Decreases in Hormone Levels Modulate Neurovascular Coupling but Not Reduced Insular Functional Connectivity in Menstrual-Related Migraine
Funding: This work was supported by Joint Research Fund of Science and Technology Department of Henan Province (222103810049).
ABSTRACT
Menstrual-related migraine (MRM) is a neurovascular disorder associated with decreased sex hormone levels. The menstrual cycle influences both cerebrovascular function and functional brain connectivity, with accumulating evidence linking migraine to altered connectivity, particularly in the insula. However, the neuropathological mechanisms underlying MRM during the menstrual cycle remain poorly understood. In this longitudinal study, 36 MRM patients and 29 healthy controls were recruited. Sex hormone levels and resting-state functional magnetic resonance imaging (fMRI) were collected during both the late-follicular phase (LFP) and the perimenstrual phase (PMP). Neurovascular function was assessed using voxel-wise hemodynamic response function (HRF) parameters. The subregions of insula-to-whole-brain phase synchronization were estimated using the HRF variations corrected phase information. Our results showed that hormone level decreases from the LFP to the PMP modulated HRF response heights. Changes in the HRF width were reversed between MRM patients and controls, with hormone fluctuations particularly affecting the superior temporal gyrus in the MRM group. Additionally, MRM patients exhibited increased insular phase synchronization in the LFP and reduced synchronization in the PMP. These findings suggest that menstrual cycle-related hormone fluctuations contribute to dysregulated neurovascular coupling in MRM. The reduced insular phase synchronization in the PMP may not be directly driven by these hormone changes.
Summary
- Sex hormones and fMRI in the late-follicular and the perimenstrual phases were assessed.
- Spontaneous hemodynamic response was estimated to characterize neurovascular coupling.
- Neuronal activity phase information was recovered from resting-state fMRI.
- Hormone fluctuations but not hormone levels contribute to the neurovascular coupling changes.
- MRM exhibits increased insular phase synchronization in the LFP, which then collapses in the PMP.
1 Introduction
Migraine is one of the most common neurological disorders worldwide, and migraines affect women more often than men (Ashina 2020; Pavlovic et al. 2017). Approximately 50% of females that experienced migraines reported associations between migraine attacks and menstruation (Martin and Lipton 2008). According to the International Classification of Headache Disorders, 3rd edition (ICHD-3), menstrual-related migraine (MRM) is a subtype of migraine that involves migraine attacks occurring within the perimenstrual phase (PMP) in at least two of three menstrual cycles, with additional migraine attacks occurring at other non-menstrual phases (Olesen 2018).
To date, the neuropathological mechanism of MRM is still unknown. Numerous neuroimaging studies employing resting-state blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) have revealed disrupted functional connectivity in patients with MRM (Huang et al. 2024; Xu et al. 2020; Zhang et al. 2021). However, most previous studies focused on the interictal phase of MRM, and the longitudinal effects of hormone variations during the menstrual cycle in MRM have not been well investigated. MRM is closely associated with the natural menstrual cycle, in particular, with the withdrawal of hormone levels (Raffaelli et al. 2023). The menstrual cycle typically consists of the follicular phase, ovulatory phase, and luteal phase. It could also be characterized by cyclical fluctuations in hormone levels, with rising estrogen levels in the late-follicular phase (LFP) and decline of progesterone and estradiol levels in the PMP (Borsook et al. 2014; Graziottin and Serafini 2016; MacGregor et al. 2006). It is likely that the sharp decline in estrogen that occurs during the PMP is associated with MRM (Raffaelli et al. 2023). Previous studies have shown that these hormonal fluctuations influence both cerebrovascular function (Korad et al. 2022; Krause et al. 2006; Krejza et al. 2013) and functional brain connectivity (Avila-Varela et al. 2024; Hidalgo-Lopez et al. 2021; Pritschet et al. 2020) in both healthy females and those with hormonal migraine (Dzator et al. 2021). It is also important to note that the BOLD signal used in functional connectivity analyses is predominantly modulated by cerebrovascular reactivity (Buxton 2012; Wu et al. 2021). Therefore, the mixed effects of hormone-driven cerebrovascular changes and MRM-related neurophysiological alterations may both contribute to observed changes in functional connectivity in MRM. This overlap makes it challenging to disentangle the specific contributions of hormone fluctuations from the underlying neurophysiological mechanism of MRM.
