Adaptive contextualization: A new role for the default mode network in affective learning
Abstract
Safety learning describes the ability to learn that certain cues predict the absence of a dangerous or threatening event. Although incidental observations of activity within the default mode network (DMN) during the processing of safety cues have been reported previously, there is as yet no evidence demonstrating that the DMN plays a functional rather than a corollary role in safety learning. Using functional magnetic resonance imaging and a Pavlovian fear conditioning and extinction paradigm, we investigated the neural correlates of danger and safety learning. Our results provide evidence for a functional role of the DMN by showing that (i) the DMN is activated by safety but not danger cues, (ii) the DMN is anti-correlated with a fear-processing network, and (iii) DMN activation increases with safety learning. Based on our results, we formulate a novel proposal, arguing that activity within the DMN supports the contextualization of safety memories, constrains the generalization of fear, and supports adaptive fear learning. Our findings have important implications for our understanding of affective and stress disorders, which are characterized by aberrant DMN activity, as they suggest that therapies targeting the DMN through mindfulness practice or brain stimulation might help prevent pathological over-generalization of fear associations. Hum Brain Mapp 38:1082–1091, 2017. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Learning that a particular sensory cue signals danger is fundamental to successful behavioural adaptation in humans and other animals and is governed by similar neural networks across species [Bechara et al., 1995; Büchel et al., 1998; Fanselow and LeDoux, 1999; Freese and Amaral, 2009; McHugh et al., 2014; Sah et al., 2003]. However, learning that a cue that was previously associated with danger is now signalling safety is equally important because it reduces the physiological consequences of stress and enables the adaptation of behaviour to changing circumstances [Lupien et al., 2009]. Safety learning can be assessed through Pavlovian fear conditioning and extinction experiments, in which participants are first trained to predict an electric shock that follows an otherwise neutral tone (conditioning) and then learn not to respond to the tone with fear when it is no longer followed by the electric shock [extinction; Greco and Liberzon, 2016; Phelps et al., 2004; Schiller et al., 2008]. Animal studies have shown that extinction and hence safety learning are facilitated by activity in the ventromedial prefrontal cortex (vmPFC), which inhibits the fear response previously associated with a danger-signalling sensory cue in the basolateral amygdala [Greco and Liberzon, 2016; Do-Monte et al., 2015; Quirk et al., 2003; for a review, see Tovote et al., 2015].
In humans, neuroimaging studies have provided evidence for a similar neural mechanism of safety learning by demonstrating increased vmPFC activity during extinction [Kalisch et al., 2006; Milad and Quirk, 2010]. However, a recent meta-analysis of neuroimaging studies showed that regions overlapping with the default mode network (DMN)—including the vmPFC—are de-activated during the processing of danger cues and activated during the processing of safety cues [Fullana et al., 2016]. This finding suggests that in addition to the vmPFC, a large-scale network, and specifically the DMN may play a functional role in safety learning. The DMN is known to be activated during internally-driven cognitive operations, for example, self-referential processing, long-term memory retrieval, and mind-wandering, which are especially likely to occur during the presentation of safety cues that do not direct attention to the environment [Anticevic et al., 2012; Buckner et al., 2008; Burianová and Grady, 2007; Burianová et al., 2010]. As such, Fullana et al.'s [2016] finding of DMN activation during the presentation of safety cues could simply reflect a corollary rather than functional role during safety learning. That is, the DMN might be an epiphenomenon of safety learning—allowing the individual to engage in mind-wandering whenever they consider themselves to be safe—rather than an integral part of the learning process that leads to the impression of safety associated with a reduced fear response. However, a number of recent studies demonstrate reduced or aberrant connectivity within the DMN associated with an impairment in safety learning in patients with post-traumatic stress and anxiety disorders, thereby suggesting that in healthy individuals, activity within the DMN may play a functional role and actively contribute to successful safety learning [DiGangi et al., 2016; Jovanovic et al., 2012; Lissek and Grillon, 2010; Lissek et al., 2014b; Patriat et al., 2016].
