Cortical Oscillatory Activity and Motor Control in Pediatric Stroke Patients With Hemidystonia
Funding: This work was supported by Canadian Institutes of Health Research, PJT173532.
ABSTRACT
Dystonia is a movement disorder characterized by repetitive muscle contractions, twisting movements, and abnormal posture, affecting 20% of pediatric arterial ischemic stroke (AIS) survivors. Recent studies have reported that children with dystonia are at higher risk of cognitive deficits. The connection between impaired motor outcomes and cognitive impairment in dystonia is not fully understood; dystonia might affect motor control alone, or it could also contribute to cognitive impairment through disruptions in higher-order motor processes. To assess the functional correlates underlying motor control in children with dystonia, we used magnetoencephalography (MEG) to measure frontal theta (4–8 Hz), motor beta (15–30 Hz), and sensorimotor gamma (60–90 Hz) activity during a “go”/“no-go” task. Beamformer-based source analysis was carried out on 19 post-stroke patients: nine with dystonia (mean age = 13.78, SD = 2.82, 8 females), 10 without dystonia (mean age = 12.90, SD = 3.54, 4 females), and 17 healthy controls (mean age = 12.82, SD = 2.72, 8 females). To evaluate inhibitory control, frontal theta activity was analyzed during correct “no-go” (successful withhold) trials. To assess motor execution and sensorimotor integration, movement time-locked beta and sensorimotor gamma activity were analyzed during correct “go” trials. Additionally, the Delis-Kaplan Executive Function System (DKEFS) color-word interference task was used as a non-motor, inhibitory control task to evaluate general cognitive inhibition abilities. During affected hand use, dystonia patients had higher “no-go” error rates (failed withhold) compared to all other groups. Dystonia patients also exhibited higher frontal theta power during correct withhold responses for both affected and unaffected hands compared to healthy controls. Furthermore, dystonia patients exhibited decreased movement-evoked gamma power and gamma peak frequency compared to non-dystonia patients and healthy controls. Movement-related beta desynchronization (ERD) activity was increased in non-dystonia patients for both hands compared to healthy participants. These results confirm that post-stroke dystonia is associated with impaired frontally mediated inhibitory control, as reflected by increased frontal theta power. Post-stroke dystonia patients also exhibited reduced motor gamma activity during movement, reflecting altered sensorimotor integration. The increased beta ERD activity in non-dystonia patients may suggest compensatory sensorimotor plasticity not observed in dystonia patients. These findings suggest that differences in motor outcomes in childhood stroke result from a combination of cognitive and motor deficits.
Summary
- Post-stroke patients with dystonia made more “no-go” errors when inhibiting use of their affected hand but showed an increased theta power during correct “no-go” withholds regardless of which hand was used.
- A greater beta ERD during correct “go” responses was observed in the non-lesioned hemisphere of dystonia patients when using their unaffected hand.
- Post-stroke patients with dystonia exhibited a smaller change in gamma power and lower gamma peak frequency during correct “go” responses when using their affected hand.
1 Introduction
Arterial ischemic stroke (AIS) is a major contributor to acquired morbidity in children, with more than 70% of survivors experiencing significant motor and cognitive disabilities (Goldenberg et al. 2009). Middle cerebral artery (MCA) strokes are the most common type of stroke and result in impaired blood supply to critical brain regions, including the lateral frontal lobe, lateral parietal lobe, superior temporal lobe, and parts of the basal ganglia and internal capsule. Disruption within those areas often leads to functional impairments, including deficits in motor and sensory functions of the upper extremities, as well as cognitive and language difficulties (Kirton and De veber 2009). As a result, patients often experience motor deficits ranging from diminished hand function to hemiparesis, dystonia, and cerebral palsy (Kukke et al. 2015; Mirkowski et al. 2019).
Diffusion MRI studies have identified correlations between white matter microstructure and motor outcomes in pediatric stroke, primarily through examining the corticospinal tract, cingulum, and superior longitudinal fasciculus (Domi et al. 2009; Ledochowski et al. 2022). Recent studies in pediatric stroke patients have reported negative motor outcomes that often coincided with challenges in cognitive functioning (Abgottspon et al. 2021; Ledochowski et al. 2020). Despite these findings, our understanding of the relationship between cognitive and motor functioning in this patient population is lacking as limited studies have explored the functional correlates of motor control in pediatric stroke patients. These insights are essential for identifying children at risk of developing maladaptive motor and cognitive outcomes, which can potentially guide interventions to optimize post-stroke recovery.
The involvement of multiple frontal areas in motor inhibitory control has been well documented in functional neuroimaging studies using techniques such as functional magnetic resonance imaging (fMRI) (Garavan et al. 1999; Luijten et al. 2014) and positron emission tomography (PET) (Obeso et al. 2013). Specifically, the inferior frontal cortex is thought to generate cognitive inhibitory processes in response to visual signals that spread to the motor cortices, resulting in response inhibition (Garavan et al. 2006). Other studies have implicated the medial frontal cortex, anterior cingulate cortex, and supplementary motor areas in movement selection and initiation during inhibitory control processes (Munakata et al. 2011; Obeso et al. 2013; Velanova et al. 2008). Assessing such response inhibition is crucial for understanding the integration of cognitive and motor control mechanisms. Successful response inhibition requires not only correct stimulus perception but also the processing of cognitive and motor control in a timely manner, as any delay can compromise the effectiveness of inhibitory control (Huster et al. 2013). Magnetoencephalography (MEG) presents a well-established avenue for investigating these processes with high spatiotemporal precision, enabling detailed examination of the specific neural regions and temporal profiles involved in motor control.
