Prediction of childhood maltreatment and subtypes with personalized functional connectome of large-scale brain networks
Funding information: National Natural Science Foundation of China, Grant/Award Numbers: 62176044, 61876114, 62103377; Sichuan University; Key Technologies Research and Development Program
Abstract
Childhood maltreatment (CM) has a long impact on physical and mental health of children. However, the neural underpinnings of CM are still unclear. In this study, we aimed to establish the associations between functional connectome of large-scale brain networks and influences of CM evaluated through Childhood Trauma Questionnaire (CTQ) at the individual level based on resting-state functional magnetic resonance imaging data of 215 adults. A novel individual functional mapping approach was employed to identify subject-specific functional networks and functional network connectivities (FNCs). A connectome-based predictive modeling (CPM) was used to estimate CM total and subscale scores using individual FNCs. The CPM established with FNCs can well predict CM total scores and subscale scores including emotion abuse, emotion neglect, physical abuse, physical neglect, and sexual abuse. These FNCs primarily involve default mode network, fronto-parietal network, visual network, limbic network, motor network, dorsal and ventral attention networks, and different networks have distinct contributions to predicting CM and subtypes. Moreover, we found that CM showed age and sex effects on individual functional connections. Taken together, the present findings revealed that different types of CM are associated with different atypical neural networks which provide new clues to understand the neurobiological consequences of childhood adversity.
1 INTRODUCTION
Childhood maltreatment (CM) is a common public health and social problem. It has a serious impact on physical and mental health across life course (Gilbert et al., 2009), including the development of behaviour problems, high risk of mental disorders, and abnormal neural development (Morgan & Gayer-Anderson, 2016). CM not only increases the risk of developing mental disorders, but also is a major risk factor for depression (Arseneault et al., 2011; Dannlowski et al., 2012; Morgan & Gayer-Anderson, 2016; Nelson et al., 2017). In addition, a previous study found that depressed patients with childhood neglect displayed a widespread reduction of functional connectivity strength suggesting CM causing damage to brain functions (Wang et al., 2014). Although the serious lifelong consequences of CM have been widely reported, the neural mechanisms underlying the consequences remain controversial. Delineating the impact of maltreatment on the developing brain paves a new way in gaining insight into how to provide protection and care for the child, as well as reduce their risk of adult mental disorders.
Emerging evidence suggests that CM experiences alter trajectories of brain development with impaired structure and function in specific regions (e.g., frontal-limbic regions and cerebellum), especially with the abnormal neural circuits involved in threat detection, emotion regulation, and cognition control (Pang, Zhao, et al., 2022; Teicher et al., 2016). Previous studies reported that CM was associated with abnormal functional connectivity (FC) within salience network (SN) and between the default mode network and SN (Marusak et al., 2015). Individuals with CM showed increased FC within theory of mind network which was associated with the severity and type of abuse (Pang, Zhao, et al., 2022; Pruessner et al., 2019; Stanton et al., 2020). Furthermore, adults with CM showed increased amygdala connectivity with the hippocampus and prefrontal cortex during emotion processing (Jedd et al. 2015). It is not surprising that a large number of brain regions and circuitry are related to CM due to the diverse influences on the social cognition-emotion processing of CM. The documented evidence demonstrated that the brain is organized as a network (Bullmore & Sporns, 2009; Li et al., 2022; Wang et al., 2021), which likely better captures the functional reality of brain activity and provides a promising new avenue for research into complex systems of disordered function in childhood trauma (Ross et al., 2021). A recent study used whole-brain functional connectivity and connectome-based predictive modeling (CPM) to succeed in predicting aggression in maladaptive childhood (Ibrahim et al., 2021). Thus, whole-brain large-scale functional network connectivities (FNCs) measurement may provide more holistic insight into how CM experiences affect functional networks' couplings.
