Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study
Abstract
Cognitive control is the process of employing executive functions, such as attention, planning or working memory, to guide appropriate behaviors in order to achieve a specific goal. Functional magnetic resonance imaging studies suggest a superordinate cognitive control network, comprising the dorsal regions of the lateral prefrontal cortex (DLPFC), anterior cingulate cortex (dACC) and parietal cortex (DPC). How gray matter structure changes across this network throughout neurodevelopment and how these changes impact cognitive control are not yet fully understood. Here we investigate changes in gray matter volume of the key nodes of the cognitive control network using structural MRI scans from 176 participants aged 8–38 years. One hundred and eleven of these also completed a longitudinal follow-up at two years. We compare these with performance on a cognitive battery also measured at these two time points. We found that volume decreases in the cognitive control network were associated with improved performance in executive function (in left DLPFC and bilateral DPC), information processing (in bilateral dACC and right DPC) and emotion identification tasks (left DLPFC). These results were significant after controlling for age. Furthermore, gray matter changes were coordinated across the network. These findings imply age-independent synaptic pruning in the cognitive control network may have a role in improving performance in cognitive domains. This study provides insight into the direct impact of structural changes on behavior within this network during neurodevelopment and provides a normative evidence base to better understand development of cognitive dysfunction in brain disorders. Hum Brain Mapp 38:631–643, 2017. © 2016 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Abbreviations
-
- CCN
-
- Cognitive control network
-
- dACC
-
- Anterior cingulate cortex
-
- DLPFC
-
- Dorsolateral prefrontal cortex
-
- DPC
-
- Dorsal/Posterior Parietal Cortex
INTRODUCTION
Cognitive control is a key aspect of normal functioning, being involved in the regulation of emotional and social cues and responses, as well as formal measures of intelligence such as memory and attention. Impairments in cognitive control have serious implications on an individual's ability to navigate both their internal and external world, as evidenced by the consistent presence of cognitive control dysfunction in psychiatric illness [Millan et al., 2012; Williams, 2016]. Recent functional magnetic resonance imaging (fMRI) findings have suggested that cognitive control is maintained through the simultaneous activation of regions in the frontal and parietal cortices, namely the dorsal anterior cingulate cortex (dACC), the dorsolateral prefrontal cortex (DLPFC) and the dorsal/posterior parietal cortex (DPC). These areas form a network that controls and co-ordinates multiple domains of cognitive control including attention, working memory, inhibition, planning and other executive functions in response to task demands [Niendam et al., 2012]. A circuit level understanding of the cognitive control network (CCN) has introduced a new paradigm for how both optimal cognitive control function is achieved in the healthy brain as well as why cognitive control impairments are seen in psychiatric disorders [Williams, 2016]. Understanding how the neuronal structure of this network changes during late childhood through to early adulthood, a key developmental period for risk of various psychiatric disorders, and how these changes relate to cognitive performance will further our understanding of the neurodevelopmental process of cognitive control.
The pattern of gray matter volume change in the developing brain has long been used as an indication of structural maturation but the relationship between this and behavioral development remains poorly understood [Casey et al., 2005]. Gray matter volume change is considered to be reflective of a combination of myelination and synaptic pruning during the neurodevelopmental period and provides a phenotype to understand various roles of predetermined genetic cues and experience dependent mechanisms in the development of higher order functions. Here we aim to use a longitudinal study design to investigate how the normal developmental trajectory of gray matter volume within the key areas of the CCN relates to cognitive control behaviors.
The pattern of cortical gray matter change throughout childhood and adolescence has been studied and replicated through a number of cross-sectional [Pfefferbaum et al., 1994; Thompson et al., 2005; Toga et al., 2006] and some longitudinal structural MRI studies [Giedd et al., 1999; Gogtay et al., 2004; Lenroot et al., 2007; Shaw et al., 2008; Sowell et al., 2003; Sowell et al., 2004]. Gray matter volume appears to increase until the pubertal years after which it begins to decline. However this pattern is staggered across the brain, with sensorimotor areas maturing first followed by areas responsible for higher cognitive functions such as the frontal lobes and dorsal parietal lobes [Giedd et al., 1999; Gogtay et al., 2004]. It has been noted that behavioral milestones follow a similar pattern, with sensorimotor abilities maturing in late childhood, and higher-order cognitive function such as language and executive function continuing to mature through adolescence into early adulthood [Anderson, 2002; Best and Miller, 2010; Clark et al., 2006; Schaie and Strother, 1968; Welsh et al., 1991].