While the impact of functional changes in motor-related areas on movement planning and execution in healthy adults is known (Cheyne et al. 2008; Isabella et al. 2015, 2021; Torrecillos et al. 2015), studies on the neurophysiological basis of impaired motor control in post-stroke children remain limited. These children represent a unique patient population to study the developmental plasticity of motor and frontal systems after a unilateral stroke. Research using MEG to investigate motor control in post-stroke children is even more scarce although one study has used MEG to study children with cerebral palsy, a condition often caused by stroke (Hoffman et al. 2019). The study showed that neuronal activities, such as movement-related beta and gamma band oscillations, were weaker in the affected hands of cerebral palsy patients compared to healthy children. However, comparisons between the affected and unaffected hands were not examined as only brain activity corresponding to the dominant hand was recorded. Another study using fMRI and EEG measures found that children with more severe motor impairments exhibited greater activation in the primary motor cortex and recruited additional brain areas, such as the contralesional primary motor cortex, when moving their affected hand relative to their unaffected hand (Weinstein et al. 2018). To the best of our knowledge, no study has examined cortical oscillatory activity during a cognitive motor task during both affected and unaffected hand use in pediatric stroke patients, nor determined if this differs in patients who have developed dystonic symptoms following their stroke. Comparing motor processing between lesioned and non-lesioned hemispheres is crucial for understanding brain recovery and the impact on the non-lesioned hemisphere following unilateral injury.
To investigate the neural correlates of motor control, MEG was used to assess the timecourse, power, and source location of theta (4–8 Hz), beta (15–30 Hz), and gamma (60–90 Hz) frequency bands. These correlates were compared to behavioral performance on the “go”/“no-go” task. This task is a widely used behavioral paradigm for testing motor control in which participants respond to “go” cues with a button press and withhold movement at “no-go” cues. We examined frontal theta to determine if children with dystonia demonstrated differing frontal-mediated cognitive control relative to healthy controls and non-dystonia patients. We also examined changes in motor beta to investigate differences in motor preparation and execution, and sensorimotor gamma activity to examine sensorimotor integration.
2 Methods
2.1 Participants
Stroke patients were recruited in-clinic from the Hospital for Sick Children (Toronto, Canada). Healthy participants were recruited from the local Toronto area. The inclusion criteria for stroke patients consisted of unilateral middle cerebral artery (MCA) stroke with basal ganglia and/or thalamus involvement and an age range of 7–18 years at the time of imaging. Dystonia patients were identified based on a positive hypertonia assessment tool (HAT) result confirmed by a pediatric neurologist, whereas non-dystonia patients were identified by a negative HAT result (Jethwa et al. 2010). Both perinatal (between 20 weeks of gestation to 28 days old) and childhood (between 28 days to 18 years old) stroke patients were included. The exclusion criteria were any individuals who would require sedation for MRI or MEG recordings, those with severe cognitive impairments (e.g., severe autism spectrum disorder), or severe motor impairments that would prevent them from performing the motor tasks. All individuals with ferromagnetic implants or items (e.g., braces, retainers, and pacemakers) that may produce MEG artifacts or be contraindicated for MRI scans were also excluded. Healthy participants were defined as normally developing children without existing neurological diagnoses. All participants capable of giving consent and/or their parents/guardians provided informed consent using protocols approved by the Hospital for Sick Children Research Ethics Board.
2.2 Experimental Design
All participants performed the “go”/“no-go” task (Figure 1A). Participants were instructed to press a MEG-compatible wooden paddle (Figure 1B) at the onset of “go” cues but withhold their movements for “no-go” cues. The “go” cue was represented as a green square on the screen whereas the “no-go” cue was a red square. These stimuli were presented for 250 ms, followed by a stimulus mask for 1500–1800 ms.
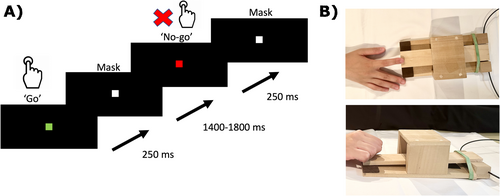
Participants were instructed to respond as quickly as possible while aiming to minimize any errors. Each block lasted 7 min and comprised of 100 “go” and 100 “no-go” trials. Each participant completed the task twice, once with each hand.
Healthy participants first performed the task using their dominant hand, and stroke patients with their unaffected hand first. Participants were given up to 10 test trials to familiarize themselves with the task, and the “go”/“no-go” block was repeated as needed for those who did not understand the task or did not fully release the paddle after each “go” trial.
To assess non-motor cognitive control abilities, participants completed the Delis-Kaplan Executive Function System (DKEFS) color-word interference task outside of the MEG (Baron 2004), guided by a trained clinical neuropsychologist. The task consisted of 50 words, each printed in a conflicting ink color, such as the word “red” printed in yellow. Participants were required to name the color of the ink each word was printed, while suppressing their tendency to read the word. Recent research has revealed that pediatric stroke patients performed significantly worse on the DKEFS color-word interference task, particularly those with poor upper limb function (Abgottspon et al. 2021), thereby indicating a potential association between impaired motor abilities and cognitive deficits.
2.3 Behavioral Measures
For the “go”/“no-go” task, two types of response errors were calculated. The number of incorrect “go” responses defined as withholding on a “go” trial (false alarms) was measured to assess the degree of sustained attention. The number of incorrect “no-go” responses (failing to withhold on a “no-go” trial) was measured to examine the ability to inhibit movement. Reaction time (RT) was measured to examine attention and speed of processing.
A high CV score would suggest less consistent motor response across trials, whereas a low CV score would suggest a more consistent, better controlled motor response.
For the DKEFS color-word interference task, the number of incorrect responses was measured and age-normalized to assess how well participants could inhibit cognitive impulses. This task focused specifically on cognitive inhibition and did not test motor inhibition.
