Radioactive iodine therapy may not improve disease-specific survival in follicular variant papillary thyroid cancer without distant metastasis: A propensity score-matched analysis
Funding information: Department of Science and Technology of Sichuan Province, Grant/Award Number: 2020YFS0166; Education Department of Sichuan Province, Grant/Award Number: 17ZA0170; Sichuan Provincial Cadre Health Department, Grant/Award Number: 2018-107
Abstract
Background
Whether radioactive iodine (RAI) therapy is effective in improving disease-specific survival (DSS) in patients with follicular variant papillary thyroid cancer (FVPTC) without distant metastasis remains unclear.
Methods
Patients with FVPTC were identified from the Surveillance, Epidemiology, and End Results (SEER) database between 2004 and 2015. The Kaplan–Meier method and the Cox proportional hazards regression model were used to evaluate DSS. Propensity score-matched analysis was performed to reduce the influence of confounding bias.
Results
RAI did not improve DSS, even in patients with aggressive features such as T4 classification (p = 0.658), extrathyroidal extension (p = 0.083), lateral lymph node metastasis (p = 0.544), and ≥5 metastatic lymph nodes (p = 0.599).
Conclusion
RAI did not affect DSS in patients with FVPTC without distant metastases in this SEER database study. Multicenter, prospective studies including recurrence and molecular information should be conducted to comprehensively evaluate the effects of RAI on FVPTC.
1 INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most common endocrine-related malignancy, and its incidence has significantly increased in recent years.1, 2 PTC can be divided into several histological subtypes according to different pathological features, such as classical, follicular, tall cell variant, columnar cell variant, hobnail variant, and solid variants.3-5 In addition to the classical variant of PTC (CPTC), the follicular variant of PTC (FVPTC) is another common subtype, accounting for 9%–41% of all PTC cases.4, 6-8
Histologically, FVPTC is characterized by the nuclear features of CPTC (such as nuclear enlargement, clearing, grooves, and pseudoinclusions) and a predominant follicular architecture. It is generally believed that its clinicopathological behavior is located between CPTC and follicular thyroid carcinoma (FTC).4, 8, 9 According to previous reports, the risk of lung metastasis in FVPTC is higher than that in CPTC, but lower than that in FTC. Extrathyroidal extension and cervical lymph node metastases are less common in FVPTC than in CPTC, but more frequent than in FTC.8, 10 Although these two PTC variants have unique clinical and pathological characteristics, several studies have shown that the overall survival of FVPTV and CPTC is similar.7, 8, 11, 12 The current guidelines cannot distinguish CPTC from FVPTC. Moreover, they did not specify whether these two variants should be managed with different treatment strategies.13
Historically, radioactive iodine (RAI) therapy was widely used in the postoperative treatment of thyroid cancer due to its high affinity and ability to target thyroid cells. However, its application has gradually become conservative with the accumulation of evidence-based medical studies in recent years. A growing body of evidence has shown that RAI was only beneficial for some patients with PTC with aggressive characteristics.14-17 Therefore, the recent American Thyroid Association (ATA) guidelines recommend that RAI therapy should be considered for intermediate-risk patients and be routinely applied to high-risk patients with PTC after total thyroidectomy (TT).13 However, to the best of our knowledge, only few studies have evaluated the efficacy of RAI on FVPTC. Whether RAI can improve the survival rate of patients with FVPTC without distant metastasis remains unclear. In order to clarify this issue, we conducted a retrospective population-based study using a large sample of patient data obtained from the Surveillance, Epidemiology, and End Results (SEER) database. Moreover, the effect of RAI on disease-specific survival (DSS) in patients with FVPTC without distant metastases was evaluated.