However, few studies have managed to link these events together. Most studies of developmental processes are solely neuroanatomical or behavioral. Additionally the majority of neurodevelopmental studies make assumptions based on cross-sectional data, which provides an incomplete and potentially misleading picture of developmental change [Kraemer et al., 2000]. Only a small number of longitudinal studies have linked cortical development with cognitive variation. Sowell et al. demonstrated that greater cortical thinning in the left dorsal frontal and parietal regions among children was associated with great improvements in a measure of verbal intelligence. However this study was limited by a small sample size (n = 45) and narrow age range (5–11 yrs) [Sowell et al., 2004]. Using a much more robust sample size (n = 307) and expansive age range (7–27 yrs), a second study [Shaw et al., 2006] found that the rate of change in cortical thickness primarily in frontal regions correlated with measures of general intelligence, a finding which has since been replicated [Burgaleta et al., 2014]. While these studies provide useful links between intelligence and change in cortical structure, only one other group has linked longitudinal changes in the brain to a specific cognitive domain. Tamnes et al. found that improved performance in working memory was related to longitudinal decreases in bilateral prefrontal and posterior parietal regions [Tamnes et al., 2013]. How gray matter volumes relate to performance on other cognitive domains such as attention, executive functioning, information processing efficiency and cognitive aspects of emotion processing still remains unclear. Additionally, while these studies address general variation in gray matter with age across the whole brain and have implicated distinct parts of the CCN, whether this variation is developmentally coordinated within the CCN is still to be established. There is evidence that there are coordinated patterns of anatomical change that are correlated with the degree of functional connectivity between different areas of the brain [Raznahan et al., 2011], which we investigate further in this study within the CCN.
This study aims to understand how changes in gray matter volume in the key nodes of the CCN relate to each other and to changes in performance across seven major cognitive domains during a crucial period of maturation through late childhood into adolescence and adulthood. We use structural MRI and cognitive data from 176 participants age 8–38 years of which 111 also completed a two year longitudinal follow-up. We used cross-sectional data to first establish the relationship between age with behavioral measures of cognitive control and gray matter structure of the CCN and whether these are retained over time longitudinally. We also test the cross-sectional relationships between cognitive behavior and gray matter structure and also investigate how the change in brain structure within the CCN is associated with changes in cognitive behavior longitudinally. We hypothesized that age would have a significant relationship with both gray matter volume and cognitive performance. Based on previous studies dealing with cognition and neuroanatomy distinctly and/or using a cross-sectional model, we predicted that decreases in gray matter in the CCN during neurodevelopment will predict an increase in cognitive ability. Additionally we reasoned that the trajectory of gray matter volume change would be coordinated throughout the CCN due to its cohesive functional role in cognitive control.
MATERIALS AND METHODS
Participants and Study Design
One hundred and seventy-nine healthy participants between the ages of 8 and 38 were recruited to complete a structural MRI scan at Westmead Hospital (Sydney, Australia) along with a well-established cognitive battery [Mathersul et al., 2009; Williams et al., 2009] at the Westmead Institute for Medical Research (Sydney, Australia). One hundred and fifteen of these same participants returned for a two year follow-up where they completed the same protocol (see Fig. 1). Three participants were excluded due to the presence of mental illness at follow-up and/or an incidental finding of structural abnormality in their brain. One participant was excluded due to the presence of movement related artifacts.
