Estimating survival after salvage surgery for recurrent salivary gland cancers: Systematic review
Funding information: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
[Correction added on 30 May 2022, after first online publication: The affiliation of Dr. Marchioni, Dr. Nocini and Dr. Molteni were incorrect in the initial publication. It has been corrected.]
Abstract
Recurrent salivary gland carcinomas (RSCs) are poorly characterized and their clinical features and treatment options have not yet been fully described. The goal of this study was to analyze the therapeutic strategies and oncological outcomes of RSC patients through a literature review analysis. This systematic review was performed according to the PRISMA statements. Inclusion criteria for the systematic review were based on the population, intervention, comparison, and outcomes according to (PICO) framework. Two thousand seven hundred and four records were selected and 1817 recurrences were studied. Three hundred and sixty-five patients underwent salvage surgery (20.1%) and their 5-year mortality rate, overall survival and disease-free survival were 35%, 70%, and 42%, respectively. RSCs are aggressive neoplasms with a high rate of distant metastases (28.9%). Salvage surgery can be considered in patients with limited local and/or regional recurrences, even in case of single distant relapse, appearing within the first 3 years of follow-up.
1 INTRODUCTION
Salivary gland cancers (SGCs) are rare neoplasms comprising approximately 3%–11% of all head and neck malignancies.1 They present an estimated age-standardized annual incidence of less than 2 new cases/100000 persons in most countries.2 Although commonly grouped together, they can develop in both major and minor salivary glands, show heterogeneous clinical presentations and be histologically diverse.3, 4 In fact, according to the most recent World Health Organization (WHO) Classification of Head and Neck Tumors, 22 different SGC histotypes can be distinguished.5
In this setting of epidemiological rarity, histological heterogeneity and unpredictable behavior, recurrent salivary cancers (RSCs) represent an extraordinarily challenging clinical scenario. While surgical resection with or without postoperative radiotherapy (RT) has been commonly recognized as the cornerstone of treatment for primary resectable SGC,6 clinical features of recurrent and/or metastatic disease have not yet been fully described, and their optimal management approach has not reached a widely shared consensus.7-9
SGCs are more likely to recur locally or at distance, and often present at an advanced stage. Since, their 5- and 10-year estimated risks of recurrence have been reported to vary between 17%–49% and 22%–55%, respectively, depending on the histology, grade, stage, and sub-site of origin,8, 9 aggressive salvage surgery is usually favored, given the morbidity associated with uncontrolled tumor progression. Unfortunately, salvage surgery with curative intent has shown to be feasible only in one third of recurrent patients and tumor control is usually obtained in approximately half of them.9, 10
The aim of this paper is to review the available evidence on indications and outcomes of surgical salvage treatment in RSC patients. In particular, we aimed at describing their patterns of failure, temporal distribution of recurrences and outcome of salvage treatment in a wide population of RSC patients reported in the literature in order to offer evidence-based treatment recommendations. Moreover, we tried to address the remaining knowledge gaps and limits, focusing on their implications for design of future studies.
2 MATERIALS AND METHODS
2.1 Search strategy
A systematic review was performed using independently developed search strategies in literature review methodology, and was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement (http://www.prisma-statement.org)11 to guarantee a scientific strategy of research thus limiting bias by a systematic assembly, and providing critical appraisal and synthesis of all the most relevant studies published on this topic.12, 13
Since SGCs represent a dynamic diagnostic-therapeutic scenario in continuous evolution, we carried out a literature search between January 2009 and January 2019, in order to try to include data as homogeneous as possible by overcoming potential biases in diagnosis, staging and treatment indications that might have arisen from a wider temporal span.14 This search was then set to automatically update until the December 31, 2020. MESH and free-text terms such as “salvage surgery,” “salivary gland tumors,” “salivary gland carcinomas,” “salivary gland malignancies,” “surgical treatment of salivary gland carcinoma recurrence,” “salivary gland carcinoma recurrence,” “recurrent salivary cancer,” “recurrent salivary tumors,” and “recurrent salivary malignancy,” were interrogated in the PubMed, Embase, and LILACS databases.
Reference lists from the identified articles were searched and cross-referenced to identify additional relevant articles, and national experts in the field were contacted to identify unpublished data. Duplicates, reviews, conference abstracts, editorial, case report, and non-English articles were excluded. All abstracts were reviewed by the authors. Those related to the subject were selected for further analysis.