The insula is widely recognized as a “hub of activity” in patients with migraine, playing a critical role in responding to hormone fluctuations during the menstrual cycle and migraine attacks (Borsook et al. 2016; De Bondt et al. 2016). Morphometric study has reported reduced insular cortex thickness with aging in females with migraine (Maleki et al. 2015). Beyond structural changes, increased anterior insular functional connectivity has been observed during the interictal phase in patients with migraine (Tso et al. 2015). Moreover, functional connectivity between the insula and the medial prefrontal cortex has been linked to pain intensity during migraine attacks. Additionally, the insular connectivity has also been shown to associate with hormone fluctuations across menstrual cycle. For instance, enhanced insular effective connectivity has been associated with heightened estradiol levels in healthy women (Hidalgo-Lopez et al. 2021). Given that different insular subregions are thought to establish connections with distinct functional brain networks and play various roles in pain processing (Borsook et al. 2016; Gollion et al. 2022; Ke et al. 2020), examining the functional connectivity between these subregions and the whole brain may provide deeper insights into the neuropathological mechanism of MRM during different phases of the menstrual cycle.
In the present study, we explored changes in neurovascular coupling and insular functional connectivity in patients with MRM during both the LFP and the PMP. The voxel-wise hemodynamic response function (HRF) was estimated to characterize the variability in cerebral hemodynamics given information on neurovascular coupling. The HRF-bias-corrected voxel-wise instantaneous phase was decoded to characterize functional connectivity between insular subregions and the whole brain. We hypothesized that both menstrual cycle and migraine modulate neurovascular coupling, as indicated by the HRF shape. Insular functional connectivity exhibited disruptions in patients with MRM.
2 Materials and Methods
2.1 Participants
A priori sample size calculations conducted using G*Power 3.1 (Faul et al. 2009) determined that a total sample size of 34 participants is required to achieve a power of 0.8 at a significance level of α = 0.05 for a repeated-measures analysis of variance (ANOVA) design with two groups, two measurements (LFP and PMP), given medium effect sizes of f = 0.25. Hence, 65 women with normal menstrual cycles, including 36 MRM patients and 29 healthy controls (HCs), were recruited between April 2019 and August 2022. The flowchart of participant recruitment in this study was illustrated in Figure 1. The inclusion criteria were as follows: (1) age between 18 and 50 years; (2) not and not having become pregnant; (3) self-reported regular menstrual cycles, that is, ranging from 21 to 35 days, without self-report of irregular cycles; and (4) being right-handed. The exclusion criteria were: (1) history of neurological/psychiatric diseases or severe head trauma and/or drug abuse; (2) abnormal anatomical MRI data; and (3) time intervals between two fMRI acquisitions exceeding 2 months.
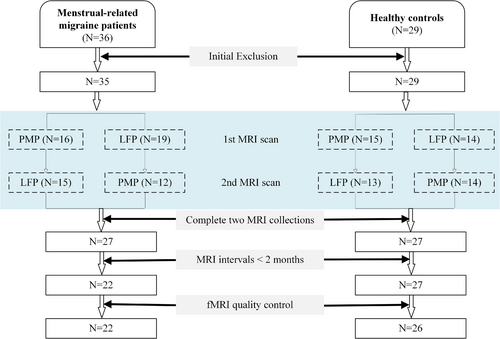
A total of 36 MRM patients, who had not received preventive treatment for migraine, were initially recruited from the Department of Neurology at the First Affiliated Hospital of Henan University of Science and Technology (HAUST). The diagnosis of MRM was based on the self-reported data, according to the ICHD-3 (Olesen 2018), included (i) fulfilling the criteria for migraine without aura; (ii) the attacks occur on 1 ± 2 days of the menstrual phase (within the PMP) in at least two out of three menstrual cycles, attacks additionally occur during other time intervals of the menstrual cycle. One MRM patient was excluded due to a history of head trauma identified. Eight patients were excluded for not completing both the fMRI sessions during the PMP and the LFP. Among the 27 MRM patients who completed both MRI sessions, five were further excluded due to extended time intervals (> 2 months) between the two fMRI scans. This resulted in a sample of 22 participants in the MRM group. In the HC group, 29 age-match female participants were recruited. Two were excluded for not completing both fMRI sessions, and one was excluded due to excessive head motion during fMRI scanning, resulting in a final sample of 26 participants. All participants provided written informed consent, and the study was approved by the Ethics Committee of the First Affiliated Hospital and College of Clinical Medicine of HAUST (Approve No. K-2025-0122).
2.2 General Experimental Procedure
As illustrated in Figure 2A, each participant was invited to visit the Imaging Center of the First Affiliated Hospital of HAUST between 7:00 pm and 8:00 pm for two separate sessions: one is LFP session (approximately 14 ± 1–2 days prior to the next menstruation, characterized by elevated sex hormone levels) and the other is the PMP session (approximately 1 ± 2 days of the menstruation, indicating low sex hormone levels). The PMP was chosen as the study period due to the fact that migraine attacks are most likely to occur within this time window for patients with MRM (Olesen 2018). The LFP was selected for testing because it coincides with the peak of estradiol levels that can be measured in this phase (Borsook et al. 2014).