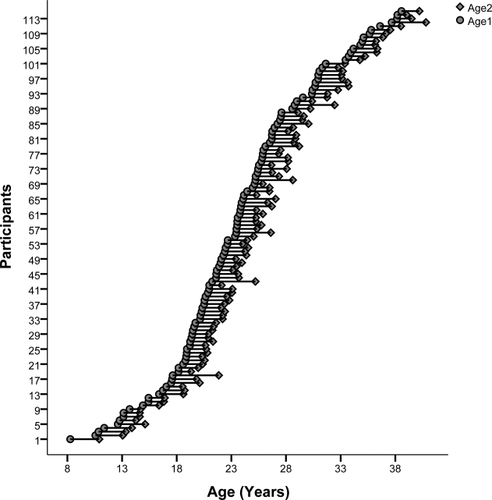
Age distribution of the longitudinal data (years). Participants aged 8 to 38 years old (n = 111, 53 females) had a 3T MRI scan and completed a cognitive test battery at two time points, an average of 1.94 years apart. The age at each scan is indicated by a circle. The first scan is the leftmost scan, with the repeated scan directly to the right and joined by a horizontal line.
Participants were required to meet eligibility criteria at both visits and were required to have no self-reported serious medical conditions, no prior or current diagnosis of major depression and/or presence of suicidal tendencies, bipolar disorders, psychosis, schizophrenia, schizoaffective disorder, primary eating disorders, post-traumatic stress disorder, obsessive compulsive disorder, personality disorder or clinical anxiety. Additional exclusion criteria included substance dependence, history of brain injury, previous loss of consciousness for greater than ten minutes and any contraindications for MRI. Participants completed the Web-Q questionnaire assessing psychological and physical health as well as the SPHERE-12 (Somatic and Psychological Health Report) and the DASS (Depression and Anxiety Stress Scale). Subjects were required to have no physical impediment that was likely to interfere with their ability to complete the testing battery and were of the mental capacity to understand and follow study procedures and instructions.
Human subjects participation was approved by the local Institutional Review Board; the University of Sydney Human Ethics Committee. After the study procedures were fully explained in accordance with the ethical guidelines of the institutional review board, participants provided written consent. For minor participants under the age of 18, assent to participate was provided by a legal guardian.
Cognitive Test Battery
This study used a computerized cognitive test battery, “WebNeuro” [Silverstein et al., 2007]. WebNeuro consists of 12 tests taking 30–40 minutes to complete. Participants were seated in front of a computer located within a sound and light attenuated room with an ambient temperature of approximated 24 degrees Celsius. Test instructions were delivered on-screen immediately prior to test practice and test materials. An examiner provided assistance where necessary, especially to the younger participants, in addition to an interactive, automated protocol used to ensure test comprehension. If the participant failed three practice trials for a test, then the participant was taken automatically to the next test.
This same test battery also runs in a dedicated touchscreen format known as “IntegNeuro” [Clark et al., 2006]. The reproducibility of WebNeuro and IntegNeuro computerized platforms has been established [Silverstein et al., 2007]. These cognitive tests (in touchscreen format) have been shown to have excellent validity in terms of relationships with conventional paper-and-pencil measures of similar constructs of cognitive performance [Paul et al., 2005; Silverstein et al., 2007]. The tests also have sound test retest reliability [Williams et al., 2005] and sound cross-site reliability [Paul et al., 2007]. WebNeuro age and sex norms across 9 decades have been established for a norm sample of 1000 participants [Mathersul et al., 2009; Williams et al., 2009]. Based on these norms, each study participant's performance on each individual aspect of each test (reaction time, accuracy etc) is expressed as a z score relative to the normative mean of 0, with positive z-scores reflecting better than average performance, and negative values reflecting poorer than average performance. Summary scores are then computed as the average of individual test scores for each test as summarized in Table 1. These summary scores were labeled according to the construct domain they represent.