Inclusion criteria for the systematic review were based on the population, intervention, comparison, and outcomes according to (PICO) framework.15
2.2 Population
The inclusion criteria were set a priori and deliberately kept wide to encompass as many articles as possible without compromising the validity of the results, and included articles: (1) published from 2009 onwards, in accordance with the abovementioned purposes; (2) reporting histological revision of their cases in accordance with the WHO classification published by Barnes et al.16; (3) reporting published series of RSC patients with at least a median 5-year follow-up; (4) reporting data about the primary SGC treatment, timing of onset, and characteristics of recurrence (i.e., single or multiple, local, regional or distant); (5) describing salvage surgical outcomes in terms of survival percentage (overall [OS] and disease free survivals [DFS]). Articles describing systemic salvage treatment only, reporting pediatric case series, or not matching our inclusion criteria were excluded from the present analysis.
2.3 Intervention and comparison
We filtered studies to ensure that only papers with comparable data were considered in the review. All recorded data underwent an unmasked double check (by G.Ma, G.Mo, L.V.C., and A.S.) and the following features were reported in a standard spread-sheet: study population characteristics (including age and gender distribution), clinical presentation of recurrent disease (recording site and staging, if single or multiple), timing of diagnosis, salvage surgical strategy, histopathological final report, and survival outcomes.
Eligibility for inclusion was separately assessed and, when in doubt, discussed and decided by consensus.
2.4 Outcomes
Abstracts were analyzed to identify papers fulfilling the above-described inclusion criteria and a first qualitative and descriptive review of the selected articles was carried out. Only publications clearly describing their aims and objectives, inclusion and exclusion criteria, and including complete statistical data were considered in this systematic analysis. All papers were graded using the NICE scoring scale for retrospective case series. Scores ≥6 were considered as indicators of a good quality study, scores between 4 and 5 as fair, and a score of 3 as poor quality (http://www.nice.org.uk/nicemedia/pdf/Appendix_04_qualityofcase_series_form_preop.pdf).
Because of the high heterogeneity of the study population, a meta-analysis was not deemed possible; thus, a systematic review was conducted.
Regarding survival, the outcomes studied were 5-year OS and DFS. From each study, the percentages of overall and disease-free surviving RSC patients were collected after 5 years from the salvage procedure. According to the availability of information, we explored the correlations between: (1) time to recurrence and histology; (2) type of recurrence and histology; (3) time to recurrence and 5-year OS and DFS; (4) type of recurrence and 5-year OS and DFS; (5) site of recurrence and 5-year OS and DFS; (6) histology and 5-year OS and DFS. Finally, in order to account for the different sample sizes across the studies, we reported weighted means when possible, weighting each study according to the number of included patients (i.e., studies with more patients had a higher weight in the mean estimation).
3 RESULTS
3.1 Study selection
The study selection scheme is shown in Figure 1. The search strategy resulted in 2704 records, of which 1208 were duplicates. The remaining 1496 abstracts were screened for eligibility, yielding 131 manuscripts. Their full text evaluation identified 64 studies meeting the inclusion criteria.17-80
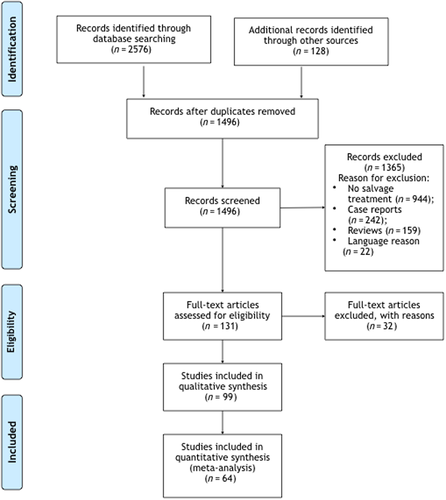
3.2 Study population and general features
Table 1 shows a summary of the 64 studies. The vast majority were published in 2011 (17.2%), 2017 (12.5%), 2014, and 2018 (11.0%, each). A high number of good (17, or 26.5%) and fair (42, or 66.7%) quality papers were reported among the 64 publications.