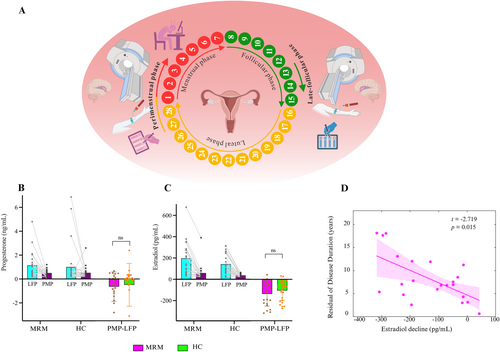
For the MRM group, participants were instructed to complete the PMP session on the same day that they reported a migraine attack. This session was intended to capture around the ictal phase. MRM patients rated their present pain intensity (PPI) index on a 0–5 intensity scale, with 0 = none, 1 = mild, 2 = discomforting, 3 = distressing, 4 = horrible, and 5 = excruciating (Dworkin et al. 2009). The LFP session was scheduled only after at least three consecutive days without any self-reported migraine attacks, corresponding to the interictal phase.
Venous blood samples were also collected during both the LFP and PMP sessions. Serum progesterone and estradiol concentrations were measured using the IMMULITE 2000 XPi Immunoassay system (Siemens Healthcare Diagnostics Inc., Germany). Subsequently, the participants underwent an fMRI scan.
2.3 MRI Data Acquisition
During the LFP and PMP sessions, MRI data were acquired using a 20-channel head coil Siemens 3 T MRI scanner. Resting-state fMRI data with a total of 197 volumes were acquired using a T2*-weighted echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 220 × 220 mm2, matrix = 64 × 64, slice thickness = 3.5 mm, and number of slices = 37. Individual T1-weighted structural MRI data were also acquired with the following parameters: TR = 2300 ms, TE = 2.27 ms, flip angle = 9°, FOV = 250 × 250 mm2, matrix = 256 × 256, slice thickness = 1 mm, and number of slices = 192.
2.4 MRI Preprocessing
The fMRI data were preprocessed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) running in MATLAB 2023a. For each participant, the first seven images collected in each fMRI session were discarded to allow MRI equilibration. The remaining 190 images from each session were first slice-time corrected and then realigned to the average image across two sessions. The individual structural image was subsequently co-registered to the mean of the time- and head motion-corrected images and segmented into grey matter, white matter, and cerebrospinal fluid. A customized template was created using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) toolbox (Ashburner 2007). Finally, the functional data were spatially normalized to Montreal Neurological Institute (MNI) space using the parameters obtained from DARTEL, resampled to 3 mm × 3 mm × 3 mm voxels, and spatially smoothed with a 6 mm Gaussian blurring kernel. Additionally, an independent component analysis based strategy for automatic removal of motion artifacts (ICA-AROMA) (Pruim et al. 2015) was employed to eliminate any remaining head motion-related artefacts, and the average signals of the white matter and cerebrospinal fluid were further regressed out after applying the ICA-AROMA approach.
2.5 Voxel-Wise HRF Estimation
The HRF shape represents the relationship between underlying neural activity and the BOLD response measured using fMRI, and can be used to model neurovascular coupling across the brain (Wu et al. 2019). With the recently proposed spontaneous HRF, we can estimate the voxel-wise HRF on the basis of resting-state BOLD fMRI data and subsequently evaluate local neurovascular coupling throughout the whole brain (Wu et al. 2019, 2021).
Given the variations in the shape of the HRF across individuals, brain regions, and menstrual phases, a data-driven approach implemented in the rsHRF toolbox (Wu et al. 2021) was adopted to estimate the voxel-wise HRF from each session of preprocessed resting-state fMRI data for each participant. Specifically, the preprocessed data were detrended and band-pass filtered, and the signal peaks with relatively large amplitudes (larger than 1.5 times the standard deviation over the mean of each voxel) were considered spontaneous neural events. The HRF for each voxel across the entire brain was estimated by fitting a general linear model to a double-gamma function with the corresponding temporal derivative (Wu et al. 2013). Three parameters, namely the time to peak, response height, and full width at half maximum (FWHM), were estimated for each voxel-wise HRF to characterize the HRF shape across the whole brain (Gao et al. 2023; Wu et al. 2019). The time-to-peak of the HRF shape has been considered to measure the latency of neuronal activity. The response height of the HRF has been used to interpret the magnitude of coupling between neural activity and vascular responses (Wu et al. 2019). The FWHM of the HRF shape has been used to indicate the duration of the BOLD response (Gao et al. 2023).
