Brain reward system's alterations in response to food and monetary stimuli in overweight and obese individuals
The authors report no biomedical financial interests or potential conflicts of interests.
Abstract
The brain's reward system is crucial to understand obesity in modern society, as increased neural responsivity to reward can fuel the unhealthy food choices that are driving the growing obesity epidemic. Brain's reward system responsivity to food and monetary rewards in individuals with excessive weight (overweight and obese) versus normal weight controls, along with the relationship between this responsivity and body mass index (BMI) were tested. The sample comprised 21 adults with obesity (BMI > 30), 21 with overweight (BMI between 25 and 30), and 39 with normal weight (BMI < 25). Participants underwent a functional magnetic resonance imaging (fMRI) session while performing two tasks that involve the processing of food (Willing to Pay) and monetary rewards (Monetary Incentive Delay). Neural activations within the brain reward system were compared across the three groups. Curve fit analyses were conducted to establish the association between BMI and brain reward system's response. Individuals with obesity had greater food-evoked responsivity in the dorsal and ventral striatum compared with overweight and normal weight groups. There was an inverted U-shape association between BMI and monetary-evoked responsivity in the ventral striatum, medial frontal cortex, and amygdala; that is, individuals with BMIs between 27 and 32 had greater responsivity to monetary stimuli. Obesity is associated with greater food-evoked responsivity in the ventral and dorsal striatum, and overweight is associated with greater monetary-evoked responsivity in the ventral striatum, the amygdala, and the medial frontal cortex. Findings suggest differential reactivity of the brain's reward system to food versus monetary rewards in obesity and overweight. Hum Brain Mapp 38:666–677, 2017. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Between 1980 and 2013 the prevalence of overweight and obesity has increased from 857 million to 2.1 billion people worldwide, becoming a major global health challenge [Ng et al., 2014]. Specifically, overweight and obesity are associated with increased risk of cardiovascular disease, stroke, type II diabetes and different types of cancer, being a consistent risk factor for these conditions when Body Mass Index (BMI) is above 23 kg/m2 [Ng et al., 2014]. In Western societies, cheap availability of high palatable foods is a primary driver of the growing obesity epidemic [Finkelstein et al., 2005]. Foods rich in sugar and fat stimulate the brain reward network, bypassing the homeostatic mechanisms that control food intake, and hence fostering eating, even in the absence of energetic needs [Stice et al., 2013; Volkow et al., 2011].
Current neurobiological theories are advocating for a “food addiction model” of obesity, given overlapping neurobiological alterations between individuals with obesity and substance addictions [Burger and Stice, 2011; Kenny, 2011; Volkow and O'Brien, 2007; Volkow et al., 2013a, 2013b]. Specifically, this model posits that individuals with overweight and obesity display increased responsivity of the brain's reward system to food stimuli, leading to a loss of control over food intake [Volkow et al., 2013a, 2013b]. In spite of the growing influence of this food addiction model, overweight and obesity are heterogeneous conditions, and more neurobiological research is needed to establish if this notion is relevant across the different manifestations of excessive weight, or to particular phenotypes [Carter et al., 2016]. Currently available functional magnetic resonance imaging (fMRI) studies have shown that sensory cues of high-palatable food evoke increased neural activation in the striatum and related regions of the brain reward network in both overweight and obese individuals versus normal weight controls [Carnell et al., 2014; Fletcher et al., 2010; Jastreboff et al., 2013; Martin et al., 2010; Rothemund et al., 2007; Stoeckel et al., 2008]. Positron Emission Tomography (PET) studies have also shown reduced striatal dopamine D2 binding potential in severely obese individuals (BMI ≥ 40) [Wang et al., 2001]. However, striatal dopamine D2 binding potential is increased in individuals with more moderate degree of excess weight for height [Guo et al., 2014].
Altogether, PET studies suggest that overweight and obesity may have unique neural underpinnings, and it has been proposed that the association between BMI and dopaminergic/reward network activity follows an inverted U-shape curve; that is, the association is positive in overweight individuals, but negative in obese individuals [Horstmann et al., 2015]. This proposed model is clinically significant and needs to be formally tested. If individuals with overweight versus obesity value food and other rewards via different brain mechanisms, delineation of these mechanisms would lead to better understanding of the underlying neurobiology of these disorders and, potentially, to more specific interventions for overweight and/or obesity.