2 METHODS
2.1 Study population
Data on all patients diagnosed with FVPTC (International Classification of Diseases for Oncology, 3rd Edition codes: 8340/3) were extracted from the SEER database between 2004 and 2015. We only included patients who underwent TT (surgery primary site codes 40 and 50), with or without RAI therapy. Patients with more than one primary tumor, distant metastasis, and tumor size >200 mm, and/or those who underwent external beam radiotherapy were excluded. Hence, only patients who underwent surgery for curative intent were included.18 Those with incomplete demographic and clinicopathological data were excluded. Due to the publicity of SEER data, this study was exempted from approval by the Institutional Review Board of Sichuan University.
We obtained data including demographic characteristics (age at diagnosis, sex, race, and year of diagnosis), clinicopathologic characteristics (tumor size, T classification, pN classification, number of metastatic nodes, multifocality, extrathyroidal extension, and whether RAI therapy was provided), and follow-up data (survival months, follow-up outcome, and cause of death). DSS was used to evaluate the prognosis of patients. The time of initial diagnosis was the starting point of DSS observation, and the end point was death due to tumor.
2.2 Statistical analysis
Continuous variables were expressed as mean ± SD, and the Student's t test was used for comparison. Categorical data were presented as frequency (%). The chi-square test or Wilcoxon rank-sum test was used for comparison. To balance baseline characteristics, we used logistic regression with a caliper width of 0.001 to match patients who received TT or TT + RAI on a one-to-one propensity score to create a subsample adjusted for all available potential confounding factors. The matching variables included age at diagnosis, sex, race, year of diagnosis, tumor size, multifocality, extrathyroidal extension, T and pN classifications, and number of metastatic nodes. The Kaplan–Meier method and the log-rank test were used to compare DSS in the overall and propensity score-matched (PSM) cohorts. The Cox proportional hazards regression model was used for multivariate prognostic analysis. A p-value of <0.05 indicates statistically significant differences. All analyses were conducted using the Statistical Package for the Social Sciences software version 22.0.
3 RESULTS
3.1 Demographic and clinicopathologic characteristics of patients
In total, 22 692 patients with FVPTC were included. Among them, 10 371 (45.7%) received TT alone and 12 321 (54.3%), TT combined with RAI therapy. The distribution of all variables between the two groups significantly differed (all p < 0.05). The TT + RAI therapy group had a higher proportion of patients with younger age (<55 years of age), male, advanced T and N classifications, multifocality, larger tumor size, and extrathyroidal extension. After adjusting for propensity scores, a new cohort of 7036 matched pairs was generated, and all variables were balanced among patients treated with TT alone and TT + RAI in the PSM cohort (Table 1).