2.4 MEG Recording
Neuromagnetic responses were recorded with a whole-head 151-channel CTF-MISL MEG system (Coquitlam, BC, Canada) located in a magnetically shielded room at the Hospital for Sick Children in Toronto, Canada. The data were collected at a rate of 1200 samples/s (filtered online at 0–300 Hz). Fiducial coils were placed at three anatomical landmarks (pre-auricular and nasion points) to monitor head position during recording. These locations also served to co-register source images to the participant's structural MRI. The recordings were carried out in the supine position, with arms resting on either side on a padded bed. Participants were instructed to remain as still as possible. Tasks were repeated if excessive head, jaw, or other large movements were observed. A non-magnetic fiber optic switch was used to collect motor responses that involved pressing a custom-designed wooden paddle to accommodate patients who had difficulty using a consistent, standard button press with their affected hand. The visual stimuli were green- or red-colored squares measuring 5 cm × 5 cm, presented on a back-projection LCD screen at a visual angle of ~5°.
2.5 MRI Acquisition
Structural MRI scans were acquired immediately following MEG recording using a Siemens 3 T Magnetom Trio scanner at the Hospital for Sick Children. MRI contrast markers were positioned on the same three anatomical landmarks to optimize MEG/MRI co-registration. Each participant underwent a standard structural T1-weighted image sequence (1-mm isotropic, magnetization prepared gradient-echo (MPRAGE) flip angle = 9°, TE = 2.88 ms, TR = 2300 ms) with a parallel acquisition technique (GRAPPA) to reduce acquisition time and motion-related artifacts.
2.6 Data Preprocessing
Trials where head motion exceeded 10 mm of mean head movement (based on trials recorded for an event type, such as correct “go” motor responses) were discarded. The data were collected at 1200 samples per second, then downsampled to 600 samples per second and bandpass filtered between 0 and 150 Hz. The datasets corresponding to “go” and “no-go” stimuli and their respective button press responses were segmented into 4-s epochs, encompassing 2 s before and after the event of interest (t = 0 s). For both correct “no-go” trials, and incorrect “go” trials, no motor response was elicited, thus these trials were segmented to the “no-go” or “go” stimulus onset, respectively.
Structural MRI images were used to co-register neuromagnetic activity with the anatomical structure of the brain in each individual. This was achieved by aligning the fiducial markers from the MEG with the radiological markers used in the MRI. The inner skull surface of the T1 images was extracted using FSL brain extraction (bet2), and a single sphere model was generated for each participant for source modeling (Cuffin and Cohen 1977; Lalancette et al. 2011). The co-registered functional images for each child were then transformed into standardized MNI space using SPM12 (Wellcome Institute of Cognitive Neurology, London, UK) for group averaging. To aid anatomical visualization of source activity relative to cortical anatomy, SAM beamformer images were interpolated onto high-resolution cortical surfaces created using the CIVET image-processing pipeline (Kim et al. 2005).
2.7 Data Analysis
2.7.1 Beamformer Analysis
The Synthetic Aperture Magnetometry (SAM) beamformer algorithm (Vrba and Robinson 2001), implemented in the BrainWave Matlab toolbox (available at https://github.com/cheynelab) xxx (Jobst et al. 2018), was used to localize and quantify frequency-specific changes across the entire brain with 4 mm resolution for three types of movement-related activity—frontal theta (4–8 Hz), motor beta (15–30 Hz), and sensorimotor gamma (60–90 Hz) (Isabella et al. 2015; Spooner et al. 2020). Source power in the baseline (pre-movement) period was subtracted from source power in predefined active time windows to generate pseudo-T images, defined separately for each frequency band, with each image reflecting within-band noise-normalized differences in power across all voxels. To select regions of interest for each frequency band, we relied on previous work on motor control in healthy adults (Isabella et al. 2015) and visually confirmed them on an MNI template brain (Collins et al. 1994). Active windows were chosen based on the activity of interest, and their duration was selected to ensure at least 2 cycles of the lowest frequency band (e.g., 500 ms at duration for 4 Hz in the theta band) were contained within the window. Baseline windows were chosen based on empirical examination to ensure that activity from the previous trial was not included (Gross et al. 2013).
2.7.2 Theta Band
To examine neural activity in the theta band, we selected an active window spanning 0–500 ms relative to the “no-go” stimulus onset. An equally sized baseline window was set from −1000 to −500 ms relative to stimulus onset. Peaks were determined by selecting the voxel corresponding to the maximum source amplitude intensity for each participant, located anatomically closest to the middle frontal gyrus. Anatomical labeling was done using the Talairach atlas by first transforming the MNI coordinates to Talairach coordinates (mni2tal function) and searching for the closest grey matter label within 5 mm of the peak. Virtual sensors were extracted from each peak voxel and a group average TFR was obtained by averaging across individual virtual sensors. The group-averaged TFR was normalized by applying baseline correction for individual TFRs. Our region of interest for the theta band was the right middle frontal gyrus and/or right medial frontal gyrus based on previous studies (Cavanagh et al. 2012; Cavanagh and Frank 2014; Cohen and Donner 2013; Van Noordt et al. 2022). However, we also searched for nearby regions such as the superior and inferior frontal gyri in stroke patients if a peak could not be reliably identified in the right middle gyrus or right medial frontal gyrus. We additionally searched the left frontal gyrus in stroke patients to account for possible functional reorganization following brain injury based on previous research (Buetefisch 2015; Crofts et al. 2020; Knopman et al. 1984).