2.6 Insula Subdivision-To-The-Whole-Brain Phase Synchronization
For the i-th voxel, the BOLD signal can be modelled as the response of a neural activity via a linear time-invariant system with a voxel-specific HRF denoted as , that is, . The convolution between and leads to bias in the phase of the neural activity. That is, the phase information encoded in is a biased version of the phase encoded in . To eliminate this bias and recover the phase of the neural activity, a temporally reversed HRF convolution was conducted, that is, (Lou et al. 2024). In the frequency domain, the phase information of is the same as . Therefore, the HRF for each voxel was temporally reversed and convolved with the unfiltered BOLD signal. The instantaneous phase was estimated as the circular mean of the phases derived from a set of equivalent band-pass filtered (0.01–0.08 Hz) signals followed by a Hilbert transform. As in our previous study (Lou et al. 2024), four types of zero-phase lag filters were employed: ten Butterworth filters with orders from 2 to 20, eight Chebyshev Type I filters with orders from 6 to 20, seven Chebyshev Type II filters with orders from 8 to 20, and seven Elliptic filters with orders from 6 to 18. The first and last 10 decoded instantaneous phases were discarded to prevent the edge effects inherent to the Hilbert transform (Ponce-Alvarez et al. 2015; Wang et al. 2019), leaving 170 time points per session for each participant. The estimated time course of the instantaneous phase was considered to reflect the phase information of the ongoing neural activity.
Subsequently, the seed-to-whole-brain phase synchronization between subregions of the insula and the whole brain was estimated to investigate functional connectivity. In the present study, six subregions of the insula identified by clustering the segregated functional connectivity (Deen et al. 2011) were adopted. These regions have also been used in previous pain-related functional connectivity studies (Wei et al. 2022; Zamorano et al. 2019). Six 6-mm radius spheres designated as the left and right sides of the dorsal anterior insula (dAI), ventral anterior insula (vAI), and posterior insula (PI) were defined as seeds. The center coordinates of these regions of interest (ROIs) were defined in MNI space, that is, left dAI (−38, 6, 2), right dAI (35, 7, 3), left vAI (−33, 13, −7), right vAI (32, 10, −6), left PI (−38, −6, 5), and right PI (35, −11, 6).
2.7 Statistical Analysis
The statistical analyses of the demographic and clinical data were performed via IBM SPSS Statistics 28. The demographic data of the MRM and HC groups were compared using the Mann–Whitney test because the data were not normally distributed. A two-way repeated-measures ANOVA with a between-subjects factor of group (MRM vs. HC) and a within-subjects factor of menstrual phase (LFP vs. PMP) was conducted to analyze the progesterone and estradiol levels separately.
The statistical analyses of the neuroimaging data were carried out using the statistical module in SPM12 and a toolbox for Data Processing & Analysis for Brain Imaging (DPABI) (Yan et al. 2016). For each of the HRF parameter maps, a two-way repeated-measures ANOVA was conducted, with age, years of education, and average head motion framewise displacement as covariates. To correct for multiple comparisons, the Gaussian random field (GRF) implemented in DPABI was used (p < 0.001 at the voxel level and p < 0.05 at the cluster level). Furthermore, to account for the six subregions of the insula in seed-to-whole-brain phase synchronization maps, the cluster level p was set to 0.0083 (i.e., 0.05/6) based on Bonferroni correction. Post hoc comparisons were conducted whenever a significant main effect or interaction effect was observed.
A general linear model was employed to examine the associations between decreases in hormone levels and neurovascular/phase synchronization changes. Specifically, each brain region with significant main or interaction effects for HRF parameters or phase synchronization was modeled as the dependent variable; decreases in progesterone and estradiol levels were modeled as independent variables, while age and years of education were modeled as nuisance covariates. Additionally, the relationships between the identified neurovascular and phase synchronization alterations and relevant clinical features, including attack frequency, disease duration, and PPI scores during the PMP session, were examined using a general linear model in the MRM group.
3 Results
3.1 Changes in Pain Intensity and Hormonal Profiles During the Menstrual Cycle
As presented in Table 1, age, education level, and the time intervals between the LFP and PMP did not differ between the MRM and HC groups (p > 0.05).
| MRM (N = 22) | HC (N = 26) | Statistic p-value | |
|---|---|---|---|
| Age (years) | 33.86 ± 6.23 | 33.00 ± 6.83 | 0.663 |
| Education level (years) | 15.77 ± 3.31 | 16.92 ± 6.15 | 0.299 |
| Time interval between LFP and PMP (days) | 16.45 ± 9.59 | 17.08 ± 8.51 | 0.377 |
| Disease duration (years) | 8.32 ± 5.57 | NA | NA |
| Attack frequency (days/month) | 4.43 ± 3.00 | NA | NA |
- Note: Data are expressed as the mean ± standard deviation. Statistical p values were obtained via the Mann–Whitney test between the MRM and HC groups.