| Characteristic | Overall cohort | PSM cohort | ||||
|---|---|---|---|---|---|---|
| TT, n = 10 371 (%) | TT + RAI, n = 12 321 (%) | p-value | TT, n = 7036 (%) | TT + RAI, n = 7036 (%) | p-value | |
| Age (years) | ||||||
| Mean ± SD | 50.4 ± 14.34 | 48.2 ± 14.28 | <0.001 | 49.1 ± 14.57 | 49.2 ± 14.05 | 0.934 |
| <55 | 6536 (63.0) | 9537 (69.3) | <0.001 | 4724 (67.1) | 4690 (66.7) | 0.542 |
| ≥55 | 3835 (37.0) | 3784 (30.7) | 2312 (32.9) | 2346 (33.3) | ||
| Sex | <0.001 | 0.156 | ||||
| Female | 8541 (82.4) | 9631 (78.2) | 5737 (81.5) | 5671 (80.6) | ||
| Male | 1830 (17.6) | 2690 (21.8) | 1299 (18.5) | 1365 (19.4) | ||
| Race | <0.001 | 0.071 | ||||
| White | 8458 (81.6) | 10 069 (81.7) | 5727 (81.4) | 5808 (82.5) | ||
| Black | 1010 (9.7) | 970 (7.9) | 635 (9.0) | 560 (8.0) | ||
| Other/unknown | 903 (8.7) | 1282 (10.4) | 674 (9.6) | 668 (9.5) | ||
| Year of diagnosis | <0.001 | 0.535 | ||||
| 2004–2009 | 3469 (33.4) | 4984 (40.5) | 2769 (39.4) | 2805 (39.9) | ||
| 2010–2015 | 6902 (66.6) | 7337 (59.5) | 4267 (60.6) | 4231 (60.1) | ||
| Tumor size (mm) | ||||||
| Mean ± SD | 14.8 ± 15.04 | 23.4 ± 16.44 | <0.001 | 19.8 ± 15.82 | 19.5 ± 15.01 | 0.211 |
| ≤10 | 5372 (51.8) | 2627 (21.3) | <0.001 | 2125 (30.2) | 2188 (31.1) | 0.149 |
| 10–20 | 2686 (25.9) | 4165 (33.8) | 2600 (37.0) | 2664 (37.8) | ||
| 20–40 | 1680 (16.2) | 3932 (31.9) | 1678 (23.8) | 1580 (22.5) | ||
| >40 | 633 (6.1) | 1597 (13.0) | 633 (9.0) | 604 (8.6) | ||
| Multifocality | <0.001 | 0.237 | ||||
| Absent | 6071 (58.5) | 5442 (44.2) | 3398 (48.3) | 3328 (47.3) | ||
| Present | 4300 (41.5) | 6879 (55.8) | 3638 (51.7) | 3708 (52.7) | ||
| Extrathyroidal extension | <0.001 | 0.275 | ||||
| Absent | 9672 (93.3) | 10 236 (83.1) | 6345 (90.2) | 6306 (89.6) | ||
| Present | 699 (6.7) | 2085 (16.9) | 691 (9.8) | 730 (10.4) | ||
| T classification | <0.001 | 0.160 | ||||
| T1 | 7639 (73.7) | 5700 (46.3) | 4314 (61.3) | 4403 (62.6) | ||
| T2 | 1513 (14.6) | 3323 (27.0) | 1513 (21.5) | 1408 (20.0) | ||
| T3 | 1131 (10.9) | 2996 (24.3) | 1127 (16.0) | 1134 (16.1) | ||
| T4 | 88 (0.8) | 302 (2.5) | 82 (1.2) | 91 (1.3) | ||
| Cervical LN metastasis | <0.001 | 0.058 | ||||
| Absent | 9749 (94.0) | 10 173 (82.6) | 6420 (91.2) | 6339 (90.1) | ||
| CLNM | 436 (4.2) | 1426 (11.6) | 430 (6.2) | 494 (7.0) | ||
| LLNM | 186 (1.8) | 722 (5.9) | 186 (2.6) | 203 (2.9) | ||
| No. of LN examined | <0.001 | 0.525 | ||||
| None | 6001 (57.9) | 6011 (48.8) | 3876 (55.1) | 3810 (54.2) | ||
| 1–10 | 3919 (37.8) | 5197 (42.2) | 2759 (39.2) | 2822 (40.1) | ||
| >10 | 451 (4.3) | 1113 (9.0) | 401 (5.7) | 404 (5.7) | ||
| No. of metastatic LN | <0.001 | 0.294 | ||||
| None | 6001 (57.9) | 8011 (48.8) | 3876 (55.1) | 3810 (54.2) | ||
| 1–4 | 4237 (40.9) | 5783 (46.9) | 3028 (43.0) | 3073 (43.7) | ||
| ≥5 | 133 (1.3) | 527 (4.3) | 132 (1.9) | 153 (2.2) | ||
- Abbreviations: CLNM, central lymph node metastasis; LLNM, lateral lymph node metastasis; LN, lymph nodes; PSM, propensity score-matched; RAI, radioactive iodine; TT, total thyroidectomy.
