Microbiosis in lung allotransplantation and xenotransplantation: State of the art and future perspective
Jiao Guohui and Wu Kun contributed equally to this study.
Abstract
The respiratory tract is known to harbor a microbial community including bacteria, viruses, and fungi. New techniques contribute enormously to the identification of unknown or culture-independent species and reveal the interaction of the community with the host immune system. The existing respiratory microbiome and substantial equilibrium of the transplanted microbiome from donor lung grafts provide an extreme bloom of dynamic changes in the microenvironment in lung transplantation (LT) recipients. Dysbiosis in grafts are not only related to the modified microbial components but also involve the kinetics of the host-graft “talk,” which signifies the destination of graft allograft injury, acute rejection, infection, and chronic allograft dysfunction development in short- and long-term survival. Microbiome-derived factors may contribute to lung xenograft survival when using genetically multimodified pig-derived organs. Here, we review the most advanced knowledge of the dynamics and resilience of microbial communities in transplanted lungs with various pretransplant indications. Conceptual and analytical points of view have been illustrated along the time series, gaining insight into the microbiome and lung grafts. Future endeavors on precise tools, sophisticated models, and novel targeted regimens are needed to improve the long-term survival in these patients.
Abbreviations
-
- AR
-
- acute rejection
-
- BAL
-
- bronchoalveolar lavage
-
- BCC
-
- burkholdheria cepacia complex
-
- BOS
-
- bronchiolitis obliterans syndrome
-
- CF
-
- cystic fibrosis
-
- CLAD
-
- chronic lung allograft dysfunction
-
- CMV
-
- cytomegalovirus
-
- LT
-
- lung transplantation
-
- MHV
-
- murine gammaherpesvirus
-
- MMF
-
- mycophenolate mofetil
-
- NGS
-
- next-generation sequencing
-
- NTM
-
- nontuberculous mycobacteria
-
- RV
-
- respiratory viruses
1 INTRODUCTION
Human populations can be clustered mainly by gut microbiome into three “enterotypes” [1]. Bacteroides, Prevotella, and Ruminococcus are symbolized species in enterotypes. Emerging studies have been conducted on the dynamics and resilience of enterotypes and pathological microbial dysbiosis [2, 3]. The gut and lungs are anatomically distinct; however, the gut and lungs form from the same embryonic tissue and bear commonalities in structure and physiology. The gut–lung axis is a hub for maintaining immune homeostasis [4]. Crosstalk between the gut and lung is achieved by lymph circulation and circulated metabolites produced by the microbiota [5]. Chronic lung disorders exhibit a dysbiotic airway and gut microbiota [6], which are further associated with dysregulated lung immune status [7].
The lung microbiota has been defined as core constituents harbored in airways, which has changed the long-term concept of the sterile airway notion in pulmonary medicine [8]. The lung microbiome varies at the lobe or segment level, leading to significant spatial heterogeneity [9]. Bronchoalveolar lavage (BAL), lung biopsies or tissue samples can yield varied results [10]. For transplanted lungs, the condition for microbes dwelling has been further altered due to the reconstruction of airway resulting in poor airway secretion clearance, cough reflex or microaspiration, posttransplantation immunosuppressive drugs, and antibiotics [11]. The microbiota in transplantation medicine has unique characteristics involving both donors and recipients [12]. The most important research tract for microbiota in recent years has been from the demonstration of the topography of the airway microbiota to discover the interaction of the host and microbe in a functional and therapeutic way [13]. To reach the goal of therapeutic manipulation of pulmonary microbiota in lung transplant (LT) patients, the causative link needs to be confirmed with more details, as demonstrated in this review.
2 MICROBIAL COMPOSITION AND DIVERSITY IN LUNG ALLOTRANSPLANTATION
Common knowledge of the airway microbiota has demonstrated five representative bacterial phyla (Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria) (Figure 1). The first three were the predominant components, while the latter two were the occasional representation. The predominance of any single bacterial phylum in the posttransplantation era was recognized to be microbial dysbiosis [14]. Most observed phyla included Bacteroidetes, Firmicutes, and Proteobacteria in lung transplant recipients; however, in nontransplant subjects, Prevotellaceae, Veillonellaceae, and Streptococcaceae were detected frequently [15]. BAL from lung transplant recipients was found to have increased levels of proinflammatory Pseudomonadaceae, Enterobacteriaceae, and Staphylococcaceae and decreased levels of Prevotellaceae, Veillonellaceae, and Streptococcaceae, aligned with remodeling gene expression [16].