General reward sensitivity has been customarily indexed in neuroimaging studies with the Monetary Incentive Delay (MID) task [Costumero et al., 2013]. In normal weight individuals, MID-evoked brain activations in the midbrain, striatum and orbitofrontal cortex have been associated with trait reward sensitivity [Costumero et al., 2013], and the food addiction model would predict a stronger involvement of these regions in people with excess weight. However, currently available studies have yielded contradictory findings. Balodis et al. [2013] showed increased reward system activation during the MID task in obese individuals versus controls, although no differences were found during reward feedback. Conversely, Simon et al. [2015] did not found a significant association between BMI and MID-evoked neural activation. Therefore, existing studies have not yet clearly ascertained the association between excess weight and brain responses to monetary stimuli, or overlapping and/or unique patterns of brain activation related to monetary versus food stimuli. The latter is relevant because the low prices of highly palatable foods have contributed to increase their subjective value, and thus to food choices leading to the obesity epidemic [Rangel, 2013].
In this study, we aimed to compare brain activations evoked by food and monetary rewards in individuals with obesity, overweight and normal weight; and to determine the association between reward-evoked brain activations and BMI. We hypothesized that, in response to high palatable foods, excess weight participants would display increased activation of key regions of the brain reward system, and particularly the striatum [Simon et al., 2015]. We also hypothesized that in response to monetary rewards, which is a biological index of generalized sensitivity to reward, there would be an inverted U-shape association between brain's reward system activation and BMI [Horstmann et al., 2015].
METHODS AND MATERIALS
Participants
Eighty-one healthy adults, aged between 25 and 45 years old were recruited for this study. They were classified in three groups on the basis of BMI: 39 Normal weights (NW); 21 Overweight (OW), and 21 Obese (OB). Participants' sociodemographic characteristics, and BMI and fat percentage data are displayed in Table 1. The inclusion criteria were defined as follows: (i) BMI falling within the intervals categorized as overweight (BMI between 25 and 30 kg/m2), obesity (BMI over 30 kg/m2), or normal weight (BMI between 19 and 25 kg/m2); (ii) right-handedness. The exclusion criteria were: (i) history or current evidence of medical or psychiatric disorders that co-occur with obesity (e.g., diabetes, hypertension, binge eating, bulimia nervosa, depression) indicated with clinical assessments conducted by professional nurses and psychologists; (iv) abnormalities on Magnetic Resonance Imaging (MRI) or any contraindications to MRI scanning (including claustrophobia and implanted ferromagnetic objects).
|
Normal weight (n = 39) Mean (SD) |
Overweight (n = 21) Mean (SD) |
Obese (n = 21) Mean (SD) |
P-value | |
|---|---|---|---|---|
| Age | 33.08 (6.73) | 35.00 (6.31) | 32.19 (5.81) | 0.345 |
| Sex (male/female) | 18/21 | 10/11 | 10/11 | 0.992 |
| Years of education | 18.18 (3.75) | 17.86 (3.58) | 17.14 (3.75) | 0.599 |
| Monthly income | ||||
| <600€ | 20.5% | 9.5% | 10.0% | |
| 601–1,000€ | 10.3% | 9.5% | 15.0% | |
| 1,001–1,500€ | 20.5% | 28.6% | 25.0% | 0.650 |
| 1,501–2,000€ | 17.9% | 14.3% | 15.0% | |
| 2,001–2,499€ | 10.3% | 9.5% | 30.0% | |
| >2,500€ | 20.5% | 28.6% | 5% | |
| BMI (kg/m2) | 22.20 (1.76) | 27.35* (1.59) | 33.43* (2.56) | <0.001 |
| Fat (%) | 19.66 (5.96) | 28.23* (7.56) | 33.99* (8.97) | <0.001 |
- BMI, body mass index; *P < 0.05 compared with Normal Weight group.
All participants had normal or corrected-to-normal vision. They were recruited through media advertisements and received a financial compensation. The study was approved by the Ethics Committee for Research in Humans of the University of Granada (Spain) and was conducted in accordance with the Declaration of Helsinki. All participants signed written informed consent.
Experimental Procedure
Participants underwent two reward related tasks during an fMRI session. Each of these tasks involved the processing of different rewards: food and money.
To ensure that every subject knew all the food stimuli to be used in the food reward fMRI task, two weeks before scanning participants attended to a catered tasting session. During that session subjects were gathered in a room and allowed to eat 18 different foods. These products had been previously classified based in their palatability: high palatable food, including sweet and fatty food (e.g., chocolate, cheese cake, burger) and plain food (e.g., yoghurt, omelet, orange). These sessions were conducted at 6:00 pm, and each participant had to taste each food and rate how much they liked these foods in a numerical scale of 1–10. All groups showed higher liking ratings for high palatable food compared with plain food (all P < 0.05).