| Author | Year of publication | Quality assessment score | Study period | Total number of patients reported | Total number of patients with recurrence |
|---|---|---|---|---|---|
| Kobayashi K et al.17 | 2009 | 5 | 1997–2009 | 20 | 20 |
| Oplatek A et al.18 | 2009 | 5 | 1990–2007 | 99 | 52 |
| Mücke et al.19 | 2010 | 6 | 1992–2006 | 9 | 9 |
| Kruse AL et al.20 | 2010 | 5 | 1999–2008 | 27 | 4 |
| Erovic BM et al.21 | 2010 | 5 | 1970–2007 | 47 | 30 |
| Iyer NG et al.22 | 2010 | 5 | 1985–2005 | 67 | 20 |
| Pederson AW et al.23 | 2010 | 4 | 1986–2007 | 14 | 14 |
| Oliveira LR et al.24 | 2010 | 5 | 1990–2009 | 63 | 15 |
| Cha W et al.25 | 2011 | 4 | 1990–2009 | 20 | 5 |
| De Angelis AF et al.26 | 2011 | 5 | 1986–2008 | 24 | 6 |
| Li Q et al.27 | 2011 | 4 | 1971–2006 | 58 | 16 |
| Shen C et al.28 | 2011 | 6 | 1996–2007 | 101 | 44 |
| Feinstein TM et al.29 | 2011 | 5 | 1990–2006 | 74 | 33 |
| Deng R et al.30 | 2012 | 5 | 1992–2010 | 28 | 14 |
| Kim JY et al.31 | 2012 | 5 | 1998–2010 | 35 | 15 |
| Li Q et al.32 | 2012 | 5 | 1968–2008 | 103 | 27 |
| Lee SY et al.33 | 2012 | 3 | 1991–2009 | 60 | 27 |
| Cao CN et al.34 | 2012 | 4 | 1963–2006 | 54 | 54 |
| Zhao J et al.35 | 2013 | 4 | 1999–2006 | 51 | 37 |
| Chen AM et al.36 | 2013 | 4 | 1998–2008 | 61 | 34 |
| Byrd SA et al.37 | 2013 | 5 | 1985–2010 | 101 | 1 |
| Shinoto M et al.38 | 2013 | 4 | 1998–2011 | 25 | 12 |
| Ali S et al.39 | 2013 | 5 | 1985–2009 | 94 | 16 |
| Lukšić I et al.40 | 2013 | 3 | 1984–2008 | 26 | 7 |
| Neskey D et al.41 | 2013 | 4 | 1990–2013 | 155 | 45 |
| Ali S et al.42 | 2013 | 5 | 1985–2009 | 301 | 70 |
| van Weert S et al.43 | 2013 | 4 | 1979–2009 | 105 | 105 |
| Michel G et al.44 | 2013 | 6 | 1998–2011 | 25 | 25 |
| Salovaara E et al.45 | 2013 | 4 | 1997–2011 | 25 | 7 |
| Kaur J et al.46 | 2014 | 5 | 1998–2008 | 65 | 26 |
| Andrade MF et al.47 | 2014 | 5 | 1997–2006 | 38 | 9 |
| Lee SY et al.48 | 2014 | 5 | 1991–2009 | 61 | 35 |
| Shi S et al.49 | 2014 | 3 | 2005–2012 | 38 | 38 |
| Iqbal H et al.50 | 2014 | 2 | 2003–2011 | 45 | 3 |
| Marcinow A et al.51 | 2014 | 4 | 1992–2009 | 87 | 87 |
| Dalgic A et al.52 | 2014 | 3 | 1994–2010 | 12 | 2 |
| Johnston ML et al.53 | 2015 | 6 | 1999–2010 | 54 | 32 |
| Li BB et al.54 | 2015 | 4 | 2001–2012 | 140 | 16 |
| Bjørndal K et al.55 | 2015 | 6 | 1990–2005 | 201 | 72 |
| Haymerle G et al.56 | 2016 | 4 | 1970–2007 | 35 | 20 |
| AL-Qahtani et al.57 | 2016 | 4 | 2007–2014 | 7 | 6 |
| Holtzman et al.58 | 2016 | 4 | 1964–2012 | 224 | 24 |
| Huang T-T et al.59 | 2016 | 5 | 1993–2008 | 7 | 3 |
| Pagh A et al.60 | 2016 | 5 | 2000–2013 | 78 | 10 |
| Ali S et al.61 | 2017 | 6 | 1985–2009 | 87 | 14 |
| Mannelli G et al.62 | 2017 | 5 | 1980–2005 | 44 | 29 |
| Yang XH et al.63 | 2017 | 6 | 2002–2012 | 155 | 35 |
| Park GC et al.64 | 2017 | 6 | 1994–2014 | 108 | 38 |
| Granic M et al.65 | 2017 | 5 | 1982–2015 | 60 | 17 |
| Cordesmeyer R et al.66 | 2017 | 5 | 1995–2016 | 68 | 21 |
| Hämetoja H et al.67 | 2017 | 6 | 1974–2012 | 64 | 35 |
| Mizrachi A et al.68 | 2017 | 6 | 1990–2010 | 20 | 7 |
| Nisa L et al.69 | 2018 | 6 | 1997–2012 | 20 | 20 |
| Forner D et al.70 | 2018 | 6 | 2010–2018 | 240 | 38 |
| Chakrabarti S et al.71 | 2018 | 5 | 2006–2015 | 165 | 61 |
| Boon E et al.72 | 2018 | 6 | 2000–2016 | 31 | 1 |
| Westergaard-Nielsen M et al.73 | 2018 | 6 | 1990–2005 | 15 | 6 |
| Park G et al.74 | 2018 | 5 | 1991–2014 | 44 | 7 |
| Ayre G et al.75 | 2018 | 5 | 1980–2010 | 22 | 2 |