2.7.3 Beta Band
Beta activity during button press movement was compared across groups by subtracting a baseline window from the active window of interest. The analysis focused on the motor response and accounted for different reaction times across groups by time-locking the MEG data to the motor response rather than the stimulus onset. For beta ERD, the window of interest was from movement onset to 300 ms during movement execution, specifically t = 0–300 ms, with the baseline set from −1500 to −1200 ms prior to movement onset. Peaks were determined by selecting the voxel corresponding to the maximum source amplitude intensity for each participant, located anatomically closest to the pre-central gyrus. Anatomical labeling was done using the Talairach atlas by first transforming the MNI coordinates to Talairach coordinates (mni2tal function) and searching for the closest grey matter label within 5 mm of the peak. Although greater beta ERD is typically found contralateral to the moving hand, motor-related beta activity is consistently present in both hemispheres (Cheyne et al. 2006, 2008, 2014; Cheyne 2013). For this reason, the bilateral pre-central cortices were selected as regions of interest for the beta band. Group average TFRs were obtained by averaging across individual virtual sensors. The baseline for the group TFR was set to −1500 to −500 ms prior to movement onset, as beta ERD has been shown to begin up to 500 ms prior to movement onset (Gaetz et al. 2010; Jurkiewicz et al. 2006; Pfurtscheller 2000; Taniguchi et al. 2000). To account for the differences in hemisphere strokes in our patient sample, peak voxels from individual SAM beamformers were identified and their corresponding virtual sensors were generated for each participant. Baseline correction was applied to individual TFRs, and the averaged TFR was visually inspected to ensure the baseline did not contain the activity of interest from the previous trial.
Beta event-related synchronization (ERS) was examined using data epoched to the button press. The active window for beta ERS was set to 700–1000 ms post-movement onset, and the baseline window was set to −1500 to −1200 ms prior to movement onset. To account for inter-patient differences, individual SAM beamformers were computed, and a beta ERS peak was identified for each patient. Virtual sensors were computed at each peak voxel, and the time-frequency changes averaged across individual virtual sensors, using a similar ROI selection method for beta ERD.
2.7.4 Gamma Band
In the gamma band analysis, data that were time-locked to correct “go” movements were used to examine high frequency (60–90 Hz) activity during active movement. Analysis of gamma power used a 300 ms active window, ranging from 150 ms prior to movement onset to 150 ms after movement onset. The baseline window was set to −800 to −500 ms relative to movement onset. Peak voxels and anatomical labels corresponding to locations of maximum gamma power were selected using the same method described for beta band peaks. Percent change in gamma power during correct “go” responses and the mean peak frequency of gamma were analyzed.
For all frequency bands of interest, the active window was shifted forward in 50 ms increments up to 4 times (e.g., for theta band: 0–500, 50–550, 100–600 ms, etc.) and images were searched for the largest peak activation within the region of interest. Through this iterative approach, we identified peaks of activation in all subjects, although some peaks did not represent the largest amplitude peak over the entire brain. For example, in cases where two gamma band peaks were observed, the peak corresponding to the sensorimotor area in the image was taken as the motor cortex peak for that individual. Single trial timecourses obtained from the resulting peak represented the spatially filtered task-related power changes and were used to generate time-frequency plots to visualize the timecourse of changes in source activity within each frequency band, using a Morlet wavelet frequency transformation in 1 Hz steps using a cycle length of seven (Tallon-Baudry et al. 1997). Mean power was subtracted from the single trial power in the time-frequency plots to image the induced oscillatory activity and exclude activity that is phase-locked to the visual cue or motor response (Tallon-Baudry et al. 1997). For each band of interest, oscillatory activity is then averaged across the frequency of interest to create a timecourse.
2.8 Statistical Analysis
One-way ANOVAs with five levels were conducted between group and hand (e.g., dystonia affected hand, dystonia unaffected hand, non-dystonia affected hand, non-dystonia unaffected hand, and healthy control dominant hand) when parametric assumptions were met according to Levene's test. Alternatively, Kruskal-Wallis tests were used for non-parametric data. In case of any significant effects for ANOVAs, post hoc tests were performed with Bonferroni correction. Significant effects for Kruskal-Wallis were followed by Dunn's post hoc test. ANCOVAs were conducted using age as a covariate to examine its effects on the frequency bands examined and to explore differences between groups. All statistical tests were performed in R. Pearson correlation was used to examine the relationship between performance on the “go”/“no-go” and the DKEFS color-word interference test.
3 Results
3.1 Participants
Overall, 19 pediatric AIS patients were included, nine with dystonia (mean age = 13.78 years old, SD = 2.82, 8 females), 10 without dystonia (mean age = 12.90 years old, SD = 3.54, 4 females), and 17 healthy participants (mean age = 12.82 years old, SD = 2.72, 8 females). Mean age and standard deviations between stroke patients and healthy controls were not significantly different. Because of the severity of dystonia in one patient, only data from their unaffected hand was reliably collected. We did not exclude this patient to maximize functional oscillatory data when using the unaffected hand. For clarity and consistency in comparison with dystonic patients, the contralesional hand for non-dystonic patients will be labeled the “affected hand,” although motor function was not compromised as with dystonic patients. Conversely, the ipsilesional hand of non-dystonia patients will be referred to as the “unaffected hand.” Patient demographic information including sex, stroke type, and stroke hemisphere is shown in Table 1, comparing dystonic and non-dystonic patients.
| Dystonia (N = 9) | Non-dystonia (N = 10) | ||
|---|---|---|---|
| Sex | F | 8 (89%) | 4 (40%) |
| M | 1 (11%) | 6 (60%) | |
| Stroke type | Perinatal | 3 (33%) | 6 (60%) |
| Childhood | 6 (67%) | 4 (40%) | |
| Stroke hemisphere | Left | 6 (67%) | 6 (60%) |
| Right | 3 (33%) | 4 (40%) |
3.2 Behavioral Results
Consistent with previous studies, healthy controls made very few errors for both “go” and “no-go” trials (Cheyne et al. 2012; Simpson and Riggs 2006). Specifically, the mean incorrect “go” rate was 2.30% and the mean incorrect “no-go” rate was 3.40%. A group effect was found for incorrect no-go trials (χ2(4) = 12.20, p = 0.016), driven by significantly higher incorrect no-go trials (failed withholds) in dystonia patients when using their affected hands compared to all other groups (p < 0.05 for all groups) (Figure 2A). These results were consistent with our hypothesis that inhibitory function is significantly compromised in dystonia.