Furthermore, fluctuations in progesterone and estradiol levels between the LFP and the PMP are shown in Figure 2B,C. Analysis of the progesterone levels revealed a significant main effect of menstrual phase (F = 6.200, p = 0.016). Progesterone levels were significantly higher in the LFP compared to the PMP. Analysis of the estradiol levels also revealed a significant main effect of the menstrual phase (F = 65.227, p < 0.001), with significantly lower estradiol levels in the PMP than in the LFP. There was no significant main effect of group or interaction effect between group and menstrual phase on the estradiol and progesterone levels. Additionally, we compared the changes in hormone levels between the PMP and LFP (i.e., PMP−LFP). However, there were no significant between-group differences in the decreased progesterone levels (p = 0.495) or the decreased estradiol levels (p = 0.396). For the MRM group, the association of hormone changes with the relevant clinical variables, including disease duration, attack frequency, and PPI scores in the PMP, was evaluated using a general linear model. Only the decreased estradiol levels were observed to have a significant relationship with the disease duration (t = −2.719, p = 0.015) (Figure 2D). These findings suggest that there are no significant differences in the fluctuation of estradiol and progesterone levels between the MRM and HC groups; however, the longer the disease duration of MRM is, the greater the decrease in estradiol levels between the PMP and the LFP.
3.2 Changes in Hemodynamic Response Function Characteristics During the Menstrual Cycle
Two-way repeated-measures ANOVA of the HRF parameters revealed that the response height and FWHM of the HRF are modulated by the menstrual cycle. A significant main effect of the menstrual phase on the response height of the estimated HRF was found in the left superior temporal gyrus (STG)/insula, the left inferior occipital lobe, and the right STG (Figure 3A). A decreased response height was observed in the PMP compared to that in the LFP. Moreover, a significant interaction effect between group and menstrual phase on the FWHM was observed in the anterior cingulate cortex (ACC) and the left STG (Figure 3B). Specifically, the FWHM values of the HRFs in the ACC and the left STG of the MRM group were significantly decreased in the PMP. Conversely, the FWHM values of the HRFs in the ACC and the left STG were greater in the MRM group than the HC group in the LFP. These results suggest altered neurovascular coupling during the menstrual cycle and migraine.
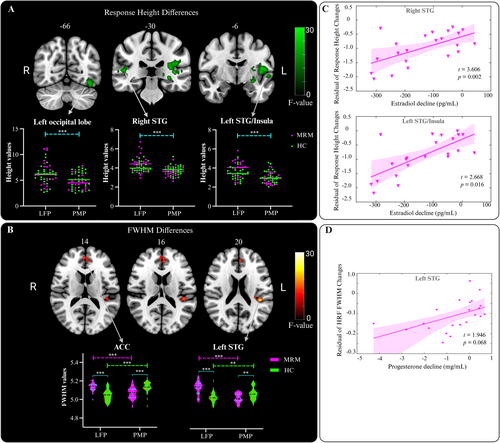
3.3 Neurovascular Function Changes With the Decreased Hormone Levels in MRM
As illustrated in Figure 3, the associations between changes in hormone levels, that is, the difference between the PMP and the LFP hormone levels, showed a positive correlation with the altered HRF parameters across these two menstrual phases in the MRM group. The reductions in estradiol levels from LFP to PMP in the MRM group were significantly associated with the reduced HRF response height in the right STG (t = 3.606, p = 0.002) and the left STG/insula (t = 2.668, p = 0.016) (Figure 3C). Additionally, a marginally significant relationship (t = 1.946, p = 0.068) was observed between decreased progesterone levels from LFP to PMP and FWHM reductions in the left STG in the MRM group (Figure 3D). No significant associations between hormone level changes and the HRF parameter alterations were observed in the HC group. Therefore, these decreases in hormone levels may modulate the neurovascular coupling in the STG and insula in patients with MRM. Furthermore, the neurovascular function alterations observed between the PMP and LFP in the MRM group were not significantly correlated with clinical variables, including attack frequency, disease duration, or PPI scores around the ictal phase.
3.4 Alterations in Anterior Insular Phase Synchronization
After accounting for the hemodynamic changes caused by the menstrual cycle and migraine, the corrected instantaneous phase information was used to estimate the PLVs between insular subregions and the whole brain. Two-way repeated-measures ANOVA of the PLVs between the left dAI and the whole brain revealed a significant interaction effect between group and menstrual phase in the middle cingulate cortex (MCC), the ACC, and the thalamus (Figure 4A). Post hoc comparisons revealed that the PLVs between the left dAI and the MCC as well as the thalamus were increased in the LFP but decreased in the PMP in the MRM group (Figure 4B). Furthermore, the PLVs between the left dAI and the aforementioned regions were significantly weaker in the PMP than in the LFP in the MRM group. Conversely, these regions exhibited stronger PLVs in the PMP than the LFP in the HC group.