| Hay Aj et al.76 | 2019 | 6 | 1985–2015 | 97 | 97 |
| Nakano T et al.77 | 2019 | 6 | 1983–2014 | 40 | 40 |
| Stodulski D et al.78 | 2019 | 5 | 1996–2015 | 40 | 23 |
| Qian K et al.79 | 2019 | 5 | 2006–2016 | 176 | 31 |
| Mimica X et al.80 | 2020 | 6 | 1985–2015 | 884 | 137 |
A total of 5344 patients were reported and 1817 (34.0%) recurrences were analyzed. Thirteen publications (20.3%) had a study period longer than 30 years.21, 27, 32, 34, 43, 56, 58, 65, 67, 75-77, 80 On the other hand, only 5 articles (7.8%)35, 49, 50, 57, 70 reported a study period shorter than 10 years. The remaining 46 papers (71.9%) presented a median study period of 16 years.17-20, 22-26, 28-31, 33, 36-42, 44-48, 51-55, 59-64, 66, 68, 69, 71-74, 78, 79
Eighteen studies (28.1%) analyzed 946 primary minor SGCs and reported treatment of 426 recurrences (45.0%).20-22, 26, 27, 32-34, 40, 44, 47, 50, 52, 56, 63, 67, 76 Twenty-five articles (39.1%) analyzed 1568 primary major SGCs and reported treatment of 570 recurrences (36.3%).17, 25, 31, 35, 36, 38, 40-42, 45, 46, 49, 57, 59, 60, 62, 64, 68, 69, 71, 73, 75, 77-79 The remaining 21 articles (32.8%) analyzed 2830 primary SGCs arising from either minor or major salivary glands, and reported treatment of 821 recurrences (29.0%).18, 23, 24, 26-30, 37, 43, 48, 51, 53-55, 58, 61, 65, 66, 70, 72, 74, 80
3.3 Patterns of recurrence
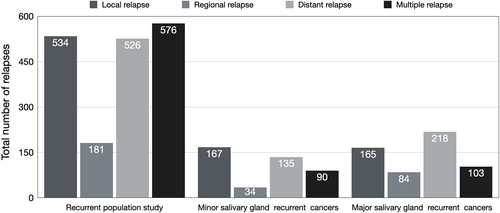
The incidence of single (1241) and multiple (576) relapses among the overall population study (5344), were 23.2% and 10.8%, respectively. Their distribution among the 1817 recurrent patients showed an incidence of single and multiple site relapses of 68.3% and 31.7%, respectively. Local (534), regional (181), and distant (526) recurrences were observed in 29.4%, 9.9%, and 28.9% of patients, respectively.
Among primary minor SGCs (946), 167 experienced single local recurrences (17.2%), 34 had single regional relapses (3.6%), and 135 reported distant metastases (14.1%). Multiple site recurrences were observed in 90 patients (9.5%).
On the other hand, patients affected by primary major SGCs (1568) reported local (165), regional (84), and distant (218) single metastasis rates of 10.5%, 5.4%, and 14.0%, respectively. This study group showed a multiple sites relapse rate of 6.6% (103).
Figure 2 reports the distribution of local, regional, distant, and multiple relapses in the three abovementioned study groups.
3.4 Salvage treatment group characteristics
Three hundred and sixty-five (20.1%) RSC patients were eligible for salvage surgical procedures, while 400 (22.0%) underwent non-surgical salvage treatment. Palliative management was planned for the remaining 1052 (57.9%) patients (2.1 male to 1 female ratio with a median age of 62.43 years). The median time to recurrence was 38 months.
The salvage surgery group was characterized by a mean time to recurrence of 41.54 ± 25.77 months (range, 5–124.38), with a median time to diagnosis of relapse of 36.08 months. A prevalence of males was recorded (2.7 males to 1 female), with a reported median age of 54.65 years. The primary tumor treatment was represented by surgery only in 95.6% (349) of cases, while adjuvant RT was delivered to 16 (4.4%) patients only. A total of 119 (32.6%) primary neck dissections was performed. Extended local re-resection and neck dissection represented the main salvage procedures. Seventy-five (14.2%) distant metastases resulted eligible for surgical resection.