All the fMRI sessions were conducted between 1 and 3 hours after lunch. At the beginning of this session BMI and fat percentage were obtained using a body composition analyzer TANITA BC-420 (GP Supplies Ltd., London). To control the satiety level, participants rated their subjective degree of appetite on a 10-cm visual analog scale (VAS) three times along the fMRI session: prior to scan, immediately before the food-stimuli task and immediately after leaving the MRI room.
fMRI Tasks
Food reward
We used a modified version of the Willingness to pay task [Plassmann et al., 2007]. Participants watched each of the 18 previously tasted foods once. Each stimulus was presented in the screen for 2 seconds and after that, they had 4 seconds to answer: “How much would you pay for it?” They could choose between four prices, ranging from 20 cents to 10 euros. Each selection was followed by a variable time between 3 and 5 seconds of baseline during which a cross fixation was presented on the screen (see Fig. 1A). Stimuli were presented in a pseudorandomized sequence to ensure that no more than two images of the same category appeared consecutively (i.e., high palatable food, plain food). Our main interest was to contrast group differences between high palatable and plain food trials.
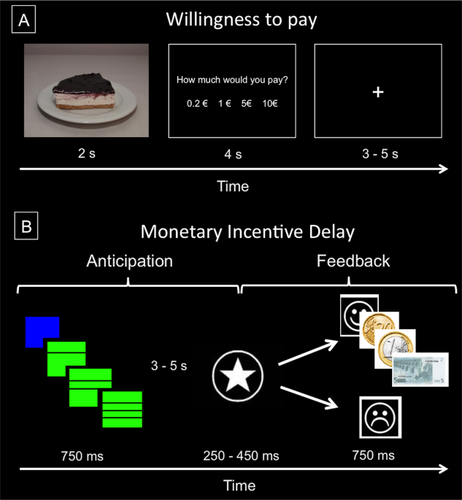
Schematic representation of the Willingness to pay (A) and the Monetary Incentive Delay (B) tasks. [Color figure can be viewed at wileyonlinelibrary.com]
Monetary reward
We used an adaptation of the Monetary Incentive Delay task [Nestor et al., 2010], based on the original task employed by Knutson et al. [2001]. At the beginning of each trial, participants were shown one of two cues (green or blue square) indicating potential winnings or no financial outcome at the end of the trial. The incentive value of each trial was signaled by means of the number of horizontal lines crossing the square (one line for 0.2€, two for 1€, and three for 5€). Each cue was presented for a fixed duration of 750 ms. Subsequently, a cross-fixation was shown during a variable period of 3–5 s, and after this interval participants had to perform a reaction-time task: respond to a white target star appearing for a variable length of time (150–450 ms) with a button press. Then participants received feedback (hit/miss) about the accuracy of their response for 750 ms, together with the information about the amount of money won in that trial (when adequate, i.e., correct responses in reward cued trials) and their cumulative total at that point of the experiment. Finally, another fixation period (750 ms) was included before the next trial. Therefore, total trial duration ranged between 5,700 and 7,000 ms. Participants performed 24 trials of each type of cue yielding a total of 96 trials (see Fig. 1B).
Imaging analyses explored brain activity changes during two periods, the reward-anticipatory period, which included the cue presentation, the variable waiting delay and the actual response period, and the reward-feedback period, involving the presentation of visual feedback (hit/miss). For the anticipatory period we defined four events of interest: (i) No outcome (0€); (ii) Low reward (0.2€); (iii) Medium reward (1€); and (iv) High reward (5€). For the feedback period we defined two events of interest: (i) Win trials; (ii) Miss trials, pooling together the different gains. Specifically, a linear contrast (High reward > Medium reward > Low reward > No outcome trials) was defined at the first level (within-subject) to explore brain activation during reward-anticipation, while a Win versus Miss contrast was used for the reward-feedback period. Therefore, this task yields two main conditions of interest: reward anticipation (High vs. Medium vs. Low vs. No reward) and reward feedback (Win vs. Miss).