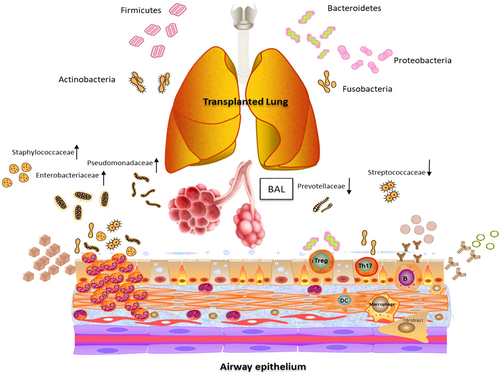
The focus of microbiome research has transitioned from solely descriptive cross-sectional analysis of composition to a longitudinal scale study [17], permitting an observation of the host response to study how the interaction benefits or destroys diversity, as well as the modulation of donor/recipient cells, alteration of the alloimmune response and posttransplant complications [18]. More precise tools, such as transcriptomics and single-cell sequencing, allow a more complementary data profile [19]. The lung microbiota should be considered together with the microbiota in the oral cavity and nasopharyngeal and upper respiratory tracts. Prevotella (Bacteroidetes) frequently dominated, while Veillonella (Firmicutes) and Neisseria (Proteobacteria) negatively correlated with typical lower airway infectious pathogens such as Staphylococcus (Firmicutes), Pseudomonas, and Klebsiella. The decreased diversity not only resulted from lung disease with functional impairment but also with antibiotic use [20]. Thus, a variety of strategies to maintain the microbial ecosystem balance have been established in the host respiratory tract in microbe–host crosstalk [21, 22]. For posttransplant patients, tracheobronchitis or lung microbiome colonization showed more diverse microbiome profiles, while pneumonia was associated with elevated cytokine responses and a higher rejection rate than colonization [23]. Each individual had a “microbiome type” representing a core functional microbiome responsible for gut diseases and extragastrointestinal tract pathological conditions [24], including drug metabolism. A commonly used immunosuppressive drug, mycophenolate mofetil (MMF), shifted the microbial cluster by increasing Proteobacteria and decreasing Bacteroidetes phyla, thus affecting drug toxicity, bioavailability, and bioavailability [25]. The Escherichia/Shigella genus was related to MMF-induced symptoms, while Bacteroidetes reduction and expansion of Actinobacteria were associated with obesity [26].
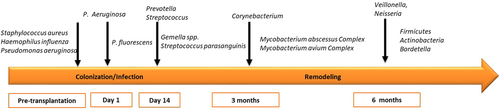
The microbiome composition in cystic fibrosis (CF) patients who received transplantation has been comprehensively demonstrated. Higher bacterial richness was found in the respiratory zone than in the conducting zone, irrespective of the time post-LT. The proportions of the phyla Firmicutes and Proteobacteria were inversely correlated in both zones. Pretransplantation colonized microbial communities included Staphylococcus aureus, Haemophilus influenzae, and Pseudomonas aeruginosa [27]. After transplantation, bacteria that remained in the sinuses, such as P. aeruginosa, seeded the allograft, which occurred within the 1st day. Patient follow-up research has shown a predominance of Prevotella and further Corynebacterium [28]. In post-LT recipients, the microbiota of the conducting zone might be more closely related to the microbiota of the recipient, whereas the microbiota of respiratory airways more closely reflected the donor microbiota [29]. Interspecies interactions have been known to occur between P. aeruginosa and microbiome constituents in CF patients [30], such as Streptococcus parasanguinis as an oral commensal, interfering with P. aeruginosa pathogenesis [31]. BAL from post-LT graft infection patients showed loss of bacterial diversity, with enrichment of Burkholderia, Corynebacterium, and Staphylococcus and reduction of anaerobes [32]. The association of P. aeruginosa and P. fluorescens with acute infection and the development of bronchiolitis obliterans syndrome (BOS) has been investigated. The risk of BOS has been found to be remarkably higher in patients with de novo Pseudomonas acquisition [33]. Nontuberculous mycobacteria (NTM), such as the Mycobacterium abscessus Complex and Mycobacterium avium Complex, are recognized as insidious opportunists, leading to increased morbidity in CF [34]. There is increasing concern about NTM in the post lung transplantation period, especially with BOS development. However, evidence is still lacking. Apart from the knowledge gap on microbial interactions with host immunity, interactions with viral, and fungal communities are still not fully understood and need to be addressed [35].