3.2 Prognostic impact of RAI therapy on DSS
The median follow-up time for the overall and PSM cohorts was 61 months (range: 1–155 months, interquartile range [IQR]: 33–97 months) and 64 months (range: 1–155 months, IQR: 34–100 months), respectively. During the follow-up period, there were 97 (0.4%) and 64 (0.5%) disease-specific deaths in the overall and PSM cohorts, respectively. The 5- and 10-year DSSs of the overall cohort were 99.7% and 99.1%, and that of the PSM cohort were 99.8% and 98.8%, respectively. There was no significant difference in terms of DSS between the TT and TT + RAI groups in the overall and PSM cohorts (both p > 0.05, using the log-rank test) (Figure 1).
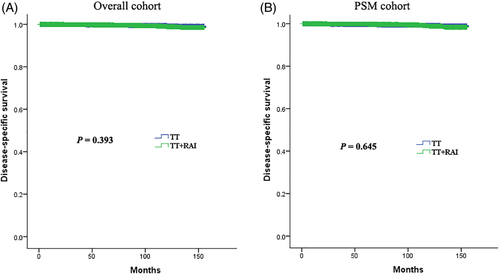
The multivariate Cox proportional hazards model analysis indicated that adjuvant RAI therapy after TT did not improve the DSS of the overall cohort (adjusted HR = 0.84, p = 0.435), nor the DSS in the PSM cohort (adjusted HR = 0.86, p = 0.535) (Table 2). After controlling for other variables, the predictors of high risk for disease-specific death in the overall cohort were old age (≥55 years), large tumor (>40 mm), extrathyroidal extension, and higher T classification (T4) (all p < 0.05). In the PSM cohort, old age (≥55 years), large tumor (>40 mm), extrathyroidal extension, higher T classification (T3/T4), and ≥5 metastatic lymph nodes were independently associated with a higher risk of disease-specific death (all p < 0.05).
| Characteristics | Overall cohort | PSM cohort | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age, years | ||||
| <55 | Ref | Ref | ||
| ≥55 | 11.83 (6.81–20.56) | <0.001 | 11.60 (5.91–22.73) | <0.001 |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 1.02 (0.65–1.61) | 0.928 | 1.03 (0.60–1.77) | 0.930 |
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.73 (0.97–3.14) | 0.062 | 1.11 (0.49–2.50) | 0.801 |
| Other/unknown | 0.45 (0.18–1.12) | 0.086 | 0.43 (0.13–1.40) | 0.162 |
| Year of diagnosis | ||||
| 2004–2009 | Ref | Ref | ||
| 2010–2015 | 0.56 (0.33–1.12) | 0.071 | 0.58 (0.30–1.12) | 0.103 |
| Tumor size (mm) | ||||
| ≤10 | Ref | Ref | ||
| 10–20 | 0.85 (0.44–1.65) | 0.624 | 0.71 (0.33–1.53) | 0.381 |
| 20–40 | 0.62 (0.25–1.54) | 0.304 | 0.51 (0.15–1.74) | 0.281 |
| >40 | 2.67 (1.19–5.97) | 0.017 | 3.03 (1.14–8.04) | 0.026 |
| Multifocality | ||||
| Absent | Ref | Ref | ||
| Present | 0.79 (0.53–1.20) | 0.273 | 0.75 (0.45–1.26) | 0.277 |
| Extrathyroidal extension | ||||
| Absent | Ref | Ref | ||
| Present | 3.80 (1.79–8.06) | 0.001 | 3.42 (1.45–8.08) | 0.005 |
| T classification | ||||
| T1 | Ref | Ref | ||
| T2 | 0.85 (0.44–1.65) | 0.624 | 1.34 (0.56–3.24) | 0.514 |
| T3 | 0.62 (0.25–1.54) | 0.304 | 6.82 (3.74–12.43) | <0.001 |
| T4 | 2.67 (1.19–5.97) | 0.017 | 9.28 (8.33–14.65) | <0.001 |
| Cervical LN metastasis | ||||
| Absent | Ref | Ref | ||
| CLNM | 1.45 (0.69–3.03) | 0.323 | 1.77 (0.67–4.66) | 0.251 |
| LLNM | 2.27 (0.95–5.46) | 0.067 | 2.41 (0.70–8.30) | 0.164 |
| No. of LN examined | ||||
| None | Ref | Ref | ||
| 1–10 | 0.66 (0.28–1.53) | 0.334 | 0.83 (0.44–1.56) | 0.555 |
| >10 | 1.48 (0.63–3.52) | 0.372 | 0.81 (0.28–2.38) | 0.709 |
| No. of metastatic LN | ||||
| None | Ref | Ref | ||
| 1–4 | 1.44 (0.57–3.65) | 0.443 | 0.94 (0.55–1.63) | 0.830 |
| ≥5 | 1.57 (0.53–4.64) | 0.413 | 2.82 (1.24–6.42) | 0.008 |
| RAI | ||||
| No | Ref | Ref | ||
| Yes | 0.84 (0.54–1.30) | 0.435 | 0.86 (0.52–1.40) | 0.535 |
- Abbreviations: CI, confidence interval; CLNM, central lymph node metastasis; HR, hazards ratio; LLNM, lateral lymph node metastasis; LN, lymph nodes; PSM, propensity score-matched; RAI, radioactive iodine.