The existing FC methods maintain the cross-subject correspondence necessary for group-level analyses while sacrificing subject-specific variation (Canario et al., 2021). Recent advance in individual functional network mapping allows for investigating brain-behaviour relationship at the individual level (Dickie et al., 2018; Wang et al., 2015). Compared with group-level analyses, individual FC has better predictive performance for cognitive abilities in healthy individuals (Li et al., 2019; Zhang et al., 2021) or clinical symptoms in psychiatric illnesses (Brennan et al., 2019; Wang, Li, et al., 2020). Moreover, group-level functional networks failed to reflect individual variation in functional topography, which is hard to characterize the real functional topography of each individual inducing differences in measure of inter-regional functional connectivity, potentially biasing both inference and interpretation (Bijsterbosch et al., 2018; Cui et al., 2020; Li et al., 2019). Recently, using non-negative matrix factorization (NMF) method (Lee & Seung, 1999), Li et al. (2017) developed a brain decomposition approach for mapping subject-specific, sparse, non-negative function networks with high functional coherence. The individual large-scale functional network mapping approach proposed by Li and colleagues has been used to establish the associations between individual functional topography variations and individual differences in brain maturity and executive function (Cui et al., 2020). Thus, using the individual-specific functional mapping may facilitate identifying functional abnormalities by CM.
In the current study, with resting-state functional magnetic resonance imaging (fMRI) data of 215 healthy adults, seventeen individual brain functional networks were first delineated using regularized NMF method and the individual functional connections were obtained between each pair of individual networks. Then, a data-driven CPM with relevance vector regression (RVR) was applied to examine the relationships between individual-level large-scale FNCs and CM experiences. As different types of maltreatment present clinical differences and likely have different effects on behavior and neurobiology (Ackerman et al., 1998), thus the effects of different CM subtypes on large-scale FNCs were further examined. Based on previous findings, we hypothesized that the CM experience can affect the large-scale FNCs involved in social cognition and emotion. In addition, the influences on large-scale FNCs were different based on different subtypes of early adversity.
2 MATERIALS AND METHODS
2.1 Subjects and behavioral assessments
A total of 215 young, healthy, right-handed volunteers (122 female, age range from 18 to 44, mean age = 25.5, SD of age = 6.3, mean CM scores = 34.6) without mental disorders were recruited. The details for all the subjects are shown in Table 1. All subjects were thoroughly examined through DSM-5-structured clinical interviews by two experienced psychiatrists to ensure lifetime absence of mental disorders. The CM experience which is before the age of 16 was assessed using a short form of the self-reported retrospective childhood trauma questionnaire (CTQ) of 28 items (25 clinical items and three validity items) (Bernstein et al., 2003). There were five CTQ sub-scales to assess five aspects of childhood trauma: emotional abuse, emotional neglect, sexual abuse, physical abuse, and physical neglect. The 5-Likert scale was used for responses ranging from 1 (never correct) to five (usually correct). The five sub-scales have their specific question items, using the previously mentioned five-level evaluation indicators, and the score of each subscale is between 5 and 25 points (Pang, Zhao, et al., 2022). When the subscale score exceeds a certain threshold value (emotional abuse ≥13, emotional neglect ≥15, sexual abuse ≥8, physical abuse ≥10, physical neglect ≥10), childhood trauma is considered moderate to severe (Bernstein et al., 1994). Totally, there are 57 subjects including six emotional abuse, 28 emotional neglect, seven sexual abuse, seven physical abuse, and 37 physical neglect were considered moderate to severe CM. If all subscales are below a certain value at the same time, childhood trauma is not considered to be accompanied (emotional abuse ≤8, physical abuse ≤7, sexual abuse ≤5, emotional neglect ≤9, physical neglect ≤7) (Bernstein et al., 1994). The reliability and validity of CTQ have previously been demonstrated (He et al., 2019). This study was approved by the local Medical Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University. Prior to the commencement of any study procedure, the written informed consents were provided and obtained from all the participants.
| Variables | CM subjects (n = 215) |
|---|---|
| Age (years) | 25.50 ± 6.29 |
| Gender (M/F) | 93/122 |
| Education (years) | 14.04 ± 2.60 |
| CTQ | |
| Total score | 34.60 ± 7.70 |
| Emotional abuse | 6.55 ± 2.10 |
| Physical abuse | 5.84 ± 1.49 |
| Sex abuse | 5.33 ± 0.83 |
| Emotional neglect | 9.64 ± 4.00 |
| Physical neglect | 7.25 ± 2.48 |
- Abbreviations: CM, childhood maltreatment; CTQ, childhood trauma questionnaire; F, female; M, male.