Imaging Data Acquisition and Preprocessing
A 3.0 T clinical MRI scanner, equipped with an eight-channel phased-array head coil, was used (Intera Achieva, Philips Medical Systems, Eindhoven, The Netherlands). During task performance, three T2*-weighted echo-planar imaging (EPI) sequences were acquired according to the following parameters: Repetition time (TR) = 2,000 ms, Echo time (TE) = 35 ms, Field of view (FOV) = 230 × 230 mm, 96 × 96 matrix, flip angle = 90°, and a total of 21 axial slices of 4 mm with a 1 mm gap. Slices were collected in sequential ascending order, paralleled with the anterior and posterior commissure. Specifically, we collected 149 scans for the food reward task and 432 scans for the monetary reward task. A sagittal three-dimensional T1-weighted turbo-gradient-echo sequence (3D-TFE) (160 slices, TR = 8.3 ms, TE = 3.8 ms, flip angle = 8°, FOV = 240 × 240, 1 mm3 voxels) was also obtained in the same experimental session for anatomical reference. Stimuli were presented through magnetic resonance-compatible liquid crystal display goggles (Resonance Technology Inc., Northridge, California), and responses were recorded through Evoke Response Pad System (Resonance Technology Inc., Northridge, California). The functional images were analyzed using Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, Institute of Neurology, Queen Square, London), running under Matlab R2009 (MathWorks, Natick, MA). Preprocessing included re-slicing to the mean image of the time series, slice timing correction, normalization, using affine and smoothly non-linear transformations, to an EPI template in the Montreal Neurological Institute (MNI) space, and spatial smoothing by convolution with a 3D Gaussian kernel [full width at half maximum (FWHM) = 8 mm]. Data were high-pass filtered to remove low-frequency noise (1/128 Hz) and corrected for temporal autocorrelation using an autoregressive AR model.
Outside Scanner Behavioral Measures
Sensitivity to Reward was measured with The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) [Torrubia et al., 2001]. The SPSRQ is a 48-item questionnaire that comprises two subscales to measure the constructs of sensitivity to reward (SR) (24 items) and sensitivity to punishment (SP) (24 items). Given our focus on sensitivity to reward, we only analyzed SR scores. SR items evaluate sensitivity to anticipation and receipt of different types of reinforcers (e.g., monetary, social). The mean SR score was 10.12 (standard deviation, 3.85; range, 2–18), and scores followed a normal distribution (Shapiro–Wilk test: P > 0.10). These scores were consistent with the results of previous studies in Spanish samples [Costumero et al., 2013; Torrubia et al., 2001]. This questionnaire has demonstrated internal consistency; construct validity, and significant associations with reward and punishment relevant brain systems [Costumero et al., 2013].
Statistical Analyses
Behavioral analyses
Behavioral data were analyzed with the Statistical Package for the Social Sciences version 19 (SPSS; Chicago, IL). We tested between-group differences in demographic, body composition and sensitivity to reward variables with one-way ANOVAs, followed by post-hoc two sample t-tests. We conducted a series of mixed-design ANOVAs to analyze putative interactions between study groups and variables of interest (i.e., fMRI tasks conditions), followed by post-hoc within- and between-group analyses.
Neuroimaging analyses
Task regressors were convolved with the SPM8 canonical hemodynamic response function. In the Willingness to pay task, we modeled two task regressors (one for each condition), including the time that the food stimulus was on the screen and the time available for the participants' response. The contrast of interest for this task was defined as High palatable trials > Plain food trials. In the monetary delay task, we modeled task regressors as the time elapsed between the presentation of each cue and the participants' response (reward anticipation), and the time in which the visual feedback was presented on the screen (reward feedback). A parametric contrast was numerically defined as (2 1 −1 −2) reflecting a High reward > Medium reward > Low reward > No outcome anticipation effect. Reward-feedback contrast of interest were defined as Win > Miss trials. To prevent motion artifacts, six head motion parameters were entered as regressors of no interest in all first-level analyses. One-sample t-tests were conducted on the resulting first-level contrast images to assess across-group activations in each of the contrasts. Next, we conducted a series of three-group ANOVAs to assess between-group differences using the same first-level contrast images.
Due to the existence of an a priori hypothesis about changes in brain activity within the reward system, all statistical analyses were spatially restricted to such region of interest. Such mask was defined empirically according to the results obtained from a large series of previous studies assessing reward system function by means of fMRI examination. Specifically, similar to other studies [Contreras-Rodríguez et al., 2016], we used the reward system mask provided by Neurosynth (www.neurosynth.org). This mask includes brain regions that have shown to be associated with rewarding processing via meta-analytic research (i.e., striatum, anterior and posterior cingulate cortices, supplementary motor area, prefrontal cortices, insula, dopaminergic midbrain, hippocampus, amygdala, and intraparietal cortices). Statistical significance threshold was corrected for multiple comparisons using a combination of voxel intensity and cluster extent thresholds. The spatial extent threshold was determined by 1,000 Monte Carlo simulations, using the AlphaSim algorithm as implemented in the SPM REST toolbox [Song et al., 2011]. Input parameters included a brain mask of 51517 voxels (the reward system mask), an individual voxel threshold probability of 0.005 and a cluster connection radius of 5 mm. At 11.0 and 9.2 mm FWHM smoothness for the food and monetary task contrasts, respectively, corresponded to a minimum cluster extent (KE) of 220 and 154 voxels to satisfy a Family-wise error (FWE) corrected P value of PFWE <0.05.