Subgroup analyses (Table 3) showed that adjuvant RAI therapy was not significantly associated with improved DSS under any other clinicopathologic characteristics, even in patients with aggressive features such as age ≥55 years (adjusted HR = 0.81, p = 0.453), T4 classification (adjusted HR = 0.71, p = 0.658), tumor size >40 mm (adjusted HR = 1.26, p = 0.583), multifocality (adjusted HR = 0.53, p = 0.086), extrathyroidal extension (adjusted HR = 0.44, p = 0.083), lateral lymph node metastasis (adjusted HR = 0.68, p = 0.544), and ≥5 metastatic lymph nodes (adjusted HR = 0.61, p = 0.599).
| Stratified subgroups | Adjusted HR of RAI therapy (95% CI)a | p-value |
|---|---|---|
| Age, years | ||
| <55 | 1.22 (0.37–4.08) | 0.746 |
| ≥55 | 0.81 (0.46–1.41) | 0.453 |
| Sex | ||
| Female | 0.92 (0.50–1.67) | 0.776 |
| Male | 0.78 (0.31–2.01) | 0.610 |
| T classification | ||
| T1 | 1.14 (0.44–2.98) | 0.785 |
| T2 | 1.11 (0.24–5.21) | 0.895 |
| T3 | 0.76 (0.37–1.57) | 0.461 |
| T4 | 0.71 (0.16–3.23) | 0.658 |
| Tumor size (mm) | ||
| ≤10 | 1.14 (0.38–3.42) | 0.814 |
| 10–20 | 0.80 (0.20–3.14) | 0.744 |
| 20–40 | 0.46 (0.13–1.62) | 0.224 |
| >40 | 1.26 (0.55–2.89) | 0.583 |
| Multifocality | ||
| Absent | 1.48 (0.71–3.08) | 0.299 |
| Present | 0.53 (0.25–1.10) | 0.086 |
| Extrathyroidal extension | ||
| Absent | 1.39 (0.71–2.73) | 0.341 |
| Present | 0.44 (0.19–3.02) | 0.083 |
| Cervical LN metastasis | ||
| Absent | 1.04 (0.63–1.72) | 0.876 |
| CLNM | 0.38 (0.12–1.18) | 0.094 |
| LLNM | 0.68 (0.20–2.34) | 0.544 |
| No. of LN examined | ||
| None | 1.47 (0.73–2.95) | 0.282 |
| 1–10 | 0.53 (0.21–1.30) | 0.165 |
| >10 | 0.30 (0.06–1.51) | 0.145 |
| No. of metastatic LN | ||
| None | 1.46 (0.73–2.95) | 0.282 |
| 1–4 | 0.61 (0.26–1.47) | 0.273 |
| ≥5 | 0.61 (0.10–3.89) | 0.599 |
- Abbreviations: CI, confidence interval; CLNM, central lymph node metastasis; HR, hazards ratio; LLNM, lateral lymph node metastasis; LN, lymph nodes; PSM, propensity score-matched; RAI, radioactive iodine.