The objective of this study was to investigate whether the DMN has a functional rather than corollary role in safety learning in healthy individuals. Learning is associated with changes in physiological and neural responses to altered cue-reinforcement contingencies, for example, if a cue were repeatedly associated with a shock, the observation of an increased BOLD response in the amygdala together with an increased physiological fear response to the cue would be interpreted as fear learning. Therefore, if the DMN were functionally related to safety learning then activity in the main nodes of the DMN would increase significantly in response to modulations of cue-reinforcement contingencies and in parallel with physiological fear responses. In contrast, if DMN activity were a consequence of safety learning, no such increases over time would be observed and the DMN would be activated to the same extent whenever safety signals are presented. To test this hypothesis, we assessed the skin conductance response (SCR) and functional brain activity during danger and safety learning with functional magnetic resonance imaging (fMRI) in a fear conditioning and extinction paradigm, in which the cue-reinforcement contingencies shift from a cue predicting danger (conditioning) to the cue predicting safety (extinction). To increase the number of changes in cue-reinforcement contingencies, a standard differential delay-conditioning paradigm was modified to include repeated conditioning and extinction phases, resulting in an A-B-A-B fear-learning paradigm. Based on previous human neuroimaging studies [Fullana et al., 2016], we hypothesized that (i) during danger cue processing, SCRs would be stronger and functional brain activity would significantly increase in the amygdala, thalamus, somatosensory cortex, anterior insula, and anterior cingulate cortex, and (ii) during safety cue processing, SCRs would be weaker and functional brain activity would significantly increase in the main nodes of the DMN, such as the vmPFC, hippocampus, inferior parietal lobule, and posterior cingulate cortex. Finally, positing that DMN activity fulfils a function in safety learning, we hypothesized that (iii) the difference between the two activation patterns associated with safety and danger learning would increase over time and that activity in DMN regions would increase with repeated extinction phases, whereas SCRs would decrease.
MATERIALS AND METHODS
Participants
Thirty right-handed adults (15 females, mean age = 26 years, age range = 21–34 years) with normal or corrected to normal vision took part in the experiment, which was approved by the Human Ethics Research Committee of the University of Queensland, after giving written consent. All participants were screened for neuropsychological disorders, brain damage, and substance abuse. Images were acquired with a Siemens Magnetom Trio 3T scanner and a 32-channel head coil at the Centre for Advanced Imaging at the University of Queensland.
Procedure
Participants took part in a partially reinforced, differential fear conditioning experiment with repeated conditioning and extinction phases (A–B–A–B paradigm; see Fig. 1). During each phase, two visual stimuli (a triangle and a circle) were repeatedly presented in randomized order. The stimuli were presented in either of two contexts (blue or orange background) associated with the different experimental phases to reduce interference from context-conditioning effects [Lang et al., 2009]. Participants were asked to identify the stimuli by pressing one of two buttons with the second and third digits of their right hand. One of the two stimuli (CS+) was paired with electro-dermal stimulation (unconditioned stimulus, UCS) during the first and third phases (conditioning) but not the second and fourth phases (extinction), while the other stimulus (CS−) was never paired with stimulation. The CS+ constitutes a danger signal during the conditioning phases and a safety signal during the extinction phases, whereas the CS− constitutes a control safety stimulus throughout the experiment. Stimuli and contexts were randomly assigned and assignments were counterbalanced across individuals. Each block started with 15 s of background presentation to allow for the electro-dermal response to settle and for the participants to habituate. During each experimental block, following 1 s of background, 20 stimuli (10 CS+, 10 CS−) were presented for 3 s and followed by 14 s of background in a randomized order. All stimuli were presented using Presentation software (Neurobehavioral Systems, Inc.) and projected onto a screen, which could be viewed with a mirror attached to the head coil.