Respiratory viruses (RVs) pose a constant risk for transplanted-lung function decline, with an incidence of 7.7%–64% [36], increasing opportunistic infections and acute rejection (AR) [37]. Picornaviruses, coronaviruses, and influenza were among the most reported viruses. The presence of an RV in the previous 3 months was associated with the appearance of AR, appearance of P. aeruginosa and cytomegalovirus (CMV) replication or diseases. CMV, EBV, and human herpesvirus have attracted attention; however, the data seem to be confounding [38]. Coinfection with CMV and Pneumocystis jirovecii correlated with worse prognosis and lung microbiota alteration in solid organ transplantation recipients [39]. Murine gammaherpesvirus (MHV) belongs to the family Herpesviridae and is related to Epstein–Barr and Herpes Saimiri virus [40]. MHV might alter the lung microbiota community composition, mediating pulmonary inflammation, and fibrosis [41]. Early reports showed that pulmonary infection correlated with influenza and lung function impairment was associated with early graft dysfunction or chronic lung allograft dysfunction [42]. The torque teno viral load increased immediately in the postoperative period, as detected in the oropharyngeal washes and BAL samples; the magnitude of viral load was associated with primary graft dysfunction development [43]. In most lung virome studies published, the DNA virus community received more attention than the RNA virus community [44]. Other viruses detected by next-generation sequencing remain unnamed and uncharacterized, which calls for further prospective longitudinal research to determine their roles.
The lung mycobiome is highly diversified in populations and conditions. Anaerobes were reported to be isolated with P. aeruginosa, and the latter was found to be more likely associated with Candida albicans than with Aspergillus fumigatus or other oral mycobiomes [45]. Candida, Aspergillus, and Cladosporium could be found in oral wash and BAL post-LT without excluding antifungal drugs as the main confounding factor [46]. Most fungal species detected in sputum samples by sequencing could not be cultured, but they remained stable through antimicrobial therapy [47]. Colonization with Aspergillus was found to be strongly associated with the development of BOS and increased BOS-related mortality in recipients who had Pseudomonas-dominated microbiomes [48]. Colonization with Aspergillus should be speculated to explain the fungi results after fully speculating the possibility of contamination with sampling techniques, environmental factors, microorganism translocation, and microaspiration via orotracheal tubes. Future mycobiota studies are suggested to reveal their roles in the context of treatment with causation other than just correlation [49]. The virome and mycobiome are not silo mentality in the lung microenvironment and should be treated as integral to lung transplantation perioperative care.
3 MICROBIAL KINETICS WITH IMMUNE SYSTEM AND GRAFT SURVIVAL IN LUNG TRANSPLANTATION
General knowledge of the microbiota recognized that the diversity of bacteria increased during the first 9 months post-LT. Immune phenotypes could be altered by individual microbial taxa, such as Bacteroides fragilis, and some Clostridium species promoted Treg development [50], and filamentous bacteria contributed to Th17 cells. Macrophages and fibroblasts with expressed genes of matrix synthesis or degradation were characterized as having the most distinct profiles post-LT [51]. The microbiota could exert its immune modular effect beyond colonization by the pathway of type I interferons and nuclear factor-ΚB pathway activation as well as induce IL-10+B cells and suppress T-cell activation [52-54]. Lower airway microbiota could intrinsically accommodate the local milieu and have been subjected to altered interaction with the local innate immune system [55]. Infection with S. aureus and Listeria monocytogenes resulted in broken tolerance, and further microbes could alter the intensity or kinetics of acute rejection [56].
An increasing number of studies have linked dysbiosis variations to recipient reactions [57]. Firmicutes- or Proteobacteria-dominated dysbiosis correlated with altered inflammation-regulated gene expression, with decreased macrophage, and elevated neutrophil percentages; Bacteroidetes-driven dysbiosis was frequently related to a pro-remodeling context and high macrophage percentage. Prevotella was believed to be less stimulatory but had an undesirable influence of promoting tissue remodeling if overrepresented [58]. This influence was almost limited to the first 12 months post-LT. A catabolic remodeling process peaked between 3 and 6 months, and then, an anabolic remodeling process was revealed from 12 months onward [59]. A catabolic remodeling background was described as driven by elevated Firmicutes or Actinobacteria and then decreased when Bacteroidetes predominated. Driven factors of post-LT dysbiosis included Firmicutes and then Bacteroidetes, thus aligning with catabolic and anabolic remodeling kinetics.