2.2 Resting-state fMRI data acquisition
Resting-state fMRI data were acquired on a 3 T Philips MRI scanner. All participants were instructed to relax with their eyes closed but stay awake, and to remain motionless. Foam pads and headphones were used to minimize head movement and scanner noise. Functional images were scanned using an echo-planar imaging sequence with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, slices = 33, matrix size = 64 × 64, flip angle = 90°, field of view = 220 × 220 mm2, thickness = 4 mm with 0.6 mm gap, and a total of 240 volumes.
2.3 Image preprocessing
The resting-state fMRI data preprocessing steps were as follows: (1) removing the first 10 time points; (2) head motion correction by realigning to the first volume; (3) registration to EPI template and resample to 3 × 3 × 3 mm3 and smoothed with 6 mm full-width half maximum Gaussian kernel; (4) nuisance regression including Friston 24-parameter model of head motion, mean white matter, cerebrospinal fluide, and global signals; (5) filtering with bandpath of 0.01–0.1 Hz; To further exclude head motion effects, scrubbing method was used to delete bad images (2 volumes before and 1 volume behind) exceeding the predefined threshold (frame-wise displacement: FD <0.5). To explore whether the number of deleted volumes affects prediction, correlation analysis between the number of deleted volumes and CTQ scores, CTQ subscale scores, and age were performed. No significant correlations were found (for details, please see Table S1 in Supporting Information).
2.4 Define subject-specific functional networks
It has been shown that the individual differences existed in the spatial distribution of large-scale functional networks in the cerebral cortex, and individualized parcellations provide a better data fitting for each participant than standard atlases that ignore functional neuroanatomical differences (Cui et al., 2020). Emerging evidence has demonstrated that the human brain could be stablly and reproduceablely parcellated into seven or fine-grained 17 functional networks belonging to visual, somatomotor, limbic, dorsal and ventral attention, frontoparietal, and default mode networks (Cui et al., 2020; Li et al., 2017; Yeo et al., 2011). Thus, in our study, regularized NMF method (Li et al., 2017) was employed to define 17 large-scale brain functional networks in each individual for further analysis. The processes to define individual large-scale brain networks mainly included three steps: group network initialization, group network atlas creation, and personalized network definition (Li et al., 2017). The first step was to randomly select 50 subjects and the time course of each voxel of the brain was first shifted linearly to make all time points have positive values if necessary, and then the time course is normalized by its maximum value so that all the time points have values in the range of 0–1. Next, the time courses of the selected subjects were combined into a matrix with 11,500 time points and 67,541 voxels. Then, an alternative optimization method and random nonnegative initialization were used to decompose the matrix (Lee & Seung, 1999). This process was repeated 50 times to enhance robustness. In the second step, 50 groups of functional networks were joined to a matrix with 850 rows (i.e., functional networks) and 67,541 columns (i.e., voxels). Then, a normalized-cut (Jianbo & Malik, 2000) divided 850 functional networks into 17 clusters based on spectral clustering method. In each cluster, the most representative functional network which has the highest overall similarity with all other FNs in the same cluster was selected. The final population network map consisted of these 17 representative FNs. All the analyses were performed using codes provided by the authors (https://github.com/hmlicas/Collaborative_Brain_Decomposition).
In the last step, we used the group networks obtained previously as the initialization networks to decompose all the voxels' time series matrix using regularized NMF to obtain the individual functional network for each individual. A network load matrix (17 × 67,541) representing the soft probability that 67,541 voxels belonged to 17 networks and a network time series matrix (230 × 17) were acquired, respectively. Finally, functional network connectivity (FNC) analyses were performed based on the time series of the identified subject-specific functional networks to generate individual-specific FNCs. A 17 × 17 FNCs matrix was obtained for each subject.
To test whether the reconstructed signal can restore the original signal, the similarity was calculated using Pearson's correlation coefficient. For each voxel, a Pearson's correlation coefficient was calculated between original and reconstructed signals in each subject. Then, the voxel was assigned to one of the 17 networks in which this voxel has the maximum load. Finally, the mean correlation coefficient was obtained across all voxels and subjects for each network.