| Task Name | Description | Function | Variables | Domain |
|---|---|---|---|---|
| Choice Reaction Time | Participants look at the computer screen as one of four target circles is illuminated in pseudorandom sequence over a series of trials. For each trial, the participant is required to use the mouse and click on the illuminated circle as quickly as possible. 20 trials are administered with a random delay between trials of 2–4 sec. | Simple decision making | Mean reaction time | Information processing efficiency |
| Digit Span (Forward) | Participants are shown a series of digits, one second apart, on the computer screen. Participant is required to recall the digits in forward order by entering them using the mouse. | Working memory | Recall span | Memory |
| Continuous Performance Test | A series of similar looking letters (B, C, D, or G) are presented to the participant on the computer screen for 200msec, separated by a 2.5 sec interval. If the same letter appears twice in a row the participant is require to press the space bar. | Sustained attention | Mean reaction time | Attention |
| False miss errors | ||||
| False alarm errors | ||||
| Switching of Attention | Participants are required to identify 13 digits (1–13) and 12 letters (A-L) in ascending sequence of alternating digits and letters (i.e., 1 A 2 B 3 C …).This requires that the participant switch focus between mental tasks (letter and number sequencing), assessing constructs equivalent to those assessed by Trail Making B (Reitan, 1958). | Cognitive flexibility | Completion time | Information processing efficiency |
| Number of errors | ||||
| Maze | A maze completion test of planning, error monitoring and decision-making. Variant of the Austin Maze. Participants navigate around the grid with the arrow keys on the key board. | Executive function | Completion time | Executive function domain |
| Number of errors | ||||
| Go/No-Go | Participants respond (by space bar) as quickly as possible to the word “press” in GREEN (to assess automatic responding) and inhibit these responses immediately when “press” is in RED. | Inhibition | Variability of reaction time | Impulsivity |
| Total errors | ||||
| Memory Recognition | The participants are presented with 12 English language words, which they are asked to memorize and later recognize from memory. A delayed memory recognition trials is completed approximately 10 min later after a number of intervening tasks. | Memory | Total immediate recall | Memory |
| Delayed recall | ||||
| Emotion Identification Test | Participants are presented with a series of faces expressing different emotions. Participants are required to immediately identify the emotion expression depicted by each by selecting the label at the bottom of the computer screen with the mouse. | Emotion recognition | Reaction time for fear, anger and sad faces | Emotion identification |
| Delayed Emotion Priming | Following an interval of 20 minutes, participants are asked to recognize which faces were presented in the previous emotion identification task. This assesses biases in recognition produced by the indirect effect of the facial emotions presented. | Implicit emotional priming | Reaction time for disgusted and happy faces | Emotion bias |
Image Acquisition
MR Imaging for this cohort was performed using an 8-channel phased-array head coil on a 3T GE Signa Twinspeed HDxT MR Scanner (GE Healthcare, Milwaukee, Wisconsin) at the Department of Radiology, Westmead Hospital. T1-weighted images were acquired in the sagittal plane using a 3D SPGR sequence (FOV = 256mm, TR = 8.3 ms; TE = 3.2 ms; Flip Angle = 11°; TI = 500 ms; NEX = 1 and ASSET = 1.5; Frequency direction: S/I). A total of 180 contiguous 1 mm slices was acquired with a 256 × 256 matrix, with an in-plane resolution of 1 mm × 1mm resulting in isotropic voxels in a total scan time of 7mins 12sec.
Voxel-Based Morphometry Analysis/Structural MRI Data Analysis
Structural brain images were visually inspected for quality control and artifact detection before processing. Images were processed with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.htmal) and the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm) running on MATLAB R2010a (Mathworks). Default parameters were used for all options. For cross-sectional analyses, data from time point 1 were segmented and normalized to standard MNI space using high-dimensional DARTEL and non-linear modulation was applied. This modulation ensures that the normalized images retained absolute gray matter volume corrected for total brain size.
For the longitudinal analysis, pre-processing was done using the standard longitudinal pre-processing stream within the VBM8 toolbox. Briefly, time point 2 (T2) scans are registered to time point 1 (T1) scans for each subject separately to create a mean image. T1 and T2 images are then realigned and corrected for signal inhomogeneity with regard to the mean image. T1, T2 and the mean image are then segmented into gray matter, white matter and cerebral spinal fluid and normalized (SPM8 default template) using the segmentations of the mean. As the effect of modulation on longitudinal data is rather subtle and comparisons are within subject, modulation is not performed in the longitudinal processing module of the toolbox. For both analyses, images were visually inspected for correct registration and segmentation and sample homogeneity was checked using covariance. All images above or below 2SDs of the sample were checked. Most errors were due to brain extraction errors. In these cases brain extraction was done using the brain extraction tool in FSL and data were re-processed. Finally, the realigned and normalized gray matter segments were smoothed with an 8 mm FWHM Gaussian kernel.