Infections as virulence factors, including S. aureus and P. aeruginosa, were enriched in the communities associated with a catabolic remodeling profile. A so-called “master of genomic flexibility” S. pneumoniae with various virulence genes helped to escape from host innate immunity [60]. Early maximal catabolic remodeling and dysbiosis between 3 and 6 months post-LT linked more to infection with highly stimulatory bacteria; later anabolic remodeling profile and dysbiosis between 12 and 24 months, stimulated by bacteria with upregulated matrix degradation products. The process was observed not only in chronic lung allograft dysfunction (CLAD) but also in stable patients or patients with other airway complications [61, 62]. A high abundance of Pseudomonas existed between days 3 and 25 post-LT. On Day 95, some of the commensal flora, such as Actinobacillus and Bordetella, had efficiently outgrown Pseudomonas and Staphylococcus in bronchial aspirate [63]. Two distinct subpopulations were further identified in the whole Pseudomonas population. The phenotypes and mutation selection of the microbiota post-LT reflected a different adaptation process of the dominant population in the grafts.
There was a systemic change in the microbiota in the newly transplanted graft (Figure 2): on post-LT Day 1, the allograft reflected the microbiota composition of the donor organ with high richness and diversity. Pseudomonas and Staphylococcus gradually invaded the graft and established a different microbiota composition with less richness and diversity, which could be similar to the pre-LT composition at approximately 10 days post-LT. There would be another community shift 3 months post-LT. Actinobacillus became dominant, especially following anti-infection treatment with imipenem. Furthermore, using bronchoalveolar lavage fluid samples to identify bacterial load and community composition states by an unsupervised machine learning approach, four “pneumotypes” were demonstrated: PneumotypeBalanced, PneumotypeMD, PneumotypeStaphylococcus, and PneumotypePseudomonas. PneumotypeMD was believed to contribute to airway remodeling, while PneumotypeStaphylococcus and PneumotypePseudomonas were linked to an inflammatory status with immune dysregulation and rejection [22]. The transplanted lung microbial ecosystem, transplanted lung function, and post-LT clinical course are believed to be intimately linked [64].
Research on microbiota did not merely find invasive pathogens as infection sources but looked into the targeted restoration of the microbial environment as a whole. The intestinal microbiome also influences the immune cells circulating and accumulating in the lungs after ischemia-reperfusion injury [65]. Chronic rejection events, such as BOS, have been the area of most focus in microbiome research [66]. The initial description presented a negative association of Pseudomonas spp. [67] and positive risk of P. aeruginosa with BOS [68]. Conflicting findings of microbiome diversity were due to the lack of consideration of clinical parameters and immune-background of the recipients [69]. A high abundance of P. aeruginosa was recognized to be associated with acute infection and lower airway colonization [70, 71]. Transplanted lungs frequently acquired sinus pathogens before donation, thus reflected by similar adaptive patterns by post-transplant pulmonary Pseudomonas strains [72].
The leading cause of death in adult lung transplant recipients beyond 1 year is chronic lung allograft dysfunction (CLAD) [55], which has been recognized as the reason for poor long-term outcomes. Respiratory infections are a widely recognized risk factor for CLAD development. The composition of the lung bacterial community in patients with CLAD showed significant differences from the composition of the lung bacterial community in patients who survived or were CLAD-free [73, 74]. Recent research has focused on the altered lung microbiota predicting post-LT outcomes. Patients infected with certain species in the Burkholderia cepacia complex before transplant have been reported to have significantly worse outcomes than patients without infection [75]. In the early stage post-LT, richness, and diversity of the oropharyngeal microbiome were restored to the level between healthy and pretransplant subjects. However, if recipients suffered infection or advanced lung disease, dysbiosis appeared [76]. Post-LT invasive pulmonary aspergilosis is among the leading causes of infection and death. Differences in bacterial diversity at the onset of invasive pulmonary aspergilosis have predictive value for infected patients [77]. Posttransplantation pulmonary grafts dominated by Proteobacteria were found to be associated with high neutrophil and proinflammatory status. Bacteroides-dominated grafts had low neutrophil and high macrophage counts with an anti-inflammatory profile [16]. Moreover, gram-positive environments correlated with a decreased risk of CLAD and were dominated by Acinetobacter [78]. Thus, post lung transplantation microbial dysbiosis has been recognized to be associated with the pretransplant microbiome when studying its effect on CLAD.