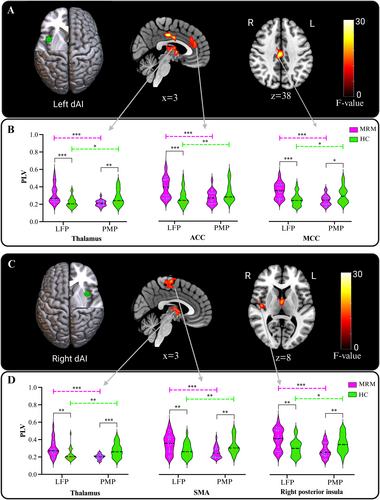
For the right dAI, an interaction effect between group and menstrual phase was also observed for the PLVs between the right dAI and the thalamus, SMA, and right PI (Figure 4C). Compared to those in the HC group, the PLVs between the right dAI and the thalamus, SMA, and right PI were significantly increased in the LFP and decreased in the PMP in the MRM group, as revealed by post hoc analysis (Figure 4D).
For the vAI, significant interaction effects between group and menstrual phase were found between the left vAI and left PI (Figure 5A), and between the right vAI and the left paracentral lobule (Figure 5B). The PLVs between those brain regions and the vAI were significantly stronger in the LFP and weaker in the PMP in the MRM group compared with that in the HC group (Figure 5C,D). However, no significant main effects of group or menstrual phase were observed. We also investigated the relationship between changes in PLVs and hormone level reductions from the LFP to the PMP, but no significant effects were found in either the MRM or the HC groups. Additionally, for the MRM patients, the PPI scores during the PMP session showed a significant negative correlation with the reductions of PLVs from the LFP to the PMP between the right vAI and the left paracentral lobule (t = −2.285, p = 0.035).
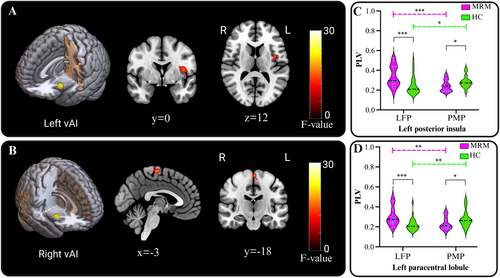
3.5 Changes in Posterior Insular Phase Synchronization
A significant interaction effect between group and menstrual phase was observed for the PLVs between the left PI and the thalamus, left amygdala, right fusiform/parahippocampal gyrus, and inferior occipital gyrus (IOG) (Figure 6A). Additionally, a significant interaction effect between group and menstrual phase was identified for the PLV between the right PI and the thalamus (Figure 6B). A post hoc analysis revealed that the PLVs between the left PI and the thalamus, left amygdala, right fusiform/parahippocampal gyrus, and IOG were significantly increased in the LFP and decreased in the PMP in the MRM group compared to the HC group (Figure 6C).
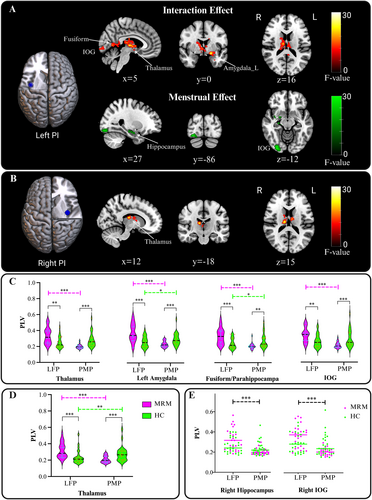
For the right PI-thalamus phases synchronization, a significantly decreased PLV in the PMP and increased PLV in the LFP were found in the MRM group compared with those in the HC group (Figure 6D). In the MRM group, a reduction in right PI-thalamus PLV from the LFP to the PMP was significantly associated with disease duration (t = 3.089, p = 0.006) and age (t = −2.310, p = 0.034), as revealed by a general linear model. However, when the interaction between age and disease duration was included in the model, the association between disease duration and reduced right PI-thalamus PLV was no longer significant (t = −0.795, p = 0.437).
In addition, a main effect of menstrual phase was found for the PLVs between the left PI and the right hippocampus and right IOG (Figure 6A). The PLVs between the left PI and the right hippocampus as well as the right IOG were significantly lower in the PMP compared to the LFP across the two groups (Figure 6E). No significant correlations were found between changes in PI-to-the-whole-brain PLVs and decreases in hormone levels in either the MRM or HC groups.