Second, we performed an ROI analysis to analyze the structural changes specific to regions in the CCN. The CCN nodes (dACC, DLPFC and DPC) were defined based on a recent meta-analysis of functional neuroimaging studies using a range of cognitive tasks [Niendam et al., 2012]: (1) DLPFC activation included nodes in the middle frontal gyrus (L: −40, 26, 28 and R: 40, 30, 28) and the inferior frontal gyrus (L: −42, 4, 30 and R: 44, 6, 32); (2) DPC activation included nodes in the superior parietal lobule (L: −28, −60, 44 and R: 28, −60, 44), inferior parietal lobule (L:−38, −52, 40 and R: 38, −50, 42) and precuneus (L: −6, −62, 44 and R: 8, −68, 46) and (3) dACC activation was in cingulate gyrus (L:−2, 16, 40 and R: 2, 16, 40) (see Fig. 2 for visual representation). Using these co-ordinates we created spherical ROIs with a radius of 8 mm and extracted raw volumes at both time points using the marsbar tool (http://marsbar.sourceforge.net/).
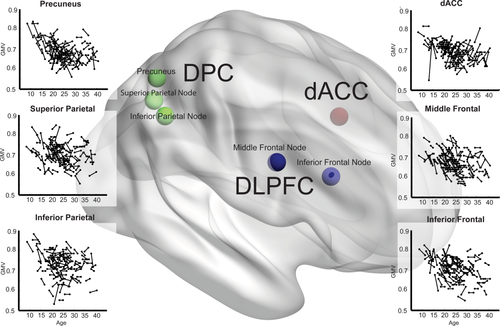
Change in gray matter volume with age in the cognitive control network. Longitudinal gray matter volume (GMV) trajectories in right DLPFC, DPC, and dorsal anterior cingulate cortex (dACC) nodes of the CCN are shown as scatter plots of gray matter volume (all y-axes) and age (all x-axes). Each scan is represented by a dot and repeat scans are connected by lines. Co-ordinates of CCN nodes are plotted within a 3D brain: DLPFC (blue), including inferior frontal (BA9) and middle frontal (BA46) nodes, dACC (red) and DPC (green), including superior(BA7), inferior (BA40) and precuneus (BA7) nodes. [Color figure can be viewed at wileyonlinelibrary.com.]
Statistical Analyses
Raw gray matter volumes and z-scores for cognitive tasks were tested for normality and plotted against age to visually inspect for outliers. To evaluate cross-sectional relationships between baseline volume measures or cognitive domain scores and age, a general linear model was used controlling for gender and age and gender interactions. As previous studies have observed a non-linear change in gray matter, this measure was re-tested with the inclusion of age2 to identify any quadratic relationships.
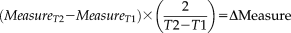
The time between scans was adjusted to two years as average time between acquisitions for the cohort was 1.94 yrs.
We also tested the cross-sectional relationships between cognitive performance and gray matter structure using the general linear model controlling for gender and with age as a covariate. In order to test this longitudinally, partial correlations, controlling for age, and gender were performed to estimate the relationship between ΔGMV in all nodes and ΔZ-score for each cognitive domain.
Finally, partial correlations controlling for age and gender were performed to investigate the relationship between changes in gray matter volume for the twelve nodes of the CCN.
For all analyses, Bonferroni correction for multiple comparisons was applied to a non-adjusted significance level of P < 0.05. As we analyzed a total of twelve nodes of the CCN (six right, six left) a value of P < 0.004 (0.05/12) was considered significant. We also report trend level findings that did not survive correction for multiple comparison (0.004 < P < 0.05).