Experimental paradigm. Participants repeatedly identify one of two stimuli (circle or triangle) through a button press. One stimulus (CS+) co-terminates with mild electric stimulation to the right wrist during the first and third (conditioning) but not during the second and fourth experimental phase (extinction). While this stimulus switches between being a danger and safety cue, the other stimulus (CS−) is never paired with stimulation and constitutes a safety cue throughout the entire experiment. CON, conditioning phase; EXT, extinction phase. [Color figure can be viewed at wileyonlinelibrary.com]
Sixty percent of CS+ presentations co-terminated with a 50 ms electro-dermal stimulation using two pre-gelled carbon snap electrodes attached to the right wrist (EL508, Biopac Systems, Inc.). Prior to scanning, stimulation strength was adjusted to individual tolerances following established procedures to ensure that stimulation was highly uncomfortable, but not painful [LaBar et al., 1998]. Stimulation was administered using a STIMISOC isolator connected to a STM100C stimulator, which was controlled by a MP150 (Biopac Systems, Inc.).
Skin conductance responses (SCRs) were sampled at 1 kHz using pre-gelled carbon snap electrodes (EL508, Biopac Systems, Inc.) attached to the medial phalanges of the second and third digits of the left hand and connected to an EDA100C module attached to a MP150 (Biopac Systems, Inc.). SCRs were defined as the peak response of the low-pass filtered (0.1 Hz) electro-dermal activity above 0.02 µS that occurred within 1–4 s after the onset of the conditioned stimuli [Lockhardt, 1966].
Image Acquisition and Preprocessing
For each participant, a T1-weighted volumetric anatomical MRI was acquired with the following parameters: 176 slices sagittal acquisition MP2-RAGE; 1 mm3 isotropic volume; repetition time (TR) = 4,000 ms; echo time (TE) = 2.89 ms; flip angle = 6°; FOV = 256 mm, GRAPPA acceleration factor = 3. Functional images were acquired using a T2*-weighted echo-planar image pulse sequence with the following parameters: 45 slices; 2.7 mm slice thickness; voxel size = 2.5 × 2.5 × 2.7 mm; TR = 3,000 ms; TE = 30 ms; FOV = 192 mm; flip angle = 90°. Brain activation was assessed using the blood oxygenation level dependent (BOLD) effect [Ogawa et al., 1990]. For functional analysis, T2*-weighted images were preprocessed with Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Images were realigned to the mean image for head-motion correction and then spatially normalized into a standard stereotaxic space with voxel size of 2 mm3 (Montreal Neurological Institute template) using segmented white and gray matter T1 maps. Head movement and rotation in the three dimensions did not exceed 1 mm and no dataset had to be excluded from analysis. Finally, the functional images were spatially smoothed with a 6-mm full width half maximum Gaussian kernel. All subsequent analysis of fMRI data was based on non-reinforced trials.
Whole-Brain Analysis
Following preprocessing, whole-brain fMRI data were analyzed with Partial Least Squares (PLS; https://www.rotman-baycrest.on.ca/index.php?section = 84). PLS is based on principal component analysis and assumes that brain function reflects the coordinated activity of groups of brain regions rather than the independent activity of any single brain region. In brief, PLS mean-centres and then decomposes the covariance matrix between brain activity and the experimental design for the whole group in a single analytic step using singular value decomposition (SVD). SVD results in separate, mutually orthogonal latent variables (LVs), which describe patterns of brain activity related to the experimental design [McIntosh et al., 2004]. SVD maximizes covariance in the partial least squares sense and generates a weight for each voxel, which designates its degree of covariance with the whole brain activity pattern. PLS then assesses the statistical significance of each LV using permutation testing with 500 permutations [McIntosh et al., 1996] and the reliability of the brain activity patterns for each voxel by using a bootstraping procedure with 100 bootstraps, resulting in an estimate of the standard error, which is used to calculate the bootstrap ratio [BSR; Efron and Tibshirani, 1985]. Peak voxels with a BSR greater than 3.0 are considered to be reliable, as this approximates P < 0.005 [Sampson et al., 1989]. Because computation of the LVs and corresponding brain images is conducted in a single analytic step across all voxels and participants, no correction for multiple comparisons is required. Finally, a brain score indicating how strongly the group pattern is expressed in each individual data set is calculated for each participant by multiplying each individual data set with the whole-brain activation loadings.