To quantify individual variations of the 17 functional networks in the brain, the median absolute deviation was used as an indicator to evaluate the variability of the brain functional networks across subjects as Cui et al. (2020). First, a load matrix with size of 67,541 × 17 representing the loadings of each voxel in 17 networks was obtained using the NMF method. The median loading of each voxel in each network was calculated across all the 215 subjects, and the absolute deviation between the load of this voxel in each subject and the median loading was calculated. Next, the median value of the absolute deviation was used to characterize individual variation for each network. Finally, the average value of the median absolute deviation across all the 17 networks was used to evaluate the variability of the brain functional networks across subjects.
2.5 Prediction analyses
Based on the FNCs among subject-specific brain networks, a RVR model was trained to predict the scores of CM (Cui & Gong, 2018). The RVR model used kernel vector as basis function, independent hyperparameters as parameter precision, and applied empirical bayes and automatic correlation decision mechanism to obtain sparse solutions. The RVR code can be found in this linkage (https://github.com/ZaixuCui/Pattern_Regression_Clean/tree/master/RVR). Before training, feature selection was carried out by identifying significantly correlated FNCs with CM scores (p < .05). The ten-fold cross-validation (CV) approach was used to avoid biased estimates and overfitting. Specially, the data set was randomly divided into ten folds. In each CV, nine folds of the data were selected as the training sets and the remaining fold was used as a testing set. After repeating the procedure ten times, predicted CM scores were obtained for all subjects. The correlation coefficient between actual and predicted scores was calculated to evaluate the prediction performance. To determine the significance of the prediction model, 5000 times permutation test which disrupted all CM scores and all subjects was performed, and the distribution of the permutated correlations between actual and predicted scores was obtained. The position of the real correlation coefficient before permutation in the distribution was recorded. The statistical p value was defined as the [1-(location/5000)]. A p < .05 was used as the cutoff for significance. To evaluate the prediction results, the R2, adjust R2, root mean square error (RMSE), and mean absolute error (MAE) were also calculated. In addition, to further evaluate whether the individual FNC could predict CM subtypes, the same procedures as predicting CM total scores were performed to predict the five subcategories of CTQ.
To determine the contribution of each network during prediction, the identified networks through NMF method were classified into eight networks including seven cortical networks (visual network (VS), motor network (Dauvermann et al., 2021), limbic network (LMB, Cheng, Roberts, et al., 2022), default mode network (Dannlowski et al., 2012), ventral attention network (Cui et al., 2020), dordal attention network (Tomoda et al., 2009), frontoparietal network (FPN), and a cerebellum network in according to the Yeo group atlas (Yeo et al., 2011). Feature weights of each FNC were assigned to the corresponding connected networks, and the positive weights which made contribution for prediction were included. Since small and negative weight indicated less contribution to predicting, thus the negative weights were not considerd in this study. Finally, the sum of positive weights of each network was calculated to obtain the contribution of each network.
For feature selection in the above prediction analyses, the FNCs correlated with behaviors were identified in all subjects as features. To evaluate the influence of feature selection for prediction, we also selected the features only based on the training dataset (nine-folds data) for prediction during ten-folds cross-validation. The correlation coefficient between real CTQ total scores and predicted CTQ scores was calculated.
To further validate the prediction model, we splitted all subjects into two-folds and one half was used for training and the other was used for testing. The correlation coefficient between real CTQ total scores and predicted CTQ scores was calculated to assess the prediction result.
2.6 Age and sex effects
Previous studies have reported age and gender effects on CM (Ancelin et al., 2021; De Bellis & Keshavan, 2003; O'Shields & Gibbs, 2021). To further investigate the age and gender effects on FNC, all the CM subjects were divided into different groups to identify FNC differences. To evaluate age effects, we divided the subjects into two groups. One is the subjects over 30 years old (44 subjects) and the other is under 20 years old (44 subjects) to better differentiate CM duration effects on the brain. Two-sample t-tests were used to identify the difference of FNCs between 17 subject-specific brain networks between the different age groups. In addition, we also divided all the subjects into female and male groups; the difference of inter-network FNCs between males and females was also evaluated through two-sample t-tests. A corrected p < .05 was set for statistical significance using false discovery rate (FDR) correction.
We also performed prediction of CM total scores by including the age and gender factors as features to test age and gender effects. The correlation between actual and predicted CM scores and the weights for all the features were also calculated (See Table 2).