The salvage non-surgical group reported a mean time to recurrence appearance of 44.25 ± 17.63 months (range, 16.9–93.2) and a median time to diagnosis of relapse of 44 months. The female to male ratio was 1 to 1.5, with a median age of 58.5 years. The primary treatment was represented by surgery only in 54.25% (217) of patients, while adjuvant RT was delivered to 185 (45.75%) patients and 5 (1.2%) received adjuvant CRT. A total of 175 (43.75%) primary neck dissections were performed.
The most common reported histology in the overall study group was adenoid cystic carcinoma (ACC) (948 patients among the 5344 starting population). The histology distribution of recurrences delineated a prevalence of ACC (124 recurrences; 50.8%), followed by mucoepidermoid carcinoma (MEC) (51 recurrences; 21.0%), secretory carcinoma (SC) (30 recurrences; 12.3%), salivary duct carcinoma (SDC) (19 recurrences; 7.9%), and adenocarcinoma not otherwise specified (ADC) (16 recurrences; 6.5%) in the salvage surgery population (365 recurrences). On the other hand, the non-surgical salvage group (400 recurrences) presented a high incidence of both ACC (69 recurrences; 43.1%) and SDC (61 recurrences; 38.1%) with respect to the other histologies (17 SC and 13 MEC recurrences, respectively). Table 2 reports the prevalence and the distribution of the most represented histologies in the two abovementioned study groups.
| Histology | Salvage surgery group number (%) | Non-surgical salvage group number (%) |
|---|---|---|
| ACC | 124 (51%) | 69 (43%) |
| MEC | 51 (21%) | 13 (8%) |
| SC | 30 (12%) | 17 (9%) |
| SDC | 19 (8%) | 61 (36%) |
| Others | 20 (8%) | 6 (4%) |
| Total | 244 (100%) | 166 (100%) |
- Abbreviations: ACC, adenoid cystic carcinoma; MEC, mucoepidermoid carcinoma; Others include, adenocarcinoma, acinic cell carcinoma; SC, secretory carcinoma; SDC, salivary duct carcinoma.
3.5 Survival analyses
The correlation between time to recurrence and the most frequently reported histologies showed that ACC and SC developed relapses not earlier than a median of 49.9 and 44.0 months after primary treatment, respectively. In contrast, SDC showed the shortest median time to relapse (15.2 months), followed by MEC (27.8 months).
In turn, the correlation between types of recurrence (i.e., local, regional, distant, or multiple) and histology, when computed over the total number of recurrences, highlighted a prevalence of local recurrence in SC patients (70.7%) and a poor trend in experiencing regional relapse in SDC (4.21%) who reported, instead, the highest incidence of distant metastases (49.5%). Table 3 summarizes the proportions of each type of recurrence over the total number of relapses considering the most common histologies.
| Type of recurrence | |||||
|---|---|---|---|---|---|
| Number of patients | Local | Regional | Distant | Multiple | |
| ACC | 1475 | 34.8% | 7.1% | 31.1% | 25.3% |
| SDC | 172 | 15.8% | 4.2% | 49.5% | 36.8% |
| MEC | 379 | 25.3% | 14.7% | 44.0% | 29.3% |
| SC | 229 | 70.7% | 19.5% | 9.8% | 17.1% |
- Abbreviations: ACC, adenoid cystic carcinoma; MEC, mucoepidermoid carcinoma; SC, secretory carcinoma; SDC, salivary duct carcinoma.
We conducted an analysis on 5-year DFS and OS of the salvage surgery group according to the following parameters: site of recurrence, type of recurrence, histology, and time to recurrence (Table 4). In addition, from each study, we extrapolated information about the number of deaths, individual surviving with disease and disease-free survival at 5-year, by distinguish salvage surgery patients from non-surgical salvage treatment group. We found a mortality rate, a 5-year survival rate with disease and 5-year disease-free survival of 35%, 15%, and 42% for the salvage surgery group against a rate of 73%, 12%, and 3% for the non-surgical salvage group, respectively.
