.2, 6 The pathophysiology of iSGS is unclear, though it is thought to be a phenomenon of aberrant and unchecked wound healing mechanisms, with proposed contributions from the effects of estrogen given the preponderance of female patients and/or acidic or enzymatic mucosal trauma secondary to laryngopharyngeal reflux. Another hypothesis postulates that an intermittent telescoping effect of the first tracheal ring into the lumen of the cricoid cartilage occurs as a consequence of trauma from coughing. This results in mechanical injury, localized ischemia, and anomalous wound repair, and ultimately fibrosis.15
4.1 Estrogen
Estrogen has long been thought to underlie the pathophysiology of iSGS, given a near absence of this disease in males. The role of estrogen in wound healing has been previously established, with evidence that post-menopausal females with insufficient estrogen levels suffer from delayed wound healing, and that topical and systemic estrogen treatments can increase the rate of acute wound healing by reducing the inflammatory response and accelerating re-epithelialization.39, 40 It has also been shown that abnormal variants of estrogen receptor beta (ER-ß) are associated with venous ulceration and poor wound healing.41 It is therefore reasonable to propose that estrogen plays an important, though poorly defined at this time, role in iSGS development.
Several early studies failed to demonstrate the presence of estrogen receptors in iSGS tissue biopsies.42, 43 This led to a bit of a detour away from this line of investigation for several years, until 2018 when Fiz et al. demonstrated a higher concentration of ER-α in stenotic tissue relative to peri-stenotic and control tissue, as well as a lower concentration of ER-ß and progesterone receptor in stenotic tissue. They also found that menopause and estrogen replacement therapy were associated with a higher grade of stenosis in iSGS patients.44 The following year, Damrose et al. demonstrated the presence of ER-α and ER-ß in subglottic tissues using immunofluorescence. Relative to control tissues, iSGS specimens demonstrated a significantly greater intensity of ER-α in the epithelium, and ER-ß in the submucosal glands and ducts.45
These recent studies provide some early support for estrogen activity in the subglottis, with differential receptor expression in iSGS patients when compared to normal controls. Given the well-described impact of estrogen on dermal wound healing, further study is warranted to better define the role of estrogen in the etiology and pathophysiology of iSGS.
4.2 Fibroinflammatory marker profile
iSGS is characterized by pathologic and excessive deposition of extracellular matrix (ECM) components, particularly collagen and fibronectin, in the subglottic lamina propria. This can be likened to a sustained inflammatory response leading to inappropriate activation of the proliferation and wound maturation phases of wound healing. ECM deposition is driven by hyperproliferative fibroblasts mediated by a number of different inflammatory factors under investigation. In 2020, Motz and Gelbard published an excellent review of the role of inflammatory cytokines in the development of iSGS, which can be referenced for a more thorough review of the data.46 The data herein is a brief summary of the existing literature.
Several recent publications have provided early data regarding inflammatory markers associated with laryngotracheal stenosis and iSGS. In 2016, Gelbard et al. published a study which demonstrated significant activation of the IL-23/IL-17A pathway in samples of tracheal mucosa from patients with iSGS, suggesting a possible role for this pathway as a driver for inflammation and fibrosis in this disease. The primary cellular source of IL-17 in this study was found to be γδ TCR+ CD3 T cells, which are known to play a role in both the adaptive and innate immune system, and have been shown to play a critical role in the pathogenesis of a more thoroughly characterized fibrotic disorder, idiopathic pulmonary fibrosis.47
In a follow-up paper published in 2019, Morrison et al. found that IL-17 is a direct driver of iSGS fibroblast proliferation, working synergistically with TGF-ß1 to amplify local inflammatory cytokine signals and to stimulate ECM production. IL-4 was found to directly stimulate the production of chemokine ligand 2, cytokine IL-6, and granulocyte-macrophage colony-stimulating factor crucial for myeloid cell recruitment and differentiation toward an inflammatory phenotype. In addition to proving a link between upregulated IL-17A, scar fibroblasts, and pathologic fibrosis in iSGS, this paper also showed some early evidence that iSGS fibroblasts not only serve to lay down new ECM, but also alter the local microenvironment with the production of their own chemokines and cytokines.48
As referenced above, Motz et al., found significantly increased expression of Coll1 and IL-4 in LTS patients, a pattern that was seen in both iLTS and iSGS patients. They also found significantly higher expression of interferon-y, an inflammatory cytokine, in iSGS patients relative to control populations, which was unique from iLTS patients.27 IL-4 is known to play a role in fibrosis, wound healing, and immune function and IFN-y has been implicated in fibrosis in several organ systems, positing both as potentially valuable therapeutics for this disease.