- a Hazards ratios are adjusted for age, race, sex, year of diagnosis, T classification, tumor size, multifocality, extrathyroidal extension, cervical LN metastasis, number of lymph nodes examined, and metastatic lymph nodes.
4 DISCUSSION
Recently, with the significant increase in the incidence of PTC, the diagnosis of FVPTC has increased.2, 19, 20 Despite the high incidence, the clinical behavior and prognosis of FVPTC remain controversial. Several studies have shown that FVPTC is an aggressive subtype of PTC because it associated with a greater risk for lung metastasis compared with CPTC.8, 10 In addition, patients with FVPTC had a lower complete remission rate and a higher rate of persistent stable lesions and progressive disease than patients with CPTC and FTC based on a long-term study.21 On the contrary, several studies have shown that FVPTC had a mild biological behavior with a low incidence of extrathyroidal extension, lymph node metastasis, and distant metastasis, and the long-term survival rate was similar or even better than that of CPTC.7, 22-24 A recent population-based study by Yu et al.8 analyzed a larger sample of cases including 21 796 patients with CPTC, 10 740 with FVPTC, and 3958 with FTC from the SEER database between 1988 and 2007. Results showed that the 10-year DSS of FVPTC was similar to that of CPTC (98% vs. 97%, p > 0.05), but better than that of FTC (98% vs. 94%, p < 0.001). These inconsistent evidences pose a significant challenge in the development of standard treatment procedures for FVPTC.
RAI therapy is routinely recommended for distant metastatic patients with PTC with RAI avidity. A few studies have indicated that RAI treatment can reduce disease-specific mortality in patients with distant metastasis.25, 26 However, the impact of RAI on DSS in patients with PTC without distant metastasis is still controversial.14, 27, 28 Recent studies have shown that RAI should be used only in some patients with PTC.17, 28-30 Most studies did not distinguish CPTC from FVPTC, without considering the potential impact of the different pathological variants of PTC. Nevertheless, whether this finding is applicable to patients with FVPTC remains unclear. Moreover, most studies on FVPTC mainly focused on clinicopathological features and prognostic evaluation, and they did not exclusively evaluate the efficacy of RAI in patients with FVPTC. Thus, the impact of RAI on the prognosis of patients with FVPTC should be urgently assessed. In the recent population-based study conducted by Yu et al.,8 multivariate analysis revealed that RAI could not improve DSS in patients with FVPTC. However, the study included patients with FVPTC with distant metastasis who benefited from RAI treatment, which may have an important impact on the overall efficacy of RAI therapy. In addition, a subgroup analysis for further analyzing the influence of different clinicopathological characteristics on the outcome of RAI treatment was not conducted.
In our study, a larger sample of patients with FVPTC (n = 22 692) without distant metastasis from the SEER database was included, and the long-term DSS of FVPTC with or without RAI after TT was compared. Results showed that patients with FVPTC had excellent DSS, and RAI had no influence on the DSS of FVPTC, even among high-risk subgroups, such as patients with old age, advanced-stage disease, large tumor, multifocality, extrathyroidal extension, lateral lymph node metastasis, and ≥5 metastatic lymph nodes. As noted in several previous studies,31-33 some of these features such as extrathyroidal extension and absolute number of lymph node metastases may not ultimately be expected to influence DSS in patients with differentiated thyroid cancer. To reduce the influence of confounding factors and make the conclusion more reliable, PSM was used to adjust baseline covariates. PSM can adjust post hoc for recognized unbalanced factors at baseline to reduce the influence of confounding and selection bias in “real-world” observational research. Before matching, the baseline covariates were quite different, and the clinicopathological characteristics between the two groups were balanced after matching. Although our findings indicated that RAI did not improve the DSS of FVPTC, it should be noted that it is possible that the slight benefit of RAI to FVPTC may be masked by a good long-term prognosis.