RESULTS
Demographics
The demographic characteristics of both the cross-sectional and longitudinal cohort are summarized in Table 2. The age distribution of longitudinal participants can be observed in Figure 1. In order to determine if the mental health of participants was stable across both time points, data collected using the DASS42 questionnaire for depression, anxiety and stress was used. The cross-sectional cohort had an average score of 3.2 ± 4.1 (depression), 2.6 ± 3.1 (anxiety) and 6.0 ± 5.1 (stress), which is within normal range for the population. For the longitudinal group, the sample had an average score of 3.4 ± 4.1 (depression), 2.9 ± 3.3 (anxiety), 6.12 ± 5.0 (stress) at time point 1 and average DASS scores of 4.3 ± 5.1, 2.5 ± 3.1, 6.5 ± 5.4 at time point 2 for each of the measures respectively. Analysis using a paired-sample T-Test showed that DASS measures at time 1 (T1) and time 2 (T2) were not significantly different. In all three groups, over 90% of the sample fell in the normal to mild range for DASS measures.
| Cross-sectional cohort | Longitudinal cohort | ||
|---|---|---|---|
| Time 1 (n = 176) | Time 1 (n = 111) | Time 2 (n = 111) | |
| Age, y, mean ± SD (range) | 24 ± 7 (8–38) | 24 ± 7 (8–38) | 26 ± 7 (10–40) |
| Sex, % male | 53 | 55 | – |
| Years of education, median (range) | 16 (2–18) | 16 (2–18) | 16 (2–18) |
| Handedness, % right | 86 | 87 | – |
| DASS42– Depression score, mean±SD | 3.18 ± 4.09 | 3.60 ± 4.39 | 4.22 ± 4.93 |
| DASS42– Anxiety score, mean ±SD | 2.61 ± 3.04 | 2.97 ± 3.28) | 2.64 ± 3.12 |
| DASS42–Stress score, mean ±SD | 6.01 ± 5.06) | 6.30 ± 5.02 | 6.59 ± 5.44 |
Cross-Sectional Relationships
Age and gray matter volume
Cross-sectionally, age was found to have a significant negative relationship (Bonferroni, P < 0.004) with all nodes of the CCN (P < 0.002), except for the left middle frontal and inferior frontal DLPFC (P = 0.012; P = 0.008), which did not survive correction for multiple comparisons. No age*gender interactions were significant in our cohort. A summary of all age, gray matter volume and behavior relationships is provided in Table 3.
| Cross-sectional gray matter volume associations with | Longitudinal gray matter volume change correlations with | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | Behavior | Age | Δ Behavior | ||||||
| Region | Node | Coefficienta | P values | P values | Coefficientb | P values (task) | Corr.c | P values | Corr.d | P values (task) |
| dACC | Left | −0.004 | <0.001** | NS | – | NS | – | NS | −0.240 | 0.030 (info processing) |
| Right | −0.004 | <0.001** | 0.028 (F>M) | – | NS | – | NS | −0.278 | 0.011 (info processing) | |
| DLPFC | Left inferior frontal | −0.003 | 0.008 | NS | 0.021 | 0.015 (executive function) | – | NS | −0.282 | 0.01 (executive function) |
| Right inferior frontal | −0.004 | 0.001** | NS | – | NS | – | NS | – | NS | |
| Left middle frontal | −0.003 | 0.012 | NS | 0.047 | 0.001** (executive function) | – | NS | −0.233, −0.252 | 0.036 (executive function), 0.022 (emotion ID) | |
| Right middle frontal | −0.003 | 0.002** | NS | – | NS | 0.234 | 0.013 | – | NS | |
| DPC | Left inferior parietal | −0.005 | <0.001** | NS | – | NS | – | NS | – | NS |
| Right inferior parietal | −0.005 | <0.001** | NS | – | NS | – | NS | −0.339 | 0.002** (executive function) | |
| Left superior parietal | −0.005 | <0.001** | NS | 0.020 | 0.01 (executive function) | – | NS | −0.302 | 0.006 (executive function) | |
| Right Superior Parietal | −0.003 | 0.001** | NS | – | NS | – | NS | −0.355 | 0.001** (executive function) | |
| Left precuneus# | −0.025 | <0.001** | 0.033 (F>M) | – | NS | – | NS | – | NS | |
| Right precuneus# | −0.022 | <0.001** | NS | – | NS | – | NS | −0.249 | 0.024 (info processing) | |
- #Showed quadratic relationships with age.