To examine the association between individual sensitivity to reward scores and brain activation during both tasks, we conducted voxel-wise correlation analyses in SPM. We used the same threshold criteria of the analyses described above.
To exclude potential confounds linked to sex differences, we replicated all contrasts of interest controlling for sex. Results were equivalent, and hence we only report results for the non-covaried analyses. We also performed specific men vs. female analysis and did not find significant between-group differences.
To examine the association between brain activations and BMI, we conducted curve fit analyses in SPSS. The peak beta eigenvalues from each cluster of significant between-group differences was extracted and related with BMI values.
RESULTS
Behavioral Measures
Appetite and sensitivity to reward measurements
We found no significant between-group differences or interactions between Group and Time for subjective measures of appetite (F(4,146) = 0.638, P = 0.599). Likewise, we did not found any significant between-group differences in sensitivity to reward scores. The relationship between BMI and sensitivity to reward scores followed a non-significant inverted U-shape curve (R2 = 0.040, P = 0.204).
fMRI Behavioral Measures
Food reward task
We found a significant “Group × Food Type” interaction (F(2,77) = 4.162, P = 0.019). Paired within-group contrasts showed that OB and OW groups paid more money for high-palatable food than for plain food (P = 0.002 and P < 0.001), unlike the NW group (P = 0.220). Paired between-group contrasts showed that OB paid significantly less money for plain food compared with NW (t(58) = 2.24, P = 0.020). We found no group differences for high palatable food.
Monetary reward
We found a significant “Group × Reward” interaction (F(6,231) = 2.67, P = 0.030). Within-group analyses showed a significant effect of cue type (F(2,7) = 4,608, P = 0.013), indicating that all participants made faster responses in high incentive trials. Between groups comparisons showed that OB had significant slower reaction time in neutral (t(57) = 2.315, P = 0.028) and low incentive trials (t(57) = 2.160, P = 0.035) compared with NW. Behavioral results are summarized in Table 2.
|
Normal weight (n = 39) Mean (SD) |
Overweight (n = 21) Mean (SD) |
Obese (n = 21) Mean (SD) |
ANOVA P-value |
|
|---|---|---|---|---|
| Sensitivity to reward | 10.31 (3.89) | 10.14 (3.81) | 9.76 (4.00) | 0.875 |
| Taste | ||||
| High-palatable food | 7.28 (1.57) | 7.74 (1.03) | 8.01 (0.79) | 0.138 |
| Plain food | 6.92 (1.38) | 7.33 (1.00) | 7.30 (0.91) | 0.397 |
| Willingness to Pay: Money paid (€) | ||||
| High-palatable food | 2.63 (1.75) | 3.03 (2.26) | 2.49 (1.25) | 0.605 |
| Plain food | 2.36 (1.63) | 1.81 (1.27) | 1.42a (1.01) | 0.045 |
| Monetary Incentive Delay: Response Time (s) | ||||
| Neutral | 0.246 (0.038) | 0.252 (0.052) | 0.279a (0.059) | 0.042 |
| Low | 0.227 (0.033) | 0.233 (0.048) | 0.249a (0.046) | 0.137 |
| Medium | 0.231 (0.037) | 0.234 (0.043) | 0.242 (0.047) | 0.612 |
| High | 0.219 (0.032) | 0.222 (0.036) | 0.230 (0.040) | 0.507 |
- a P < 0.05 in relation to Normal Weight group.
Neuroimaging
Food reward task
During high-palatable versus plain food participants significantly activated bilaterally the dorsal caudate, the nucleus accumbens, the ventral putamen, the ventral tegmental area, the intraparietal, ventromedial and dorsolateral prefrontal and anterior cingulate cortices, and the anterior insula extending to the lateral orbitofrontal gyrus (Supporting Information Table SI and Fig. 2). We found no significant correlations with sensitivity to reward scores.