4.3 Laryngopharyngeal reflux
Chronic and repetitive microtrauma from gastric acid and enzymes in the setting of laryngopharyngeal reflux has also long been considered a driving force behind the development of iSGS, and exposure to pepsin and acid has been shown to have deleterious effects on glottic wound healing, with increased inflammation and collagen deposition in the setting of exposure.49 Early studies supported this proposition by demonstrating a high rate of co-morbid laryngopharyngeal reflux in iSGS patients.50, 51 In 2011, Blumin et al. found detectable levels of pepsin in subglottic biopsy specimens from 13/22 (59%) iSGS patients compared to undetectable levels in five control patients, though this did not correlate with extra-esophageal reflux presence on dual pH probe studies.52
Recently, McCann et al. published a contradictory in vitro study examining the effects of pepsin on subglottic fibroblasts and healthy vocal fold fibroblasts in culture. Each group of fibroblasts was exposed to pepsin at neutral or low pH or to TGF-ß as a positive control. qPCR and ELISA were used to measure gene and protein expression for a variety of fibroinflammatory markers, collagen, and fibronectin. Likewise, a basic wound healing/cell migration assay was performed using vocal fold fibroblasts exposed to pepsin at low and neutral pH. Pepsin exposure had no effect on gene or protein expression or on cell migration for either group of cells. This study therefore did not support acute pepsin exposure as a direct cause of iSGS.53
Clinically, Bianchi et al. reported on 175 patients with tracheal stenosis who underwent esophageal manometry and dual-probe 24-hour ambulatory esophageal pH study. Patients with an abnormal pH study were managed laparoscopically with a modified Nissen fundoplication or medically (omeprazole 80 mg/day, orally). Patients with normal pH study results were observed. Patients were followed for 24 months and a satisfactory outcome was defined as achieving stability of tracheal stenosis by resection, without need for further dilation, and allowing for definitive decannulation. This study demonstrated that patients with pathologic acid reflux who underwent fundoplication had a similar outcome of the stenosis compared with subjects without pathologic reflux.54 In a cohort of 95 patients undergoing laryngotracheal reconstruction, Tawfik et al. found that the factors associated with decannulation failure 1 year after surgery were GERD, Cotton-Myer grade 4 stenosis and diabetes mellitus.55 While there appears to be an association between reflux and iSGS, causation has yet to be defined.
4.4 Microbiology
The role of the microbiome in iSGS is not well-understood at this point, and the study of the microbiome's role in inflammation and wound healing is yet in its infancy. However, Gelbard and Hillel have both previously reported on microbiological findings in iSGS, providing a basis for future investigations into this topic.
In 2019, Hillel et al. found that bacteria within the Moraxellaceae family, which includes the genera Moraxella and Acinetobacter were more prevalent in swab samples from iSGS patients than controls to a statistically significant degree. These bacteria are known inflammatory pathogens within the respiratory tract, contributing to COPD exacerbations, asthma, and bronchopulmonary infection, therefore supporting a potential role for the pathogenesis of iSGS. Though the clinical relevance is somewhat unclear, this group also noted decreased prevalence of a commensal bacterial Prevotella in iSGS samples relative to controls.38 In 2017, Gebard it al. found that a distinct form of Mycobacterium was associated with iSGS specimens, unique from iLTS specimens.36 These data taken together may suggest that a disruption of the normal laryngeal microbiota contributes to the pathogenesis of iSGS by promoting a pro-inflammatory state, or perhaps these particular specimens thrive under the altered microenvironment and state of chronic inflammation seen in iSGS and are rather a consequence of the iSGS itself. Further investigation in this field of study will help to elucidate these details and could potentially lead to new therapeutic targets for this disease.
5 CONCLUSION
Laryngotracheal stenosis is considered to be the result of abnormal and dysregulated wound healing mechanisms, leading to hypertrophic scar formation and airway narrowing. The development of mature scar and clinically symptomatic stenosis mirrors the time-course of the classic phases of wound healing. Current management of LTS is primarily surgical and this review highlights the limited adaptation of pathophysiological review in exploring etiology and treatment. Therefore, it is important to understand the basic mechanisms that underlie the healing process in order to identify and intervene on the process early in its development and discover future therapies that target individual wound healing mechanisms limiting the incidence of this recalcitrant disease process.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.