3 RESULTS
3.1 Individual functional networks
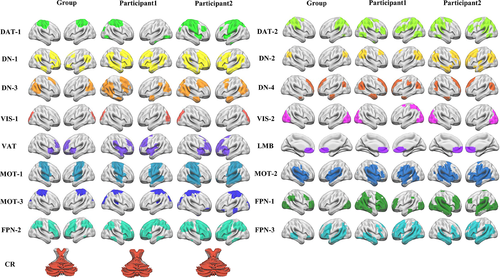
Using NMF approach, we identified 17 individual-level large-scale functional networks for each subject. By comparing with the Yeo atlas, these identified networks were classified into the visual network (VIS: VIS-1 and VIS-2), somatomotor network (MOT: MOT1, MOT2, and MOT3), dorsal attention network (DAT: DAT1 and DAT2), ventral attention network, frontoparietal network (FPN: FPN1, FPN2, and FPN3), defalt mode network (DN: DN1, DN2, DN3, and DN4), limbic network (LMB), and cerebellum network (Figure 1). The parcellation results were compared at the group level and individual level, respectively. At the individual level, two subjects, including one participant with minimum CM scores and the other participant with maximum CM scores, were selected to show the individual-level functional networks. There were obvious differences in most of the parcellation results of the 17 brain networks at the group level and individual level except VIS, LMB, and CR networks. In addition, there were also obvious differences in the DAT, DN, VIS, VAT, and FPN networks between the two participants with minimum or maximum CM scores (Figure 1).
To determine whether the reconstructed signals could restore the original signals, the mean Pearson's correlation coefficient for each network was obtained. For all the 17 networks, the similarities were higher than 0.7 suggesting that the reconstructed signals could well mimic the original signals (for details, please see Figure S1 in Supporting Information).
To quantify networks' variability, cross-subject variability of functional network topography was shown, and the primary visual and sensorimotor cortices showed small while high-order cortices such as prefrontal, parietal, and temporal cortices showed large individual variations (for details, please see Figure S2 in Supporting Information).
3.2 Individual-level FNCs predict CM scores
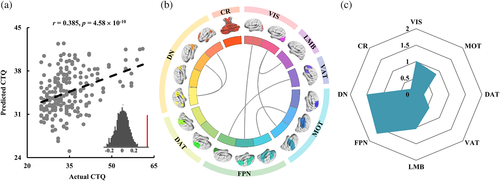
With a set of individual FNCs, the total CM scores of each participant could be predicted (r = .385, p = 4.58 × 10−10) (Figure 2a). Individual-specific connections that largely contributed to the total CM score prediction were mainly involved in FNCs within DN, between VIS and FPN, MOT, LMB, and between FPN and VAT, MOT (Figure 2b). In all these networks, the contributions of FPN, DN, and VIS networks were relatively higher while the contributions of CR and DAT networks were relatively lower during prediction (Figure 2C). The prediction results evaluated by R2, adjust R2, RMSE, and MAE were also shown in Table 2.
| CTQ | R2 | Adjust-R2 | RMSE | MAE |
|---|---|---|---|---|
| Total score | .2125 | .1779 | 3.07 | 5.56 |
| Emotional abuse | .1196 | .0809 | 2.02 | 1.44 |
| Physical abuse | .1030 | .0636 | 1.45 | 1.03 |
| Sex abuse | .6278 | .0216 | 0.82 | 0.50 |
| Emotional neglect | .1776 | .1415 | 3.70 | 2.96 |
| Physical neglect | .0644 | .0233 | 2.45 | 3.03 |
- Abbreviations: CTQ, childhood trauma questionnaire; RMSE, root mean square error; MAE, mean absolute error.
To test the influence of feature selection, we performed feature selection in each CV. Although the prediction result is a little lower than that obtained using the features selected with all the subjects, the predicted scores still showed a significant correlation with actual CTQ scores (for details, please see Figure S3 in Supporting Information).
Using half subjects as test, we found that the prediction model still works (predicted CTQ scores were significantly correlated with actual CTQ scores), but the prediction accuracy is much lower than that using ten-folds cross-validation (for details, please see Figure S4 in Supporting Information).