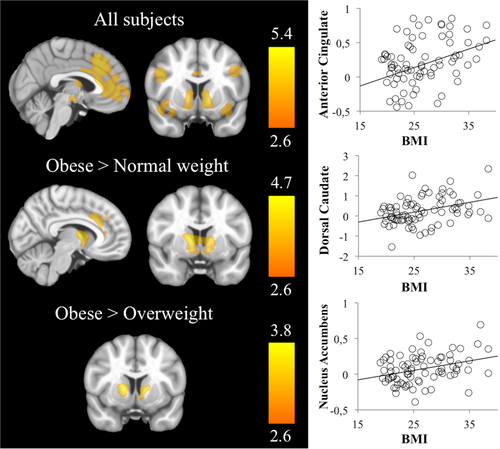
Left panel: Brain evoked activation and between-group differences during the food reward task. Right hemisphere is displayed on the right. The color bar indicates t-value. Right panel: Scatter plots showing a linear relationship between BMI and the peak activations from regions showing significant between-group differences. [Color figure can be viewed at wileyonlinelibrary.com]
Group comparisons showed that OB subjects displayed significantly increased activations bilaterally in the dorsal caudate and nucleus accumbens compared with both NW and OW participants. In addition, OB group had significantly increased activation in the anterior cingulate cortex compared with the NW group (Supporting Information Table SI and Fig. 2).
Post hoc analyses showed a linear and positive correlation between BMI and bilaterally activation in the dorsal caudate (Right: r = 0.408, R2 = 0.166, P < 0.001; Left: r = 0.299, R2 = 0.089, P = 0.007), the nucleus accumbens (Right: r = 0.333, R2 = 0.111, P = 0.003; Left: r = 0.312, R2 = 0.097, P = 0.005) and the dorsal anterior cingulate gyrus (r = 0.351, R2 = 0.123, P = 0.002).
Monetary Reward
Reward anticipation contrast
Parametric increases in reward magnitude cues were associated with higher activations in bilateral dorsal and ventral striatum, midbrain (including ventral tegmental area), thalamus, amygdala-hippocampal complex, orbitofrontal cortex, middle frontal gyrus, anterior insula, and anterior and posterior cingulate and intraparietal and cortices (Supporting Information Table SII, Fig. 3). We observed a positive correlation between sensitivity to reward scores and anterior cingulate gyrus (r = 0.395, P < 0.001) and supplementary motor area (r = 0.355, P = 0.001).
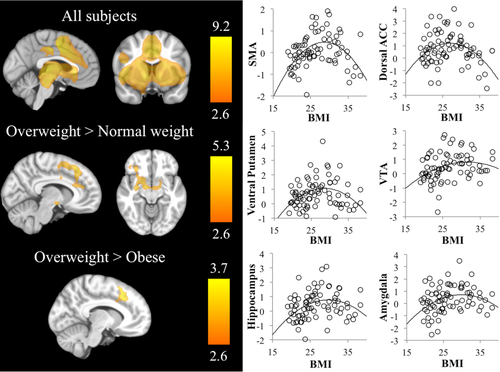
Left panel: Brain evoked activation and between-groups differences during monetary anticipation contrast. Right hemisphere is displayed on the right. The color bar indicates t-value. Right panel: Scatter plots showing a quadratic relationship (inverted U-shape) between BMI and the peak activations from regions showing significant between-group differences. [Color figure can be viewed at wileyonlinelibrary.com]
Group comparisons showed that OW individuals displayed significantly increased activation in the anterior cingulate cortex/supplementary motor area in comparison with both OB and NW groups. Likewise, OW individuals (but not OB individuals) showed a significantly increased activation in the ventral tegmental area, the ventral putamen, the lateral orbitofrontal cortex, and the hippocampus–amygdala complex in comparison with NW participants (Supporting Information Table SII, Fig. 3).
Curve fit analyses of the association between BMI and peak activations from the above analyses showed inverted-U associations for the supplementary motor area (R2 = 0.240, P < 0.001), dorsal anterior cingulate (R2 = 0.144, P = 0.003), ventral tegmental area (R2 = 0.103, P = 0.016), ventral putamen (right: R2 = 0.137, P = 0.004; left: R2 = 0.079, P = 0.043), hippocampus (R2 = 0.135, P = 0.004) and amygdala (R2 = 0.115, P = 0.009). Post-hoc analyses showed that the peaks of the inverted U ranged between 27 and 32 kg/m2.
Reward feedback contrast
In win versus miss trials participants significantly activated the bilateral ventral and dorsal striatum, the amygdala–hippocampal complex, the orbitofrontal cortex, the middle frontal gyrus, the posterior cingulate, and the intraparietal cortices. Miss compared with win trials evoked activations including the anterior insula, the dorsal anterior cingulate cortex and the supplementary motor area. (Supporting Information Table SIII, Fig. 4). We found no significant correlations with sensitivity to reward.