- **Significant at corrected level (Bonferroni, P < 0.004).
- NS—not significant.
- a Age and gender effects with GMV were estimated within the same general linear model.
- b General linear model estimating association between each behavioral measure and GMV, age not controlled for.
- c Pearson's correlation.
- d Bivariate correlation, controlling for age and gender.
Age and cognitive behavioral measures
There were no significant associations between cognitive scores and age and no gender or age*gender effects for either of the cognitive domains.
Gray matter volume and cognitive behavioral measures
There was a significant relationship between gray matter volume in the left middle frontal node (P = 0.001) and also trends for the left inferior frontal node (P = 0.015) of the DLPFC and left superior parietal node of the DPC (P = 0.01) with executive function. However these relationships did not survive controlling for age.
Longitudinal Relationships
Correlation of change in gray matter volume with age
Figure 2 shows individual volumes for right nodes of the CCN at T1 and T2 against age. Only the right middle frontal node of the DLPFC had a trending relationship with age, with less volume change as age increased (P = 0.013). None of the other CCN nodes showed a significant relationship between age and change in gray matter volume longitudinally.
Correlation of change in cognitive behavioral measures with age
There appeared to be no correlations between change in cognitive performance and age in any domain.
Correlation of change in gray matter volume with change in behavioral performance
Executive function performance was significantly correlated with gray matter volume of the right inferior (P = 0.002) and superior parietal lobules (P = 0.001) of the DPC. There were also trends observed with a negative correlation in the left inferior frontal lobule (P = 0.010) and the left middle frontal lobule (P = 0.036) in the DLPFC and the left superior parietal node of the DPC (P = 0.006) (see Fig. 3A). Additionally, there were trends of negative correlation between information processing efficiency scores and gray matter volume in the bilateral dACC (L: P = 0.03, R: 0.011) and in the right precuneus in the DPC (P = 0.024) (see Fig. 3B); and for emotion identification performance and gray matter volume in the left middle frontal lobule of the DLPFC (P = 0.022). For all regions, volume decreases with improvement in cognitive performance were observed.
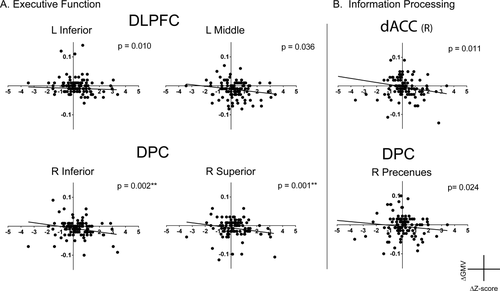
Association between change in gray matter volume and cognitive performance. Relationship between change in gray matter volume (ΔGMV) and change in cognitive scores (ΔZ-score), controlling for age and gender, a) shows changes is gray matter volume in the dorsolateral prefrontal cortex (DLPFC) and dorsal parietal cortex (DPC) associated with improvement in executive function and b) shows changes in gray matter volume changes in the dorsal anterior cingulate cortex (dACC) and DPC associated with improved information processing. For both tasks a greater decrease in gray matter volume over two years is significantly associated with an increase in cognitive performance.
Is There Co-Ordinated Anatomical Change across the CCN?
Partial correlation analysis, controlling for age and gender across the regions of the CCN were highly significant (P < 0.0001) between bilateral dACC nodes, bilateral inferior frontal and left mid frontal nodes of the DLPFC and bilateral inferior and superior parietal nodes of the DPC. The left precuneus had a significant correlation (P < 0.0031) with bilateral cingulate, bilateral inferior frontal nodes, left midfrontal node, bilateral inferior parietal nodes and bilateral superior parietal nodes and with right precuneus. The right precuneus had a significant correlation (P < 0.001) with all but the left inferior, and bilateral middle frontal DLPFC nodes. The least correlated to all other areas in the CCN was the right middle frontal node of the CCN, which was significantly correlated (P < 0.003) with only right inferior and left middle frontal DLPFC nodes.