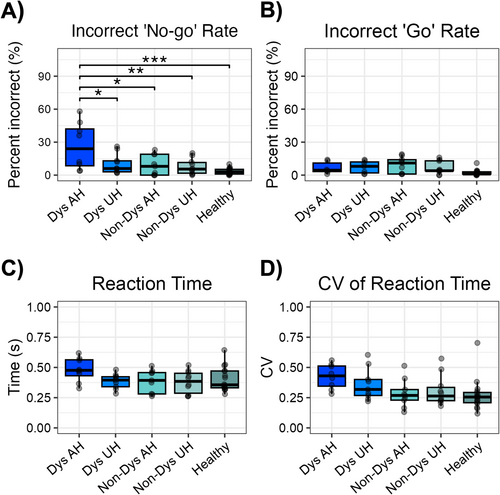
Two patients were excluded from this comparison because they did not fully release the wooden paddle after each press, which affected the accuracy of subsequent measurements. A significant group effect was found for “go” errors (χ2(4) = 12.21, p = 0.024), but post hoc tests revealed no significant differences between groups (Figure 2B). Dystonia patients did not differ in “go” errors when comparing their affected and unaffected hands, nor were hand differences seen in non-dystonic patients. The fact that incorrect “go” rates did not differ between hands in dystonia patients suggests that motor initiation was not significantly impaired for the dystonic hand.
No group effect was found for mean reaction time (RT) (F(4,49) = 1.70, p = 0.165), nor did RT differ significantly between hands in dystonia and non-dystonia patients (Figure 2C).
No significant group effects were found when separating between hands in dystonic and non-dystonic groups (F(4,49) = 2.44, p = 0.06) (Figure 2D), although trends indicated that dystonia patients exhibited less consistent motor response times compared to healthy controls, while non-dystonic patients were comparable to healthy controls.
The DKEFS color-word interference task was used as a non-motor, cognitive inhibitory control task to evaluate general inhibition in stroke patients and healthy participants. The test is age-normalized, with higher DKEFS scores indicating better inhibitory control and higher cognitive control abilities. Significant group differences were observed among dystonia patients, non-dystonia patients, and healthy participants (F(2,34) = 27.29, p < 0.001). Dystonia patients had significantly lower DKEFS scores compared to both non-dystonia patients (p < 0.001) and healthy participants (p < 0.001). However, there was no significant difference in DKEFS scores between non-dystonia patients and healthy controls (p = 0.061).
In comparing cognitive effort to overt response, a significant correlation was found between higher DKEFS scores and fewer incorrect “no-go” responses on the “go”/“no-go” task for healthy participants (r = −0.75, p < 0.001). However, there was no correlation between DKEFS scores and the ability to withhold movements on the “go”/“no-go” task for stroke patients, regardless of dystonia (r = −0.39, p = 0.38 for dystonia; r = 0.53, p = 0.14 for non-dystonia).
3.3 Theta Activity
We examined theta activity (4–8 Hz) during the “go” condition but did not observe consistent frontal peaks across participants, which prevented us from reliably assessing theta amplitude during “go” trials. Similarly, when computing “go”/“no-go” contrast images (“no-go” minus “go” difference images), we did not observe stable peak frontal theta sources likely due to spurious intensity differences in other brain regions between the two conditions.
Theta activity (4–8 Hz) during successful “no-go” trials in stroke patients (dystonia and non-dystonia) was examined based on affected and unaffected hands and compared to the dominant hand of healthy controls. The maximum peak voxels with the largest pseudo-t value were found in the inferior, middle and medial frontal gyri in all healthy controls, while the equivalent locations were less consistent in all stroke patients—regardless of dystonic severity. Theta activity increased prior to stimulus onset, peaked following stimulus presentation, and returned slowly to baseline. A significant difference between groups was found in percent change in theta peak power (F(4,49) = 5.54, p = 0.001), with the effect driven by higher percent change in theta power in dystonia patients (when using either their dystonic hand or unaffected hand) compared to non-dystonia patients when using their affected hand (both p < 0.01) (Figure 3A).
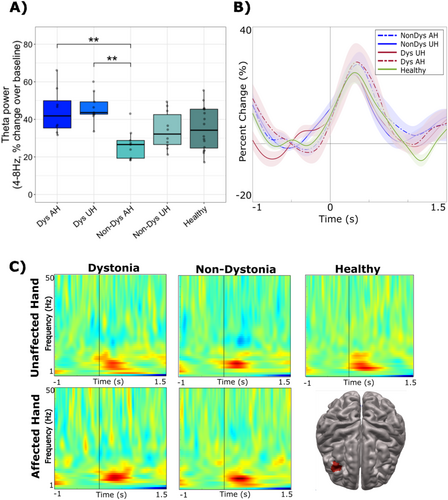
Theta peak latency did not differ significantly between groups (F(4,49) = 0.323, p = 0.861), however, the affected hand for both dystonia and non-dystonia patients showed trends of exhibiting later peak latencies compared to their unaffected hand. The timecourse (Figure 3B) and TFR (Figure 3C) demonstrate the averaged percent change in theta activity between groups and its corresponding latency relative to the onset of the “no-go” stimulus.