3.3 Individual-level FNCs predict CM subtypes
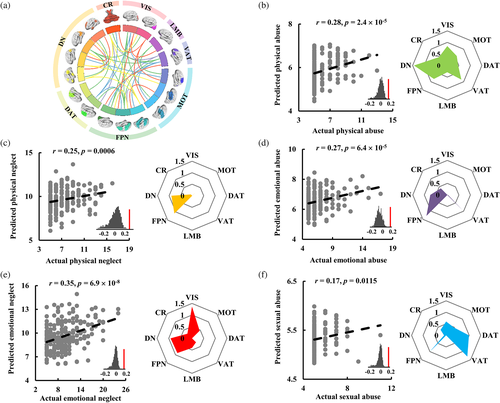
With individual-level FNCs of the large-scale functional networks, the five subtypes of CM can be well predicted: physical neglect (r = .25, p = .0006), physical abuse (r = .28, p = 2.4 × 10−5), emotional neglect (r = .35, p = 6.9 × 10−8), emotional abuse (r = .27, p = 6.4 × 10−5), and sexual abuse (r = .17, p = .0115) (Figure 3). By calculating the contribution weights of each network, we found that FPN and DN networks made large contribution to predicting emotional abuse and physical neglect, FPN, DN, and VIS networks made large contribution to predicting emotional neglect, FPN, DAT, and VAT networks made large contribution to predicting sexual abuse, and VIS, DN, FPN, and VAT showed large contribution to predicting physical abuse. These results demonstrated that each subtype of CM was associated with specific networks. The prediction results for all the CM subtypes evaluated by R2, adjust R2, RMSE, and MAE were also shown in Table 2.
3.4 Age and sex effects on functional connectivities in CM
To identify the age effects on functional couplings of large-scale functional networks, all the participants were divided into two groups, one group with age < =20 years, and the other group with age > =30 years. The old age group showed higher functional connectivities within MOT, and between MOT and VIS networks than young age groups (Figure 4a).
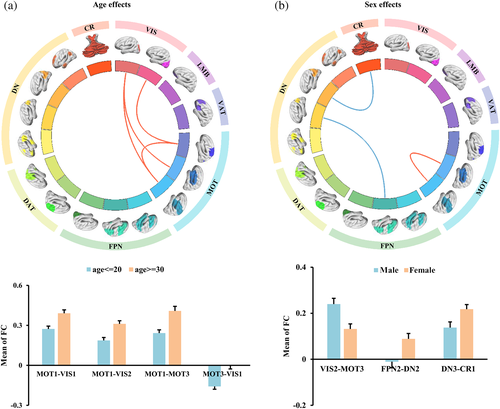
We also divided the participants into male and female groups to explore the sex effects on functional couplings between large-scale networks. FNC analyses identified significantly increased FNCs between VIS and MOT networks and significantly decreased functional connectivities between DN and FPN, CR in male groups compared to female groups (Figure 4b).
In addition, with age and gender added into features for prediction, we found that the correlation coefficient of the predicted and actual CTQ scores was a little lower than that not taking age and gender as features. The weight distribution showed that age and gender had very low weight for prediction suggesting that the two factors had little influence on the results (for details, please see Figure S5 in Supporting Information).
4 DISCUSSION
Combining individual-specific large-scale FNCs and multivariate pattern analysis techniques, we evaluated the effect of CM on individual FNCs of 17 large-scale brain networks. We demonstrated that the FNCs of individual-specific functional networks could predict the CM total scores and subscale scores, and different types of CM are associated with differentially atypical neural networks. Emotional abuse and physical neglect are primarily associated with FPN and DN networks; emotion neglect is mainly associated with FPN, DN, and VIS networks; sexual abuse is mainly related to FPN, DAT, and VAT networks, and physical abuse primarily links to VIS, DN, FPN, and VAT. These findings shed light on the underlaying mechanism for effects of CM impairing functional interactions of brain networks, and highlight the importance of accounting for interindividual variability in investigating functional brain organization in individuals.