4 GUT MICROBIOTA IN LUNG XENOTRANSPLANTATION: PERSPECTIVE
Gut microbiota-derived a1,3-galactose (Gal) antigen content has been reported to contribute to inflammation in immune-related disorders [79]. Humans have been discovered to have high levels of Gal against circulating antibodies [80]. The microbiota synthesizes the α1,3-Gal epitope and interacts with the human microbiome and immune response. The identified gut bacteria bearing α1,3-GT genes mostly belonged to the Enterobacteriaceae family, Haemophilus influenzae, Pasteurellaceae genera, and Lactobacillus species. Anti-Gal antibodies have also been identified as the major components contributing to hyperacute vascular xenograft rejection in xenotransplantation. Genome editing has revolutionized genetically modified pigs [81]. Genetically multi-modified pig-derived organs have been put into clinical transplantation trials, which lack Gal and express human membrane cofactor protein (CD46) and thrombomodulin [82]. Nonhuman primates have been reported to survive for over a year with kidneys [83] and for over 6 months with heart xenografts [84]. However, prominent inflammation and graft loss still beset lung xenotransplantation practice. Rapid failure could be seen in Gal-transferase knockout pig lungs transplanted into baboons. Combined Gal-transferase knockout, with the expression of human complement, coagulation regulatory proteins, anti-inflammatory enzymes, and self-recognition receptors, as well as blocking multiple proinflammatory innate and adaptive immune mechanisms, has successfully extended lung xenograft recipient survival to 1 month [85]. However, anti-non-Gal antibodies could not be eliminated even upon immunosuppression. Whether these antibodies are associated with the gut–lung axis and microbe–host crosstalk warrants further investigation. To date, there is no direct evidence from studies focusing on the role of microbiota from xenografts. However, another perspective could be to cope with complex posttransplantation complications, other than new immunosuppression drugs and gene-editing tools.
5 LIMITATION AND FUTURE DIRECTION
Currently, most studies have been performed in relatively small numbers of lung transplant patients but with highly innovative insights. Mostly, the studies described the composition of microbial communities and their distribution in airways. Generations of microbial identification techniques have contributed to the analysis of microbe communities in patients with airway diseases [86]. The most significant problem related to the inadequacy of techniques was contamination via the respiratory tract during sampling. Modification of testing methods and analysis methods would overcome these barriers to some extent. For the detection of lung-specific bacteria, it is important to identify oral &QJ0;and nasal sampling, thus excluding the impact of sedation-related aspiration or bronchoscopy carryover.
Moreover, how microbial species contribute to clinical disease is still unknown. High-throughput sequencing has expanded the knowledge of microbes; moreover, it is critical to move this field forward to investigate the functional properties of these organisms and their relationship to disease progression. Methodologies such as transcriptomics, proteomics and metabolomics are currently being used to understand how these organisms affect and influence the immune response of the host [87, 88]. Based on clinical manifestations, comprehensive evaluation tools have been used on the infection and rejection risk by performing on-site imprint cytology of biopsy, sequencing, and histologic examination. However, how this coinfection affects the long-term prognosis of patients remains to be observed. The follow-up period was relatively short, and it was difficult to determine whether the patients were stable or had chronic rejection, which may require further longitudinal observation.
While most studies have focused on single group of microbes, the interaction of various infectious agents, cross-talk of microbial community, such as gut–lung axis, together with fully understand resident and transient species, when and how dysbiosis affecting the host organism are more complex and fascinating questions [89, 90]. Novel clinical trials of immune regulation regimes rather than mixed probiotic supplementation in an uncontrollable way need to be explored with insight. The eminent effect of probiotics in children or early in life [91] will inspire their further development in pediatric transplantation.
AUTHOR CONTRIBUTIONS
Jiao Guohui: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal). Wu Kun: Conceptualization (equal); data curation (equal); formal analysis (equal). Tian Dong: Conceptualization (equal); data curation (equal); formal analysis (equal). Zhang Ji: Conceptualization (equal); data curation (equal); formal analysis (equal). Liu Dong: Conceptualization (equal); data curation (equal); formal analysis (equal). Wei Dong: Conceptualization (equal); data curation (equal); formal analysis (equal). Chen Jingyu: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal).
ACKNOWLEDGMENTS
None. There are no funders to report for this submission.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
None.
INFORMED CONSENT
None.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