3.4 Beta Activity
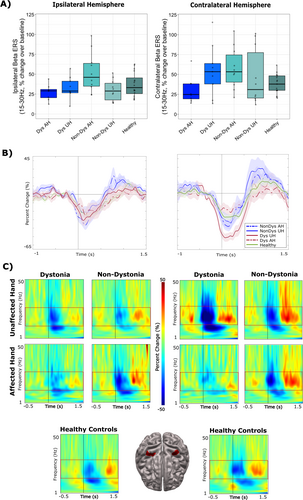
Significant differences between groups were found for percent change over baseline (−1500 to −500 ms) in contralateral beta ERD activity during correct “go” responses (F(4, 49) = 5.85, p < 0.001). Post hoc t-tests revealed that this effect was driven by the unaffected hand in dystonic patients (p < 0.001). Specifically, the change in beta ERD in the contralateral sensorimotor cortex was significantly greater in the unaffected hand compared to the dystonic hand and healthy controls (Figure 4A, right panel). Significant group effects were also found for percent change in ipsilateral beta activity for “go” responses (F(4,49) = 3.12, p = 0.024). Post hoc t-tests revealed that the affected hand in non-dystonia patients exhibited significantly greater percent change in ipsilateral beta ERD compared to healthy controls (p = 0.034) (Figure 4A, left panel). The timecourses (Figure 4B) and TFRs (Figure 4C) demonstrate the averaged percent change in ipsilateral beta ERD (left panel) and contralateral beta ERD (right panel) between groups.
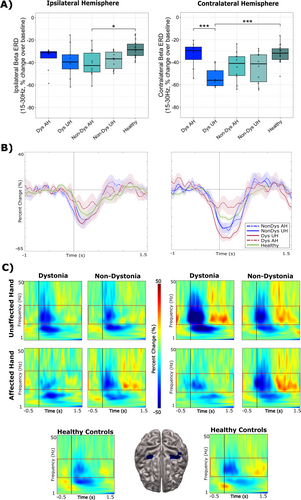
No significant group effects were found for contralateral beta ERS activity following correct “go” responses, although trends indicated lower ERS patterns in dystonia patients using their affected hand (F(3,44) = 2.06, p = 0.100) (Figure 5A). Similarly, no significant group effects were noted for changes in ipsilateral beta ERS activity following “go” responses, although the analysis approached significance (F(3,44) = 2.47, p = 0.057). Non-dystonia patients, when using their affected hand, displayed higher ipsilateral ERS activity compared to all other groups (Figure 5A). The timecourses (Figure 5B) and TFRs (Figure 5C) demonstrate the averaged percent change over baseline (−1500 to −1200 ms) in ipsilateral beta ERS (left panel) and contralateral beta ERS (right panel) between groups.
3.5 Gamma Activity
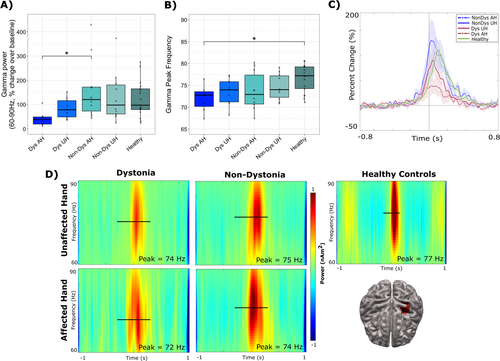
Significant differences between groups were observed in movement-evoked gamma power (percent change) during correct “go” responses (F(4,49) = 2.70, p = 0.041) (Figure 6A). Post hoc t-tests revealed that the affected hand in dystonia patients exhibited significantly lower percent change in gamma power compared to the affected hand in non-dystonia patients (p = 0.046). Significant group differences were observed for peak frequency in gamma power (F(4,49) = 3.02, p = 0.027) (Figure 6B). Post hoc t-tests revealed that the affected hand in dystonia patients exhibited significantly lower gamma peak frequency compared to healthy control participants (p = 0.035). The timecourses (Figure 6C) and TFRs (Figure 6D) demonstrate the averaged percent change over baseline in gamma activity between groups.
3.6 Effect of Age
Age was used as a covariate for all oscillatory power analyses. Significant age effects were found for beta ERD (F(3, 48) = 16.705, p < 0.001), showing that percent change over baseline in beta ERD decreased with increasing age. Significant age-related effects were also found for beta ERS (F(3, 48) = 19.85, p < 0.001), showing that percent change in beta ERS increased with increasing age. However, no age effects were found for percent change in theta power, percent change in gamma power, or gamma peak frequency. No significant group differences were found for age.
4 Discussion
In this study, MEG was used to investigate differences in brain activity between stroke patients (dystonia and non-dystonia) and healthy participants during a motor control task. The findings revealed that dystonic patients made significantly more errors on “no-go” trials while using their affected hand compared to their unaffected hand, and to non-dystonia patients and healthy controls. Interestingly, dystonia patients exhibited higher task-related theta power when using either their affected or unaffected hand. Changes in movement-related beta ERD during correct “go” responses were significantly greater in the non-lesioned hemisphere of dystonia patients, compared to the lesioned hemisphere when moving the contralateral hand. Percent change in beta ERS power was reduced in dystonia patients when using their affected hand, in both hemispheres. Additionally, dystonia patients exhibited reduced gamma power and peak gamma frequency during correct “go” responses when using their affected hand.