4 Discussion
In the present study, we investigated the modulation of neurovascular coupling and insular-subdivisions-to-the-whole-brain functional connectivity by the menstrual cycle in patients with MRM. The principal findings of this study can be summarized as follows: (i) Neither progesterone nor estradiol levels nor fluctuations in these hormone levels differed between the MRM and HC groups. However, estradiol fluctuations were significantly correlated with the disease duration in patients with MRM. (ii) Alterations in neurovascular coupling, particularly in the insula, were evident from the altered HRF shapes observed in MRM patients during different menstrual phases. Additionally, hormone fluctuations in patients with MRM were positively correlated with changes in HRF shapes in the STG and insula. (iii) The activity of the insula in patients with MRM exhibited a strong coupling with brain regions involved in pain processing during the LFP as revealed by PLVs. Conversely, these phase locking patterns were reversed during the PMP.
Decreases in estrogens and progesterone levels during the premenstrual phase have been hypothesized to trigger migraine attacks and modulate pain in migraine patients (Raffaelli et al. 2023; Somerville 1971). Ibrahimi et al. (2015) reported that MRM patients had similar estradiol and progesterone levels during the menstrual phase but reduced estradiol levels during the luteal phase. However, Rustichelli et al. (2020, 2021) reported comparable estradiol and progesterone levels between MRM patients and HCs during the follicular phase. In the present study, progesterone and estradiol levels were examined in both the PMP and the LFP. The results confirmed that serum levels of progesterone and estradiol were comparable between MRM patients and HCs in both the LFP and the PMP. It is unlikely that the levels of estradiol and progesterone in the PMP and the LFP, or their fluctuations, directly contribute to MRM. However, decreases in estradiol levels are significantly associated with the disease duration of migraine. This suggests that the longer the disease duration, the greater the decrease in estradiol levels in the PMP observed in patients with MRM. It is possible that the fluctuations in estradiol levels between the PMP and LFP may indirectly link to migraine pathophysiology (Raffaelli et al. 2023).
The HRF shape has been reported to be linked to a set of neurovascular factors. Specifically, a lower response height indicates less brain activation, while a smaller FWHM indicates a shorter duration of neuronal activity. In this study, we observed reduced neurovascular coupling responses in the bilateral STG/insula and the left occipital lobe in the PMP compared with that in the LFP. These findings suggest that changes in the serum levels of progesterone and estradiol from the LFP to the PMP would modulate the neurovascular response, particularly in the MRM group. To further explore this relationship, we examined the associations between changes in hormone levels and changes in HRF response height across the two groups using a linear mixed-effects model. As shown in the Table S1, reductions in HRF response height were significantly or marginally positively associated with decreases in serum levels from the LFP to the PMP across both groups.
Additionally, an interaction effect in FWHM revealed distinct patterns of neurovascular function change between the MRM and the HC groups. It is likely that the reduced duration of neural activity in the ACC and left STG contributes to the altered neurovascular function observed in patients with MRM. We found the differences in BOLD response duration between the PMP and LFP, as indicated by the FWHM of the HRF in the ACC and the left STG. The durations of the BOLD responses in these brain regions are longer during the LFP but shorter during the PMP for MRM patients. The alterations in neurovascular coupling in the STG have been previously demonstrated in patients with episodic migraine using cerebral blood flow (CBF) (Z. Y. Chen et al. 2018). The ACC is believed to be the origin of altered laser-evoked potentials during the menstrual cycle in migraine patients (de Tommaso et al. 2009) and has recently been identified as a crucial region in pain management (Xiao et al. 2021). The altered FWHM of the HRF shapes in patients with MRM can be attributed to abnormal neuronal excitability in the ACC and left STG. The decrease in hormone levels during menstruation appears to be associated with a reduced duration of neuronal activity in the STG in patients with MRM. The present study provides evidence of altered neurovascular coupling in patients with MRM. Moreover, these hormone-induced changes in neurovascular coupling could be indicated by the HRF estimated from resting-state fMRI data.
To ensure unbiased phase synchronization analysis, voxel-wise HRF variations were corrected to eliminate phase distortion for the phase synchronization between insular subregions and the whole brain. In the MRM patients, significantly increased synchronization between the anterior insula and brain regions involved in somatosensory, emotional, and cognitive processes was found in the LFP. This enhanced synchronization significantly decreased in the PMP. The anterior insula is believed to integrate sensory information with the cognitive and emotional processes of pain (Kong et al. 2006; Labrakakis 2023; Roy et al. 2009). Electrophysiology studies have demonstrated that information flows from the PI to the anterior insula during pain processing (Bastuji et al. 2018; Frot et al. 2014; Gehrlach et al. 2020). This coincides with the abnormal phase synchronization observed between the anterior insula and PI in the MRM patients. The SMA and paracentral lobule are key regions of the sensorimotor network and constitute parts of pain intensity pathways (Qin et al. 2020). The negative correlation between the reduction in right vAI-paracentral lobule PLV and PPI scores during the PMP session suggests that the greater decreases in PLV from the LFP to the PMP in MRM are associated with higher pain intensity during migraine. The ACC and MCC are involved in the emotional processing of pain (Houde et al. 2020; Vogt 2005). Additionally, the thalamus is implicated in pain modulation in migraine patients (Younis et al. 2019). Notably, the functional connectivity between the anterior insula and thalamus was preferentially enhanced by painful stimuli (Tu et al. 2024). Therefore, the aberrant phase synchronization between the anterior insula and the brain regions identified in the present study suggests that patients with MRM exhibit abnormal integration of sensory and affective/emotional information in both the LFP and PMP.