Although previous studies have reported that early CM induced abnormal development of neural circuits, these results were inconsistent (Dannlowski et al., 2013; Hart et al., 2018; Jedd et al., 2015; Rakesh et al., 2021; van der Werff et al., 2012). It is speculated that using group-level atlas-based FC might underestimate the correspondence between the connectome and behavior (Li et al., 2019). Recently, using individual FC mapping, Wang, Tian, et al. (2020) demonstrated that FC defined using the individually specified functional regions was able to predict baseline verbal memory performance and electroconvulsive therapy-induced verbal memory impairments in major depressive disorder patients, but the same models using connectivity derived from a group-level atlas completely lost the ability to predict these behavioral measures. Moreover, Cui et al. (2020) revealed that individual variability of functional topography was associated with fundamental properties of brain organization, including evolutionary expansion, cortical myelination, and cerebral blood flow. Recently, Zhang and colleagues developed a new individual functional parcellation approach to define individual hippocampus subregions and found that individual functional connections could better predict aging effect than group-level functional connections (Zhang et al., 2021). All the evidence indicated that individual functional connectivity mapping might be better to establish brain-behavior relationship than group-level. In our study, we further demonstrated individual-specific functional connectivity mapping is a useful tool to predict the effects of early CM.
For CM prediction, we found that the functional connections of DN, FPN, and VIS networks showed a large contribution. DN network is involved in internally oriented cognitive functions (Cheng, Wang, et al., 2022; Pang, Wei, et al., 2022; Perkins et al., 2015; Wang et al., 2019) while FPN is implicated in cognitive control process during externally oriented tasks (Cheng et al., 2021; Marek & Dosenbach, 2018; Wang, Wei, et al., 2018; Wang, Wei, et al., 2020), sustained attention, and working memory (Liu et al., 2021; Ptak, 2012). The VIS network is involved in visual information processing. The relationships between CM and these networks have been documented in previous studies. For example, CM was associated with increased DN deactivation during working memory (Philip et al., 2013), reduced resting-state FNCs within DN and FPN (Dauvermann et al., 2021), and enhanced morphometric network centrality in DN regions involved in internal emotional perception, self-awareness (Teicher et al., 2014). Physical abuse/neglect was associated with the FNCs between VIS and cingulo-opercular network in patients with major depressive disorder (Yu et al., 2019). Young adults who witnessed domestic violence during childhood showed reduced gray matter volume and thickness in the visual cortex (Tomoda et al., 2012). Childhood sexual abuse was associated with reduced gray matter volume in the visual cortex involved in facial recognition in young women (Tomoda et al., 2009). These previous reports showed that the early adversity experience leads to deleterious development of primary and high-order cortical regions. Our findings further demonstrated that this effect of early CM continues into adulthood and spread to other systems showing connectivities with these regions. The identification of a potentially sensitive period during which the brain is maximally vulnerable to CM argues for the value of early intervention or prevention strategies.
In addition, distinct functional networks had different contributions to the prediction of different subtypes of CM. In general, the networks showing major contributions to prediction were concentrated in the DN, FPN, VIS, and VAT networks which were also identified for the prediction of CM total scores. As two core networks of triple network models (Menon, 2011), FPN and DN exerted large impacts on almost all subtypes of CM. The DN and the FPN habitually work in opposing directions which are involved in attentional demands: FPN activation increases while DN activation decreases when attentional demands increase; conversely, FPN activation decreases while DN activation increases during periods of rest or internally focused cognitions (Fox et al., 2005). By extending previous findings, our result further suggested that the DN and FPN as a basic “target of attack” no matter what form of CM occurred, with the generally observed maladaptive high-order cognition function in adults with CM (Heledd Hart et al., 2017). The VIS network contributed most to the prediction of physical abuse and emotional neglect. VIS network is the main channel for processing external information, and process and conveys the adverse sensory input (such as the facial expressions of emotion) of CM (Kitada et al., 2010; Tomoda et al., 2009; Wang, Feng, et al., 2018). The VAT network made major contributions to the prediction model of physical, emotional, and sexual abuse. Child abuse is associated with increasingly abnormal threat-related attention bias (Pine et al., 2005), and significantly reduced activation in the attention network implicated in responding to external attention demands (Asplund et al., 2010; Scalf et al., 2014; Serences et al., 2005) during sustained attention were found in young people with childhood abuse experience (Lim et al., 2016). Our results explained the neural circuit correlates of different types of CM while controlling for psychiatric conditions. The novel findings highlighted the different neurobiology effects of different CM types, and proposed that the impact on the brain development of CM subtypes should ideally be considered separately.