In order to test the overlap of our results with the DMN, a DMN mask was generated from 820 resting state datasets published by the Human Connectome Project (http://humanconnectome.org). The DMN was identified as component #2 of the HCP900-PTN data that was preprocessed and analysed following the HCP pipeline with FMRIB Software Library (FSL, http://fsl.fmrib.ox.ac.uk) using a 25-dimensional independent component analysis (ICA). Each 15-minute run of each subject's four fMRI scans was processed according to Smith et al. [2013]. It was minimally-preprocessed [Glasser et al., 2013], and had artefacts removed using ICA + FIX [Griffanti et al., 2014; Salimi-Khorshidi et al., 2014]. Each dataset was then temporally demeaned and had variance normalisation applied according to [Beckmann and Smith, 2004]. Group-PCA output was generated by MIGP (MELODIC's Incremental Group-PCA) from 820 subjects. This comprises the top 4,500 weighted spatial eigenvectors from a group-averaged PCA [a very close approximation to concatenating all subjects’ timeseries and then applying PCA; Smith et al., 2014]. The MIGP output was fed into group-ICA using FSL's MELODIC tool [Beckmann and Smith, 2004; Hyvärinen, 1999].
RESULTS
There were no significant differences in reaction times in response to CS+ and CS− presentations. However, a trend suggested slower reaction times in responses to CS+ than to CS− in the initial conditioning phase (t(29) = 1.9, P = 0.07, d = 0.39; see Fig. 2a). Psychophysiological evidence of successful differential fear conditioning was provided by a 2 × 2 × 2 analysis of variance of the SCRs with factors stimulus (CS+, CS−), context (conditioning, extinction), and time (first, second presentations) that yielded significant main effects for the factors stimulus (F(1,1) = 5.4, P < 0.05) and context (F(1,1) = 17.2, P < 0.001). Repeated, two-sided t-tests demonstrated significantly larger SCRs to CS+ than CS− presentations as well as to CS presentations during the conditioning than during the extinction phases (all t(29) > 2.1, P < 0.05, d > 0.27; see Figure 2b).
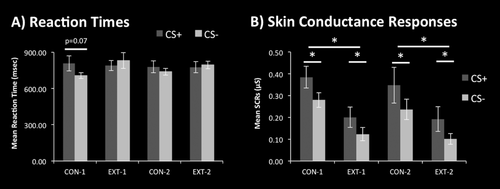
Behavioural results. (A) Average reaction times (±S.E.M.) for button presses in response to the CS+ and CS− presentation. B) Average skin conductance responses (±S.E.M.) in response to presentations of the CS+ and CS−. CON, conditioning phase; EXT, extinction phase, * P < 0.05.
Whole-brain fMRI analysis resulted in one significant LV (P < 0.001) accounting for 51.4% of the variance in the data. The LV differentiated activity related to danger cues from activity related to safety cues. In contrast to safety cues, the presentation of danger cues (i.e., CS+ during the first and second conditioning phase) engaged dorsal anterior cingulate cortex, bilateral insula, middle temporal gyrus, temporal pole, ventrolateral prefrontal and inferior parietal cortex, thalamus, brainstem, and medial cerebellum (see cool coloured regions in Figure 3). In contrast to danger cues, the presentation of safety cues (i.e., CS+ and CS− during the first and second extinction phase as well as CS− during the second conditioning phase) engaged orbitofrontal cortex, ventromedial prefrontal cortex, subgenual anterior cingulate cortex, bilateral hippocampus, precuneus, superior parietal gyrus, right fusiform gyrus, medial superior frontal gyrus, posterior cingulate gyrus, ventral striatum, and lateral cerebellum (see warm coloured regions in Fig. 3). The results further show that this pattern increased over time and repeated measures, two-tailed t-tests revealed significant differences between presentations of CS− during the first and the second conditioning and extinction phases (for both t(29) > 2.8, P < 0.008, d > 0.57) but not between presentations of CS+ during the first and second extinction phase (t(29) = 0.7, P = 0.5, d = 0.16).