Inhibitory control is crucial to suppress inappropriate movements and execute correct responses (Dowsett and Livesey 2000; Theeuwes 2010), an aspect of movement that was predicted to be compromised in dystonia patients. It was found that dystonia patients had significantly higher errors on “no-go” trials when using their affected hand, which indicated greater challenge in inhibiting movements. When examining whether dystonia diagnosis affected the observed frontal theta power differences, dystonia patients exhibited significantly higher theta power when correctly inhibiting a movement, while non-dystonia patients did not differ from healthy controls. Higher frontal theta power was observed for both hands, suggesting that cognitive control is globally compromised in dystonia patients rather than being specifically impaired when using the affected hand. These results align with findings that indicate that frontal theta power is associated with cognitive effort and inhibitory control (Isabella et al. 2021). Notably, similar abnormalities in task-related theta power have been observed in patient populations with known deficits in inhibitory control, such as those with autism spectrum disorder, ADHD, and addiction (Chan et al. 2011; Deiber et al. 2020; Kamarajan et al. 2004). From a developmental standpoint, the observed increase in theta power in patient populations can be explained by altered brain development, as theta power decreases with age and reflects changes in cognitive processing (Cellier et al. 2021; Clarke et al. 2001; Liu et al. 2014).
Peak locations were selected individually for each participant for the analyses to account for potential anatomical displacement in patients with larger lesions (Figure S1). An intriguing observation from our investigation of stroke patients is the functional reorganization of the anatomical location of the largest changes in theta power relative to their stroke lesions. Although the sample was too small to conduct statistical analyses, dystonia patients exhibited frontal theta activity only on the non-lesioned hemisphere, for both their affected and unaffected hands. The mechanism underlying contralesional functional reorganization remains unclear; however, it has been shown that patients who experience functional recovery with activation of the motor cortex on the non-lesioned hemisphere tend to have worse neurological outcomes than those who recover through perilesional reorganization (Ko and Yoon 2013; Ward 2005). Thus, it is possible that the correlation between contralesional functional reorganization and poorer outcomes also extends to the frontal cortex. A larger sample would be required to establish the correlation between anatomical location and frontal theta activity.
Greater beta ERD during correct “go” responses was observed in the contralateral hemisphere of dystonia patients when using their unaffected hand compared to their affected hand and healthy controls. These results are in accordance with a recent investigation revealing stronger beta ERD in the non-lesioned hemisphere when moving the unaffected hand in adult stroke patients (Shim et al. 2023) and replicate findings that weaker beta ERD corresponding to the affected hand is associated with worse motor outcomes (Kulasingham et al. 2022; Zhu et al. 2019). Explanations for these findings include compromised disinhibition in the non-lesioned hemisphere during motor planning and execution (Graziadio et al. 2012). Furthermore, beta ERD was generally greater in non-dystonia patients when using either hand compared to healthy controls. This is consistent with previous work suggesting that stronger beta ERD is correlated with improved post-stroke motor function (Shiner et al. 2015). Given these findings, further studies examining post-stroke interhemispheric connectivity and its relation to motor outcome are warranted.
Although the statistical analyses did not reach significance, an increase in contralateral ERS was observed when using the unaffected hand for both dystonia and non-dystonia patients compared to healthy controls. Similarly, increased contralateral ERS was evident when using the affected hand for non-dystonia patients but was absent in dystonia patients when using their affected hand. Previous research has linked stronger ERS to better post-stroke motor outcomes (Shiner et al. 2015). Conversely, decreased beta ERS has been associated with post-stroke sensory loss rather than loss of motor execution (Laaksonen et al. 2012). These findings suggest that dystonia, particularly in the affected hand, may be associated with impaired sensory input related to movement processing and ongoing motor activity during active movement. Beta ERS is also thought to play a role in motor inhibition and modulating sensory feedback during movement (Heinrichs-Graham et al. 2017; Tan et al. 2016). The observed upregulation of beta ERS in non-dystonia patients could reflect a compensatory mechanism to support movement control.
In our study, dystonia patients displayed significantly reduced gamma power when moving their affected hand compared to non-dystonia patients. Dystonia patients also exhibited significantly reduced gamma peak frequency when moving their affected hand compared to healthy controls. Overall, gamma power and peak frequency were observed to correlate with motor outcome, reflected by the lowest values of gamma power and peak frequency in the dystonic hand, followed by the unaffected hand of dystonia patients, the affected hand of non-dystonia patients, the unaffected hand of non-dystonia patients, and the highest power and frequency in healthy participants. While lower gamma power has been consistently linked to reduced sensorimotor abilities (Paggiaro et al. 2016; Tedesco Triccas et al. 2019), this is the first study to show a link between lower gamma peak frequency and reduced motor outcomes after stroke. The reduced gamma peak frequency exhibited by dystonia patients could be attributed to less developed sensorimotor integration abilities based on a recent study reporting that gamma peak frequency increases across pre-school age through adulthood, reflecting the maturation of neural networks underlying motor development (Johnson et al. 2020). This finding is also consistent with prior research showing that gamma activity increases with age and motor skill acquisition in school-age children (Trevarrow et al. 2019; Wilson et al. 2010). Although the exact role of gamma activity in motor function remains unclear, studies have suggested that gamma activity is linked to cognitive aspects of movement such as movement awareness and effort and is not elicited by passive movements (Isabella et al. 2015, 2021; Muthukumaraswamy 2010; Nowak et al. 2018). Additionally, gamma activity has been found to be essential for action monitoring, rather than being directly involved in movement execution (Cheyne and Ferrari 2013). It has been shown to be sensitive to cognitive factors such as predictability of ‘go’ cues and motor learning (Isabella et al. 2021; Zich et al. 2021). This finding is of particular interest given the nature of these patients' hypertonicity, which has been hypothesized to involve abnormal processing of proprioceptive and tactile feedback (Avanzino and Fiorio 2014; Desrochers et al. 2019; McClelland and Lin 2021). Taken together, our findings contribute to the existing knowledge of the role of motor gamma activity in sensorimotor integration and suggest that gamma peak frequency may serve as a potential marker of altered sensorimotor integration and control in pediatric dystonic patients.