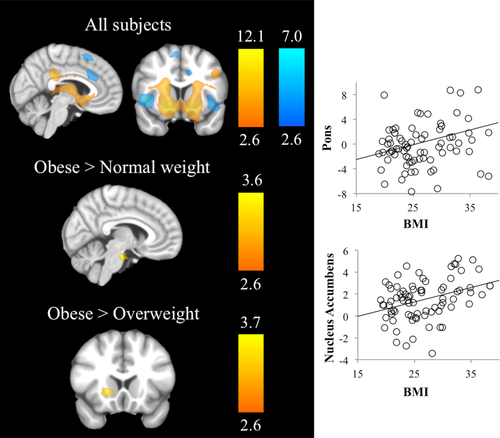
Left panel: Brain evoked activation and between-groups differences during monetary feedback contrast. Right hemisphere is displayed on the right. The color bar indicates t-value (hot colors for the win vs. miss contrast and cold colors for the miss vs. win contrast). Right panel: Scatter plots showing a linear relationship between BMI and the peak activations from regions showing significant between-group differences. [Color figure can be viewed at wileyonlinelibrary.com]
Group comparisons in Win versus Miss trials showed that OB individuals compared with NW had increased activation in the rostral–ventral pons. Likewise, OB individuals compared with OW had increased activation in the nucleus accumbens. Curve fit analyses showed a linear and positive association between nucleus accumbens and pons activations and BMI scores (r = 0.363, R2 = 0.132, P = 0.001, r = 0.276, R2 = 0.076, P = 0.014). (Supporting Information Table SIII, Fig. 4).
In addition, we conducted a whole brain analysis to ascertain between-group differences in brain activation that were outside the reward system identified by Neurosynth. We only found two significant clusters of activation. In the food task, one cluster comprising the left frontal operculum extending to the anterior insula was more activated in obese versus healthy weight participants. In the anticipation phase of the monetary task, a cluster located in the intraparietal cortex was more activated in overweight versus healthy weight participants.
DISCUSSION
We found that individuals with obesity and overweight have unique patterns of brain activation in response to food and monetary rewards. Specifically, individuals with obesity display enhanced food-evoked ventral and dorsal striatal activations compared with individuals with overweight and normal weight. Conversely, individuals with overweight display increased monetary-reward anticipation activations in widespread regions across the brain reward network. Monetary reward feedback, however, evoked greater responses in the rostral-ventral pons and nucleus accumbens in obese individuals versus normal weight and overweight subjects, respectively. Food and monetary-feedback evoked neural activations showed a linear positive relationship with BMI, whereas monetary-reward-anticipation evoked neural activations showed an inverted U-shape association with BMI.
Behavioral measures showed that individuals with overweight and obesity had greater sensitivity to high palatable versus plain food. This pattern indicates that individuals with excess weight have increased reward sensitivity in relation to palatable food, which is consistent with greater striatal activation in the food task [Passamonti et al., 2009]. In addition, individuals with obesity showed slower reaction times in low monetary incentive trials, suggesting reduced reward sensitivity and/or weaker reward learning. Based on recent theoretical work, reduced reward learning may contribute to explain their decreased brain responsivity during the cue phase, coupled with increased responsivity during the feedback phase [Kroemer and Small, 2016].
The increased responsivity of the ventral and dorsal striatum to high-palatable food in obese individuals is consistent with previous fMRI studies showing increased striatal activation in response to food cues [Rothemund et al., 2007; Simon et al., 2014]. Critically, we show that these alterations are specific to individuals with obesity (relative to overweight), and therefore they may reflect severity related neuroadaptations. This notion is consistent with food addiction models of obesity, which propose that this disorder is associated with ventral striatal neuroadaptations leading to incentive sensitization of food, and dorsal striatal neuroadaptations leading to food-related habits [Tomasi and Volkow, 2013]. Our findings also extend available evidence by showing alterations in a food choice task, with greater ecological validity than passive observation of food cues [Fletcher et al., 2010]. In fact, imaging findings were paralleled by behavioral results, which show that obese individuals assign less value to standard food, which may bias their food choices toward highly palatable unhealthy food [Rangel, 2013].
The increased responsivity of the VTA/striatum, amygdala, orbitofrontal cortex, and medial prefrontal cortex in overweight individuals to anticipation of monetary rewards, and the inverted U-shaped relationship between activation of these regions and BMI is consistent with findings of dopamine-PET studies [Horstmann et al., 2015]. Indeed, brain activation in the MID task is regarded as a biological index of general sensitivity of the brain reward system [Costumero et al., 2013]. Our findings clearly indicate that brain response to monetary-reward anticipation is increased in individuals with overweight, and comparatively decreased in individuals with obesity. This finding is relevant, as it indicates that strategies to prevent overweight might need to focus on downplaying general hyper-reactivity of the brain reward system, whereas strategies to prevent obesity might need to stimulate the brain reward system's responsivity to alternative reinforcers that can compete with food. It remains to be determined if overweight-specific reward system hyper-reactivity represents a different biological phenotype, or an “en-route” state leading to obesity. In any case, our results have theoretical implications for the understanding and prevention of overweight versus obesity.