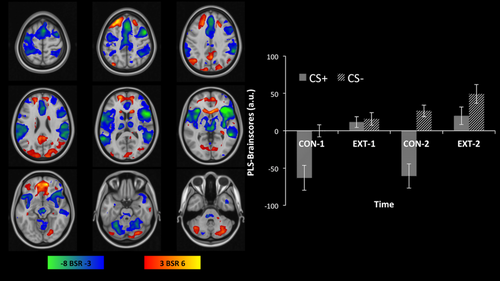
Multivariate whole-brain analysis results showing safety learning in the default mode network. PLS is a data-driven method using principal components analysis, which does not depend on a priori definitions of experimental contrasts but extracts the most salient and robust patterns based solely on the variance contained within the data. PLS first identifies anti-correlated patterns of brain activity, that is, when one set of regions is activated, the other is deactivated and vice versa, and then assesses their relation to the experimental design by computing the contribution of the experimental manipulation to each activity pattern in the sample (brain scores). Left: PLS analysis identified two anti-correlated patterns of brain activation comprising of regions overlapping with the DMN (warm colours) and regions overlapping with the salience, somatosensory, and ventral attention networks (cool colours). The patterns are thresholded at a bootstrap ratio (BSR) estimate of the standard error of 3 (P < 0.05). Right: The bar graph shows how the brain activation patterns are related to the fear conditioning and extinction task. PLS brain scores indicate the contribution of each stimulus and context to the two anti-correlated patterns across the four phases of the experiment. Positive brain scores (related to the activation pattern depicted in warm colours) are related to safety cues (CS+ in extinction and CS− in all phases). Negative brain scores (related to the activation pattern depicted in cool colours) are related to cues predicting shock (CS+ in conditioning phases). Increasing brain scores indicate learning and provide evidence for safety learning in the default mode network. [Color figure can be viewed at wileyonlinelibrary.com]
The comparison between the DMN mask derived from 820 resting-state data sets and our results demonstrate an overlap of 42% of the pattern associated with safety learning and regions considered to form the core of the DMN [Andrews-Hanna et al., 2014] including the ventromedial prefrontal cortex, anterior and posterior cingulate cortex, superior frontal gyrus, and angular gyrus (see Fig. 4).