| Salvage surgery patient group | ||||
|---|---|---|---|---|
| 5-Year DFS | 5-Year OS | Number of studies (number of patients) | ||
| Site | Minor salivary gland | 34.3% | 65.0% | 9 (565) |
| Major salivary gland | 14.1% | 64.6% | 15 (910) | |
| Type of recurrence | Local | 100% | 75.3% | 3 (172) |
| Regional | n.e. | 91.8% | 1 (101) | |
| Distant | 23.8% | 62.1% | 6 (260) | |
| Histotype | ACC | 33.7% | 74.1% | 12 (759) |
| MEC | n.e. | 78.9% | 5 (360) | |
| SC | 80.3% | 75.2% | 3 (302) | |
| SDC | 8.1% | 48.1% | 4 (154) | |
| Time to recurrence | <24 months | 26.6% | 64.1% | 9 (811) |
| >24 months | 35.6% | 73.5% | 16 (1443) | |
| <38 months | 34.3% | 69.1% | 13 (1431) | |
| >38 months | 30.8% | 70.1% | 11 (722) | |
- Abbreviations: ACC, adenoid cystic carcinoma; MEC, mucoepidermoid carcinoma; SC, secretory carcinoma; SDC, salivary duct carcinoma; n.e., not enough data.
A second recurrence was described in 51 patients after salvage treatment (6.6%) who experienced 13 local, 16 regional relapses, and 22 distant metastases. The median time to re-recurrence was 45.73 months. Treatment modality of re-recurrences was not reported.
4 DISCUSSION
SGC is a rare and very heterogeneous disease with a tendency to give late recurrence. The 5-, 10-, and 15-year crude survival rates of SGCs are 66%, 51%, and 42%, respectively, where disease-related death can occur even decades after the index treatment.8, 81, 82 Primary SGCs have been well documented in terms of clinical features and risk factors for recurrence.7, 9 On the other hand, management of RSCs have not been fully described nor extensively reported so far. Since the literature reports mainly single institution and retrospective studies, collecting small series with follow-up generally shorter than 10 years, this makes knowledge on survival rates scarce among the global scientific community at large. In this setting, the continuous refining and implementation of the WHO classification since its first publication in 197283 up to its latest update,5 together with the absence of a homogenous data collection and distribution among different institutions and countries,55 contribute to give a partial and incomplete picture of management of recurrent disease.
In our study, we reviewed the clinical features and management approaches of 1817 RSC patients. Our main findings were: (a) RSCs are aggressive neoplasms that present at significantly more advanced stages compared to initial tumors; (b) there is a high rate of distant metastases (28.9%) developing after the index treatment and even following successful salvage approaches (43.1%); (c) a limited number of local, regional or distant recurrences (20.1%) can be managed surgically; and (d) a significant difference in terms of survival in patients managed by salvage surgery versus salvage non-surgical approaches can be demonstrated.
The recurrence rate (34.0%) observed in the population of 5344 primary SGC patients along a median follow-up of 83 months, together with the distribution of the different types of relapse, were very close to those reported elsewhere.8, 9, 24, 55, 84, 85 Regarding the site of relapse, minor SGCs had a higher recurrence rate (45.03%) in comparison to the major salivary glands (36.35%), but the latter presented twice the percentage of distant metastases (20%) in comparison to minor RSCs (10%), together with a threefold higher rate of multiple relapses than the latter (12% vs. 4%). In this setting, the submandibular gland presented a significantly higher incidence of distant metastases in comparison to the parotid (p < 0.05), in accordance with previous reports.68
There is no significant difference in terms of recurrence rate among histotypes in the literature.24, 84 However, this was not confirmed in our investigation which, in agreement with others, suggests that some histologies are more likely to give local (i.e., SC, 70.73%) or distant (i.e., SDC, 49.47%) recurrences, while regional relapses seem to be less common in SDC (4.21%) than in SC (19.51%).86, 87 At the same time, according to our results, multiple relapses are more frequently seen in SDC (36.84%) than in ACC (25.32%) or MEC (29.33%). Some of these discrepancies may be due to revisions in diagnostic criteria for histopathological classification of SGCs, potentially contributing to apparent changes in their prevalence.16 This is a bias that could influence identification of the most frequent histopathological subtypes and hinder comparison among publications over the last two decades. Above all, the most frequently diagnosed RSC in our cohort was ACC, in accordance with previous reports.24
RSC patient rate eligible for salvage treatment was 42.1%, but only 365 received salvage surgery, while 400 underwent non-surgical salvage strategy. The remaining 1052 recurrent patients were addressed by palliative management. There were some differences between the two salvage strategy groups of our study. First of all, the median time to recurrence was 12 months longer in the non-surgical salvage group than in the surgical one (44 vs. 36 months; p = 0.31). This diagnostic delay might explain the non-eligibility for salvage surgery for those 400 patients. Therefore, the vast majority of patients who were potentially eligible for salvage surgery received a diagnosis of recurrence within the first 3 years from their primary treatment.