Both beta ERD and beta ERS increased with age across all participants. Similar age-related findings were observed in a study of preschool and older children (Johnson et al. 2020) as well as in older children and young adults (Heinrichs-Graham et al. 2018). These results suggest that developmental changes in beta ERD and ERS, reflecting age-related shifts in both motor preparation and performance monitoring, may persist despite the occurrence of a stroke.
An important consideration when interpreting our findings is the potential impact of the heterogenous clinical characteristics of stroke patients. For instance, patients differed in age of stroke onset, age at study participation, location and size of infarct, and causes of stroke. To account for this, the inclusion criterion was narrowed considerably. Specifically, only patients with MCA territory stroke with basal ganglia and/or thalamus involvement were recruited. These regions are clinical predictors of poor affected hand function after stroke (Ilves et al. 2022; Kirton et al. 2008; Li et al. 2021) and cognitive impairment (Westmacott et al. 2018). Also, patients with significant behavioral issues associated with autism spectrum disorder and/or ADHD were excluded from the study due to requirements for task compliance and the ability to remain still during MEG and MRI acquisition and may have reduced the generalizability of our results to stroke patients with cognitive comorbidities. In addition, while we used the term “affected hand” to refer to the dystonic hand, it is worth noting that hand function varied across patients, with some only demonstrating hypercontractivity with sensory stimulation, while others were unable to move individual fingers independently. As a result, there was some variability in how participants interacted with the response device. While all non-dystonic participants used their index fingers, some dystonia patients used multiple digits or their entire hand due to restricted or clenched hand postures. To prioritize patient comfort during testing, we allowed participants to choose the most natural hand posture. To offset this inconsistency, we emphasized the importance of (and practiced) limiting any other movements during the button press prior to each scan. While it is known that mirrored movements are likely to occur in this population (Tallet 2019), it is notable that we observed no ipsilateral gamma peaks, which are typically associated with contralateral motor cortex activity during active movement. This suggests that any mirror movements, if present, were either absent or insufficient to elicit a detectable gamma peak. Future studies should incorporate EMG or similar force measures to evaluate the absence of such movements more rigorously.
Other limitations would be the unavoidable variances in the demographics that make up this unique patient group and differences in their abilities to perform tasks, as some patients had more affected limbs than others. Furthermore, the highly restrictive recruitment criteria (e.g., exclusion of individuals with co-morbidities such as severe ASD, which might affect participant safety in the scanner or task adherence) resulted in a sex imbalance, with females outnumbering males in the dystonia group. This has been previously reported (Soman et al. 2006), but the significance of this finding is not well understood. For the aforementioned reasons, hand dominance was not matched across groups. In stroke patients, handedness depended on stroke location, with hand dominance being ipsilateral to the stroke hemisphere. Healthy controls, however, were balanced in sex and all right-handed. In addition, dystonia patients made significantly more errors, especially with their affected hand, and thus had less trials compared to non-dystonic and healthy control participants. This may have affected the sensitivity of the analyses by masking true differences between groups. Incorrect trials were not analyzed due to the small sample size in the non-dystonia and healthy control groups. As a result, there was insufficient statistical power to reliably measure error-related functional correlates, for example, error-related frontal activity during incorrect ‘no-go’ responses. Lastly, it is important to note that the short ISI in our study may have allowed some overlap of beta oscillatory changes between trials. To mitigate this, we applied baseline correction and visually inspected time-frequency representations, but potential overlap cannot be entirely ruled out.
5 Conclusion
Pediatric stroke remains largely understudied compared to adult stroke, and the underlying reasons why some children develop dystonia after a stroke while others do not remain unclear. To address this, we conducted the first study that integrates functional and behavioral data to examine differences among pediatric stroke patients with dystonia, those without dystonia, and healthy control participants. The study specifically examined how differences in inhibitory control related to motor deficits by employing a ‘go’/‘no-go’ task performed with both affected and unaffected hands. Dystonia patients exhibited greater theta power when using either hand, indicating a generalized impact on cognitive control that extends beyond their motor impairment. Dystonia patients also exhibited reduced motor cortex gamma power and frequency during correct ‘go’ responses, consistent with impairment in sensorimotor integration during movement compared to their non-dystonic counterparts and healthy controls. These findings suggest that specific functional differences in brain activity, such as higher theta power when inhibiting movement and reduced gamma power during correct movement, may contribute to differences in higher-order motor processing. Furthermore, the observed impairments in sensorimotor integration and cognitive control in dystonia patients may help explain why this group is more at risk of experiencing both motor and cognitive sequelae compared to their non-dystonic counterparts. These findings were made possible by using a comprehensive approach that combines behavioral data (i.e., ‘go’/ ‘no-go’ and DKEFS) with functional (i.e., MEG) and structural (i.e., MRI) imaging methods. Such an approach holds promise to better predict the outcomes of pediatric stroke patients, in particular their risk for developing dystonic symptoms which often persist throughout life. By understanding the complex interplay between cognitive and motor deficits, clinicians and researchers may be better equipped to design interventions that can also address cognitive sequelae, given that dystonia patients are also more likely to experience cognitive impairment.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research (CIHR) grant (PJT173532). The authors would like to thank Jessica Schultz and Fatemeh Mollaei for their help with data collection, Erum Syed for their support with participant recruitment, Samantha Feldman and Justine Ledochowski for facilitating and scoring the neuropsychology tests, and Amanda Robertson for administrative matters.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