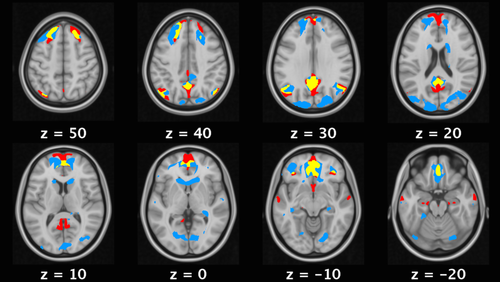
A comparison of the regions associated with safety learning (blue) with the DMN identified through group independent component analysis of resting state data from 820 participants (red) shows overlapping activations (yellow) in core DMN regions such as anterior and posterior cingulate cortex, angular gyrus, and superior frontal gyrus. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
The aim of this study was to investigate whether the activation of the DMN that has been observed incidentally in previous fear conditioning studies [Fullana et al., 2016] is functionally related to safety learning. Based on patient studies, which link impaired safety learning to a reduction in DMN activity, we hypothesized that in healthy adults, the DMN would be activated in response to safety cues and that DMN activity would increase as learning progresses with repeated presentations of safety cues, whereas physiological fear measures would decrease [DiGangi et al., 2016; Jovanovic et al., 2012; Lissek and Grillon, 2010; Lissek et al., 2014b; Patriat et al., 2016]. Our results demonstrate activity within the core nodes of the DMN (ventromedial prefrontal cortex, anterior and posterior cingulate cortex, superior frontal gyrus, and angular gyrus) in response to safety cues. Importantly and in contrast to previous studies [Fullana et al., 2016], the multivariate, data-driven method used in our analysis was able to identify the hidden factors or latent variables underlying the co-variation within brain regions, thereby yielding two activation patterns that are negatively correlated with each other [Abdi and Williams, 2010]. As a consequence, our results extend previous findings by showing that DMN activity associated with safety cues is directly anti-correlated with activity in regions associated with fear learning. In other words, these findings strongly suggest that the DMN is deactivated whenever the fear-learning network is activated and vice versa. Furthermore, we show increases in DMN activity over time in response to safety cues during extinction (CS+ and CS−) and also, importantly, during the conditioning phases (CS−), while the physiological indicator of fear (skin conductance response) decreases over time in response to safety cues during extinction (CS+ and CS−) as well as during the conditioning phases (CS−). These parallel changes over time not only demonstrate that the DMN is related to safety learning but also suggest that activation of the DMN is not an epiphenomenon of safety learning but that it performs the function of safety learning. Taken together, the findings of this study show that the DMN is activated during safety learning, directly anti-correlated with fear learning and, its activity increases over time in parallel to decreasing physiological indicators of fear. Thus, our study provides strong evidence for a functional rather than a corollary role of the DMN.
Based on these findings, we propose that it may be the DMN's function during affective learning to contextualize safety memories and that such contextualization of safety memories inhibits fear responses and thereby contributes to the adaptive generalization of fear associations. At the core of our proposal is the idea that the engagement of the DMN during safety learning reflects the contextualization of safety memories. The DMN is mainly associated with perceptually decoupled and internally-driven cognitive processes, such as mind-wandering, self-referential processing, and autobiographical long-term memory retrieval [Andrews-Hanna et al., 2010; Binder et al., 1999; Herold et al., 2016; Mantini and Vanduffel, 2013; Mason et al., 2007; Smallwood et al., 2013; Spreng and Grady, 2010]. However, a number of studies have reported DMN activity related to behavioural performance in stimulus-directed tasks [Chen et al., 2013; Elton and Gao, 2015; Gilbert et al., 2007; Hahn et al., 2007; Smallwood et al., 2012; Vatansever et al., 2015], suggesting that the DMN is also involved in unfocused attention and broad monitoring of the external environment for unexpected events [Buckner et al., 2008; Leech and Sharp, 2014; Pearson et al., 2011; Mantini and Vanduffel, 2013]. The convergence of these two different aspects of the DMN is plausible because stimulus-independent processes (such as self-generated thoughts) often occur in conjunction with unfocused monitoring of the environment (as when one travels on the bus, for instance). In a recent proposal, Hasson et al. [2015] provide evidence for the view that these two aspects—internally-driven processing and unfocused environmental monitoring—are even more integrated and argue that the DMN is engaged in the formation of a ‘process memory’ that constitutes the meaningful continuous context of our subjective experience over time on the scale of several minutes. As such, the DMN seems to be responsible for the maintenance of a cohesive sense of self across time that forms a reference point for the interpretation of novel information [Andrews-Hanna et al., 2014; Kajimura et al., 2016; Northoff and Bermpohl, 2004; Roy et al., 2012]. Our finding that the DMN is engaged during safety learning might thus reflect the process of embedding safety cues that predict the absence of shock within the context of self-generated thought.