The increased responsivity of the nucleus accumbens to monetary reward feedback in obese individuals is also consistent with the incentive sensitization model, although in this case with the “liking” or hedonic aspects of reward (and not the “wanting” or anticipation aspects) [Robinson and Berridge, 2003]. The nucleus accumbens is the key “liking” hotspot of the brain, which is involved among other functions in amplifying the taste of food [Berridge et al., 2010]. Alternatively, it may be explained by reinforcement learning theory, as individuals with obesity may have weaker learning signals linked to monetary reward (cue phase) and, subsequently, greater responsivity when the reward value is updated (feedback phase) [Kroemer and Small, 2016]. Likewise, our finding is similar to previous results in cocaine dependent users, which have greater activation of the nucleus accumbens during feedback processing in the MID task [Bustamante et al., 2014; Jia et al., 2011]. Therefore, our findings indicate that obese individuals have similar alterations in reward feedback processing to those observed among addiction populations.
Whole-brain results identified two additional clusters that showed between-group differences. These clusters were consistent with the main findings, as they involve brain regions that have been previously associated with reward processing which showed increased activation in the obese and overweight groups compared with normal weight participants. Increased activation of the frontal operculum/anterior insula has been previously found in obese participants in response to visual stimuli of high calorie food [Rothemund et al., 2007]. Both regions are involved in the processing of the gustatory aspects of food [Ziauddeen et al., 2012]. The greater activation of the intraparietal cortex in overweight individuals during the monetary task is consistent with the key role of this region on subjective valuation of reward, as shown in monkey studies [Kubanek and Snyder, 2015; Louie and Glimcher, 2010].
This study has important strengths. The groups were well matched in key sociodemographic characteristics, such as age, years of education and socioeconomic-status. We applied strict eligibility criteria, which ruled out the presence of obesity related comorbid conditions, including medical comorbidities (i.e., diabetes, hypertension) and mental health problems (i.e., depression or eating disorders, such as binge eating or bulimia nervosa). We also maximized the ecological validity of assessments by pre-exposing participants to the food products of the neuroimaging task in a pre-scanner buffet session. Nevertheless, our findings also need to be understood in the context of some limitations. First, we used different tasks to assess food-related reward (Willingness to Pay) and monetary reward (Monetary Incentive Delay), and therefore we could not analyze interaction effects of food and monetary rewards on the brain reward system. Nonetheless, both tasks are well-validated measures of reward processing in relation to food and money stimuli. Moreover, the number of participants in each group was unequal: Obese and overweight groups were smaller than the normal weight group. We addressed this limitation by performing post-hoc tests of homogeneity of variance for all significant findings, which showed non-significant results (i.e., homogenous variances across groups) in all cases. Another potential limitation is the use of BMI as the main independent variable. Recent evidence has shown that measures of body fat, particularly visceral fat, are more sensitive to brain health specifically among adolescents [Schwartz et al., 2014]. We chose BMI over body fat because our measure of fat (bioelectrical impedance) does not allow reliable estimations of visceral versus subcutaneous fat, and BMI was more adequate than total body fat to classify adult participants of both sexes. Furthermore, BMI is regarded as a reliable index of weight-to-height ratio and is the key indicator of overweight and obesity in population-based studies [Ng et al., 2014]. An additional limitation is the non-significant curvilinear relationship between BMI and the behavioral measure of sensitivity to reward (SPSRQ). This negative finding can be explained by methodological differences between self-report and biological (neuroimaging) measures—the latter more objective and sensitive, and/or by the strict inclusion/exclusion criteria, which resulted in a narrow BMI range. This relationship has been previously demonstrated in a behavioral study with a broader BMI range (17–51 kg/m2) relative to ours (19–38 kg/m2) [Davis and Fox, 2008]. Finally, we analyzed neuroimaging activations within discrete regions of the brain reward system, although these regions are known to be part of an integrated network. Therefore, future studies performing functional connectivity assessments of the reward system during food and monetary reward processing will probably be a relevant add-on to present findings.
In conclusion, our results support the food addiction model and previous evidence showing an increased food-cue reactivity in striatal areas and a greater subjective value of high palatable foods in excess weight adults. Conversely, a different pattern of activation was found during monetary reward anticipation, with an inverted U-shape relationship between brain reward system activation and BMI. These reinforcement-dependent differential processing should be confirmed using other natural reinforces, and further studies in overweight populations should also investigate whether overweight-specific reward system alterations represents a distinctive feature of this group or an “en route” state to obesity.