Furthermore, patients with MRM exhibited disrupted posterior insular PLVs in both the LFP and PMP. The PI has been previously reported to exhibit the highest CBF levels during migraine attacks (Stankewitz et al. 2021). The looped interactions between the PI and amygdala, thalamus in the limbic system have been demonstrated to play crucial roles in affective pain processing (Labrakakis 2023; Shi and Cassell 1998). Additionally, these regions are likely specific to females who experience migraine (Maleki et al. 2012). Consequently, the disrupted posterior insular phase synchronization suggests that pain-modulating circuits are disturbed in patients with MRM in both the PMP and LFP.
It is noteworthy that both the anterior and posterior insular phase synchronizations were increased in the LFP but reduced in the PMP in MRM patients. Accumulating evidence suggests increased functional connectivity of the anterior insula in interictal migraine patients with (Gollion et al. 2022) and without aura (Tso et al. 2015), as well as in chronic pain patients (Ferraro et al. 2022). In a recent study, enhanced connectivity between the right insula and the middle and superior temporal gyri was reported in the interictal period of MRM compared with that in non-menstrual migraine (non-MRM) patients (Huang et al. 2024). Notably, the increased functional connectivity decreased significantly during the PMP in the present study, which is consistent with the results of recent longitudinal studies. Reduced hypothalamic-limbic connectivity has also been observed during the migraine attack phase when assessed across the migraine cycle (Stankewitz et al. 2021). Considering that migraine attacks most likely occur in the PMP, the reduced phase synchronization in patients with MRM during the PMP may be linked to the pathophysiology of MRM, which supports the pathophysiological model of the disconnectome in patients with migraine (Silvestro et al. 2021).
Several limitations should be considered when interpreting the findings in this study. First, the relatively small sample size may have limited the generalizability of our findings and reduced the sensitivity to detect subtle changes in the HRF and phase synchronization in patients with MRM. The findings of this study should be validated in future studies with larger MRM cohorts. Second, the migraine attacks and menstrual cycle data were self-reported, potentially introducing reporting bias. Regular headache diaries and menstruation calendars prior to data collection could enhance the reliability of the results (Verhagen et al. 2022). Third, the present longitudinal study primarily focused on the LFP (i.e., with the lowest risk of migraine attacks) and the PMP (i.e., with the highest risk of migraine attacks in patients with MRM), which may have limited the scope of the investigation. Further research involving more menstrual phases during the menstrual cycle is warranted to reveal the modulatory effects of the menstrual cycle on migraine attacks. Additionally, this study did not include a non-MRM control group. Including such a group in future research would help distinguish the effects of hormonal fluctuations from general migraine-related brain alterations. Fourth, in addition to estradiol and progesterone, other types of hormones, such as pregnanolone and pregnenolone sulfate (Rustichelli et al. 2021), should be considered in future studies. Finally, the neurovascular coupling abnormalities in patients with MRM were examined on the basis of the HRF shapes derived from resting-state fMRI in the present study. Future studies evaluating the associations between CBF and functional connectivity might provide detailed evidence of abnormal trigeminal neurovascular coupling in migraine patients (Z. W. Chen et al. 2024).
5 Conclusion
Our findings provide evidence that both dysregulated neurovascular coupling and the dysfunctional brain connectome contribute to the neuropathology of MRM. The increased insular phase synchronization during the LFP and reduced synchronization during the PMP could indicate that the menstrual cycle modulates disruptions in the integration of sensory and affective information in patients with MRM. Furthermore, the findings suggest that hormone fluctuations are likely associated with changes in neurovascular function only.
Author Contributions
Xinyu Li: conception and study design, data acquisition, results interpretation, manuscript drafting. Lisa W. C. Au: results interpretation, manuscript drafting, manuscript revision. Huifen Hao: data acquisition, results interpretation. Yingying Li: data acquisition, results interpretation. Xiuju Gao: data acquisition. Junqiang Yan: data acquisition. Raymond K. Y. Tong: conception and study design, manuscript revision. Wutao Lou: conception and study design, data analysis, results interpretation, manuscript drafting and revision. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