Finally, the age and gender effects were assessed. The older subjects exhibited higher FNCs within MOT and between MOT and VIS networks than younger individuals. During development, sensory and motor integration is critical for brain and behavior typical maturity (Chicoine et al., 1992; Kagerer & Clark, 2014). The increased FNCs within MOT and between MOT and VIS networks observed in older individuals might show a compensatory mechanism for impaired sensorimotor integration. In addition, a large number of previous studies have reported significant gender effects on major depressive disorder (Grigoriadis & Erlick Robinson, 2007; Piccinelli & Wilkinson, 2018). As a high-risk factor for mental disorders, our study also revealed significant gender differences between men and women. We found that women showed higher FNCs between DN and FPN, CR networks while lower FNCs between MOT and VIS networks than that in men. The high FNCs indicated abnormal functional swithcing between internal and external states, while low FNCs between MOT and VIS suggested impaired sensorimotor integration in women. The differences in FNCs between DN and FPN, CR, and between MOT and VIS may serve as an early biomarker to predict the progression of depression state (Cheng, Roberts, et al., 2022; Cheng, Wang, et al., 2022).
The current study has a few limitations. First, the sample includes adults with CM scores but without current psychopathology, which may be a “resilient” sample. Therefore, the findings related to CM may be specific to those who have experienced CM but lack psychopathology. Second, the CTQ questionnaire only assessed the trauma or adversity experience before age 16. The experience after 16 or current was not included in this questionnaire and was not evaluated in our study. Third, the CTQ scores were obtained by subjective evaluation. Due to the relatively large age span of the participants, recall bias is inevitable and maybe not so objective to reflect CM. Fourth, to explore the age effects, we simply divided all the participants into ages below 20 and above 30 groups to identify FNC differences. Whether the identified differences reflect age effects need to be further validated. Fourth, for the prediction of sexual abuse, the data distribution was relatively concentrated, which may affect the prediction results. The data with uniform distribution is needed to further validate our model. Fifth, inconsistent distribution of CTQ total scores may also affect the accuracy of prediction results and generalization of the prediction model. The number of participants with high CTQ total scores is fewer than that with low CTQ total scores leading to the prediction model may be only suitable for prediction of low CTQ total scores. Finally, although there are several methods to define individual network, whether the large variations in functional network topology could well characterize individual behavioral differences is still doubtful. Validating the effectiveness is important for future individual functional network mapping.
5 CONCLUSION
This study established the neural correlates of large-scale networks for CM or its subtypes based on individual-level functional mapping to better explain the individual differences during development with childhood adversity experience. Furthermore, the current findings shed light on the neurofunctional basis of CM or subtypes and highlight different types of CM have different impacts on brain development, which may provide neuromarkers for the classification of CM subtypes. Further efforts are needed to identify the sensitive period for each CM subytpe and promote the development of effective prevention and treatment strategies to normalize the CM-induced brain atypical mature trajectory.
AUTHORS CONTRIBUTIONS
J.J. Wang designed and conceived the study; H.W. Wu performed MRI scanning and behavioral data collection. J. Zhang, T.Y. Zhao, J.Y. Zhang, Z.W. Zhang, B.C. Cheng analyzed the data. H.M. Li provided the method tutor. J. Zhang, T.Y. Zhao, Y.J. Pang, and J.J. Wang wrote the paper. Y.J. Pang and J.J. Wang edited the paper and all the authors discussed the results.
ACKNOWLEGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 62176044, 61876114, and 62103377), the Key Technologies Research and Development Program of Henan Province (222102210076), the Key Scientific Research Program of the Higher Education Institutions of Henan Province (22A416013), and the Sichuan Science and Technology Program (2021YJ0186, 2022YFS0178), Natural Science Foundation of Yunnan Province (202001BC070001, 202102AA100053) and Med-X Center for Informatics funding project of Sichuan University (YGJC002).
CONFLICT OF INTEREST
This work has no any competing financial interests to disclose.
Open Research
DATA AVAILABILITY STATEMENT
All the data and codes will be shared by request to the corresponding authors.