An interesting finding was that the vast majority of surgically salvaged patients had surgery only as the primary treatment (95.6%), reporting a rate of primary neck dissections of 32.6%. On the contrary, the non-surgical salvage group recorded a much higher rate of primary surgery followed by adjuvant RT (45.75%) together with a percentage of primary neck dissection of 48%. This might be linked to what already published in the literature, suggesting prophylactic neck RT regardless of the status of primary nodal metastasis34, 88 to reduce the risk of recurrence and death.29, 54 However, the significance and influence of postoperative RT on relapse risk and DFS is still uncertain.89-93 A matched-pair analysis from Memorial Sloan-Kettering Cancer Center showed that local control for Stages III and IV in patients receiving combined-modality therapy (i.e., surgery followed by RT) versus patients who underwent surgery alone at 5 years was 51% versus 17%, respectively.94 Based on our results, even though the prognostic significance of postoperative RT was not assessed, the 5-year rates of mortality, OS, and DFS in the salvage surgery group were 35%, 70%, and 42%, respectively. In contrast, the non-surgical salvage group presented a much higher 5-year mortality rate (73%) and a very low DFS (3%) with 12% of patients alive with disease at 5 year from the salvage treatment. The consequent main inference may be that a primary combined-modality therapy might affect the salvage surgical strategy making both local and regional relapse diagnosis challenging due to RT-induced effects, and significantly increasing local morbidity in case of salvage re-resection. For these reasons, selecting patients appropriately for salvage surgery might be challenging because disease presentation is variable and consideration of pre-existing co-morbidities, previous treatments and patient preferences might result in complex decision making. In addition, nodal involvement has been proven to significantly affect OS, but the proportion of nodal metastases is inconsistently discussed in the literature. There is, in fact, consensus that neck dissection should be performed in patients with clinically confirmed neck metastasis. Nevertheless, no agreement has been reached regarding the indications for elective neck dissection (END) in patients with cN0 SGC. The estimated rate of occult metastases in primary tumors is about 25%,33 but the risk of nodal metastases at presentation is rarely described, especially in minor SGCs, and thus END is often considered not necessary.9 The possibility to get a precise pN-staging by END could therefore be important and may be a predictive factor for survival.58, 60 Although patients with clinically or radiographically positive neck disease should undergo a therapeutic neck dissection, those with a clinically negative neck who are at high risk for regional disease achieve high rates of control with elective neck irradiation.95 This underlines that a more appropriate analysis about the prognostic significance of END or irradiation should be addressed in the future.
In terms of type of histology distribution among the two salvage groups, there was an almost statistically significant prevalence of recurrent MEC in the surgical group (p = 0.06), and the most frequent histotype diagnosed in the former was ACC (50.8%). The latter reported a very high incidence rate of both recurrent ACC (43.1%) and SDC (38.1%). Recurrent SC was the third most common histotype in both groups (12.3% and 10.6%, respectively).96
Concerning salvage surgery group only, 5-year OS was superimposable for both major and minor RSCs (64.6% and 65%, respectively). Moreover, salvage surgery resulted in a very high 5-year OS in case of both regional (91.7%), local (75.3%), and distant (62.1%) relapse re-resection, thus ensuring a very high success rate in case of single relapse management.
Distant metastases (DMs) are the primary cause of treatment failure and death in patients with SGCs.80 Our DM rate of 28.9% is comparable to those previously reported, ranging between 18% and 40%.97-100 Accordingly, it is well known that distant relapse appearance needs urgent management given its poor prognosis, with an estimated median OS for all types of SGC of about 15 months and OS rates at 1, 3, and 5 years of 54.5%, 28.4%, and 14.8%, respectively.34, 100 The National Comprehensive Cancer Network guidelines list multiple options for treatment of DM (https://www.nccn.org/), including observation, metastasectomy, targeted therapies and best supportive care. However, there is no universal consensus even for the most common histological variants (i.e., ACC), and the discussion has been traditionally focused mainly on when to start treatment (i.e., at symptoms appearance or before that).
Salvage surgery reached the highest 5-year DFS rate in recurrent SC (80.3%) and the lowest one for recurrent SDC (8.1%). At the same time, recurrent ACC and MEC showed a very high 5-year OS (74% and 78.8%, respectively) (see Table 4). These results advocate an aggressive treatment strategy of the first recurrence in a selected group of patients.