The contextualisation of safety memories is important for fear learning in general, as adaptive fear learning depends not only on the generation of appropriate fear responses but also on the inhibition of inappropriate fear responses [Rescorla and Wagner, 1972]. Fear associations readily generalize to similar stimuli, whereas the inhibition of fear responses is highly context-dependent, which suggests that context-dependent inhibition plays a crucial role in preventing the maladaptive overgeneralization of fear associations [Bouton, 2004; Quirk et al., 2003]. Recent evidence implicates a role of the DMN in the regulation of fear generalization by showing that the generalization of fear is counterbalanced by activity within the DMN [Lissek et al., 2014a]. Such a regulatory function is commonly associated with the ventromedial prefrontal cortex (vmPFC)—the frontal node of the DMN, which shows the strongest overlap with the areas activated during safety learning in our results. Animal and human studies have demonstrated that the vmPFC is engaged in the inhibition of fear responses [Todd et al., 2014; Quirk and Beer, 2006] and that it plays a major role in regulating emotions and decision-making [Bechara et al., 2000; Etkin et al., 2011]. The engagement of the vmPFC and the DMN during safety learning suggests that the contextualisation of safety memories contributes to the inhibition of inappropriate fear responses and consequently to the adaptive generalization of fear.
Importantly, our findings demonstrate that the DMN is engaged in safety learning during both extinction and differential conditioning, that is, learning that both a previously conditioned stimulus (CS+) after a period of non-reinforcement and a non-reinforced conditioned stimulus (CS−) within the context of threat signal safety. It is yet unclear how these different forms of safety learning are related to each other and more research is necessary to address this question. Based on our results, we suggest that the DMN might constitute a high-level form of safety learning that integrates other cognitive functions involved in threat and safety processing, such as perception, attention, cognitive inhibition, and higher-order reasoning, as well as working and long-term memory [Anderson et al., 2016; Dayan et al., 2016; Elton and Gao, 2015]. The DMN's functional profile, the rich-club properties of its hub regions (especially the medial prefrontal and medial temporal cortices), as well as its effective, global structural connectivity make it well suited for such an integrated, contextual form of safety learning [Van den Heuvel and Sporns, 2011; Vatansever et al., 2015].
Our proposal that the DMN's function in adaptive, affective learning may be the contextualization of safety memories has important implications for pathologies that are characterized by an over-generalization of fear associations, more specifically for anxiety and stress disorders [Dymond et al., 2015; Jovanovic et al., 2012]. The findings of this study suggest that these patient populations might be impaired in their ability to appropriately contextualize and integrate safety cues into their current experience as a result of insufficient recruitment of the DMN or less sustained activation within the DMN. Our proposal might help improve our understanding of aberrant DMN activity and connectivity commonly found in patients suffering from anxiety disorders [Zhao et al., 2007], post-traumatic stress disorder [Bluhm et al., 2009; Reuveni et al., 2016], or bipolar disorder [Vargas et al., 2013], as these disorders are also characterized by an impairment in safety learning [Grillon, 2002; Jovanovic et al., 2012; Kim et al., 2011; Woody and Rachman, 1994] and pathological over-activation of limbic regions and over-generalization of fear associations [Dymond et al., 2015; Grillon and Morgan, 1999; Lissek and Grillon, 2010; Lissek et al., 2014b]. Based on the findings of this study, we suggest that disruptions in functional and structural connectivity within the DMN might be a potential biomarker for the early detection of insufficient contextualization of safety memories in affective, mood, and stress disorders, informing effective therapeutic and pharmacological interventions. In addition, our results support emerging evidence that patients with affective disorders might benefit from therapeutic interventions, which target the DMN through mindfulness techniques or brain stimulation [Dichter et al., 2014; Fox et al., 2014; Hölzel et al., 2011; King et al., 2016; Liston et al., 2014; Taylor et al., 2013].
ACKNOWLEDGEMENTS
The authors declare no conflicts of interest.