In addition to clinicopathological differences, FVPTC and CPTC have varying molecular profiles.34-36 Several studies have reported that FVPTC carried a high frequency of RAS mutation, but BRAF mutation was not common.34, 36 In contrast, CPTC harbored a higher BRAF mutation rate and a lower RAS mutation rate.34-36 In addition, rearranged during transfection (RET) proto-oncogene/papillary thyroid carcinoma (RET-PTC) and paired box-8 (PAX8)/peroxisome proliferator-activated receptor γ (PPARγ) fusion protein (PAX8-PPARγ) rearrangements with variable frequency have been reported in FVPTC.34, 37, 38 These mutations and rearrangements may influence the iodine affinity of thyroid cells, thereby affecting the efficacy of RAI.39, 40 This may be one of the reasons why RAI has different effects on CPTC and FVPTC.
Recent studies have shown that FVPTC may be a heterogeneous disease with three distinct types: encapsulated, nonencapsulated, and infiltrative subtypes (EFVPTC, NFVPTC, and IFVPTC), each of which has different clinicopathologic aggressiveness.41-43 EFVPTC and NFVPTC may be indolent, with a lower rate of extrathyroidal extension and lymph node metastasis, particularly in the absence of angioinvasion and capsular invasion. While IFVPTC is regarded as a more aggressive subtype, it had a higher propensity for extrathyroidal extension and lymph node metastasis.42 In this study, although FVPTC could not be classified into distinct subtypes due to lack of data, our subgroup analyses revealed that RAI did not significantly improve DSS in patients with FVPTC with aggressive features, such as T4 classification (p = 0.658), extrathyroidal extension (p = 0.083), lateral lymph node metastasis (p = 0.544), and ≥5 metastatic lymph nodes (p = 0.599). However, whether RAI is effective in improving DSS in patients with IFVPTC should be further assessed.
The current study had several limitations. First, our study is unable to evaluate the effect of RAI on disease free survival given the lack of recurrence data in the SEER database. Second, there might be classification errors in the SEER database because a centralized review of pathologic data was not performed and the diagnostic criteria of FVPTC had always been controversial. Third, the current study is retrospective in nature. Thus, our results are limited by unstandardized of RAI treatment, including potential differences in the mode of preparation of RAI, and variability in practices in terms of therapy dosing. Although PSM was performed, not all bias could be controlled, and bias in the selection of patients with advanced-stage disease who are receiving RAI might still exist. That is, patients undergoing RAI might have seen with more advanced-stage disease. Fourth, the usual limitations inherent to a large national database such as SEER, where there could be attribution bias in attributing death to thyroid cancer, when death could have been from another unreported cause in a patient with history of thyroid cancer. Finally, these data were obtained from United States and might not be extrapolated worldwide.
In summary, we evaluated the impact of RAI treatment on DSS in patients with FVPTC based on a large, population-based, and PSM study with a long follow-up time. Results showed that patients with FVPTC without distant metastasis had an excellent DSS, and RAI did not affect DSS, even among high-risk subgroups, such as patients with old age, advanced-stage disease, large tumor, multifocality, extrathyroidal extension, lateral lymph node metastasis, and ≥5 metastatic lymph nodes. Considering the potential limitations, multicenter, prospective studies including recurrence and molecular information should be conducted to comprehensively evaluate the effects of RAI on FVPTC.
ACKNOWLEDGMENTS
This study was supported by the Project of Sichuan Provincial Science and Technology Department (2020YFS0166), Project of Sichuan Provincial Education Department (17ZA0170), and Project of Sichuan Provincial Cadre Health Department (2018-107). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
All authors contributed on data analysis, drafting, and critically revising the paper and they are accountable for all aspects of the work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in software package SEER*Stat 8.3.6 (https://seer.cancer.gov/seerstat/).