Unfortunately, the aggressive nature of loco-regionally RSCs and the high rate of DM combined with the effect of previous RT impose a non-curative approach in a substantial number of patients. Even our cohort had a high rate of patients who underwent salvage non-surgical procedure (22%) and palliative treatments (58%), according to previously published reports.23, 88, 101
Indeed, among non-surgical approaches, reirradiation of RSCs poses a considerable therapeutic challenge: in fact, the outcomes using conventional RT both in postoperative and definitive settings are dismal.23 In this scenario, modern techniques, including intensity modulated radiotherapy and particle therapy, may play a prominent role. Recently, Orlandi et al.102 analyzed reirradiation outcomes on a cohort of 159 patients, of which 45% were non-squamous cell carcinomas (in particular, 16% ACC and 5% other SGCs). With a median follow-up of 49.9 months, 5-year OS and progression-free survival (PFS) were 43.5% and 20.9%, respectively. Although it is hard to draw conclusions due to the limited number of patients and the mixed nature of such a cohort, outcomes seem satisfactory. In recent years, use of particles, especially by carbon ion therapy, has been investigated to overcome some of the limitations of conventional RT in the treatment of SGCs.103 Thanks to their physical and biological properties, in fact, they may spare normal tissues outside the target and are able to overcome the hypo-oxygenation observed in recurrent SGCs. A recent experience reported excellent clinical results for 51 consecutive un-resectable RSC patients, two thirds with ACC, treated with carbon ions: 2-year PFS and OS were 52.2% and 64%, respectively, with acceptable rates of acute and late toxicities.104
An open question remains about whether current treatment strategies for RSCs impact long-term outcomes in an era of more targeted or locally ablative therapies. For this reason, a multidisciplinary approach is highly warranted whenever dealing with RSCs. However, even though oncological results in surgically salvaged RSCs remain poor, it is important to note that, in the present systematic review, 42% were still disease-free and 70% alive at 5 years after treatment. For these reasons, surgical salvage for RSCs should be individually taken into account in patients with limited local and/or regional recurrences and/or single distant relapses.
This study was primarily limited by the high heterogeneity of the study population. In addition, the vast majority of the included studies were retrospective, non-randomized patient cohorts, sometimes focused on one histotype only, and we were not able to control them by a comprehensive meta-analysis. There were considerable differences in the surgical and postoperative follow-up protocols, as well as in the length of surveillance time. Other weaknesses of our analysis were the relatively small sample size, the heterogeneity in inclusion and exclusion criteria and the wide variety of treatments used over a 30-year period.
5 CONCLUSION
RSC remains a difficult condition to diagnose and treat. We reported on presentation, management, and outcomes of a series of 1817 patients. RSCs are aggressive and highly infiltrative neoplasms, with a high rate of DM. Despite careful follow-up and appropriate recurrence re-staging, many patients are not amenable to surgical salvage.
It is worth pointing out that the lack of high levels of evidence in salvage surgery indication for RSC management has precluded the development of standardized follow-up protocols. According to our results, salvage surgery is required in almost all cases of single local, regional, or distant resectable SGC relapse. Salvage surgery, when the recurrence is timely diagnosed, represents an option of providing a higher chance of oncological success in comparison to salvage non-surgical approaches. Thus, one of the main objectives of patient education should be to enable them to react to alarm symptoms. In fact, this study confirmed the hypothesis that patients benefit from close follow-up, as the vast majority of those amenable to salvage treatment were diagnosed within the first 3 years from their index treatment.
In conclusion, the outcomes of salvage surgery for RSCs are encouraging. Aggressive surgical salvage is justified and should be positively considered for RSC treatment. Our observations can be potentially valuable in the design of prospective studies in RSCs by ensuring proper selection of patients and stratification in future randomized clinical trials.
AUTHOR CONTRIBUTIONS
All authors have participated by (1) giving substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; AND (2) drafting the article or revising it critically for important intellectual content; AND (3) approving the final version of the manuscript to be published; AND (4) agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Giuditta Mannelliand Gabriele Molteni: Conceptualization, Methodology, Data curation, Investigation, Visualization, Writing—original draft & review & editing, Project administration; Lara Valentina Comini and Andrea Sacchetto: Methodology, Resources, Writing—review & editing. Roberto Santoro, Giuseppe Spinelli, Pierluigi Bonomo, Isacco Desideri, Cesare Piazza, Paolo Bossi, Ester Orlandi, Alessandro Franchi, Annarita Palomba, Daniele Marchioni, Riccardo Nocini, and Albino Eccher: Writing—review & editing. Giammarco Alderotti: Methodology, Statistical analysis, Writing—review & editing.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Firenze within the CRUI-CARE Agreement.
CONFLICT OF INTEREST
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.