DISCUSSION
This study provides the first direct evidence that structural maturation of regions of the CCN supports executive function development. By mapping cognitive performance and structural brain changes over time in a longitudinal study, we not only observed coordinated gray matter change in this network but also a significant association of this change with improvement in cognitive abilities during a key period of neurodevelopment. We found that independent of age, decreases in gray matter volume in the posterior parietal and dorsolateral prefrontal cortices were correlated with improved performance in the executive function domain. Additionally, a decrease in gray matter volume in bilateral cingulate cortex and the right precuneus was associated with improved performance in the information-processing domain and in the left dorsolateral prefrontal cortex with emotion processing (both at trend levels). Somewhat surprisingly the longitudinal change in both gray matter volume in the CCN and cognitive performance were unrelated to age within this sample.
The human brain has been observed to change in gray matter structure throughout the entire life-span. Cross-sectional findings have described a non-linear decline in gray matter from around age 7 to age 60 [Grieve et al., 2005, 2011; Pfefferbaum et al., 1994; Sowell et al., 2003]. However while longitudinal studies thus far support a decline in gray matter through adolescence into the twenties, there is limited longitudinal data for gray matter changes in the second, third and fourth decades of life [Gogtay et al., 2004; Lenroot et al., 2007]. Two prior longitudinal studies with large cohorts comprising subjects ranging in age from first through to the third decades of life describe a period of initial increase in childhood, followed by a decline in adolescence and then subsequent stabilization of gray matter change [Lenroot et al., 2007; Shaw et al., 2008]. Additionally, differences in maturation trends based on gender have also been identified [Lenroot et al., 2007]. This pattern of cross-sectional gray matter decline with age was also observed in our cohort, however the trend was observed to be mostly linear. In contrast observed longitudinal findings show that the rate of change in gray matter volume was mostly independent of age, except for a moderate trend in the right middle frontal node of the DLPFC. One possible explanation for these differences is the high proportion of participants in their 20s and 30s, especially in comparison to under-18 year olds, which had a minimum age of eight years old. This disproportion of younger participants could have reduced the impact of their data. This is especially relevant if looking at the data in comparison to the two previously mentioned longitudinal studies which had a predominantly younger cohort with minimum age of three years old. This could also have led to an absence of gender effects which are thought to be age dependent and affect gray matter trajectories throughout the cortex [Lenroot et al., 2007]. Another likely reason is that our findings are based on data for only two time points, whereas the studies of both Lenroot et al., and Shaw et al., had included MRI data from 3 or 4 time points in over 30–40% of their large samples (387 and 375 respectively) which better captures a developmental trajectory over time [Lenroot et al., 2007; Shaw et al., 2008]. Additionally, our study focused on gray matter regions only within the CCN, brain regions that have been found to maintain the most plasticity throughout life [Toga et al., 2006].
It has been previously observed that structural changes in the brain, largely parallel cognitive milestones [Casey et al., 2005]. In this study, we evaluated these relationships across seven cognitive domains. We found a relationship between DPC and DLPFC volumes and executive function, and also with the DPC and the dACC and information processing. The executive function domain captures a general performance of cognitive control functions. Information processing also accesses a broader range of specifically attentional control functions which have been previously linked to dACC and parietal cortex connectivity [Mesulam, 1983]. Our finding lends support to previous longitudinal findings by two studies, one of which saw improvement in verbal intelligence associated with declining gray matter in frontal areas [Sowell et al., 2004] and the second observed improvement in working memory associated with frontal and parietal gray matter volume decreases [Tamnes et al., 2013]. Both findings were also independent of age [Sowell et al., 2004; Tamnes et al., 2013]. We also observed a trend in the relationship between DLPFC and emotion identification, supporting the role of this region in cognitive regulation and processing of emotions [Erk et al., 2010]. Despite the known role of a coordinated functional activation of the entire CCN for a range of cognitive tasks, we did not find any relationships between CCN volumes and domains of memory, attention and impulsivity or significant relationship for all the regions of the CCN with executive function and information processing. Despite the functional activation of CCN regions during a range of cognitive tasks [Niendam et al., 2012], it is still possible that discrete components and connections within the network maintain specific behavioral correlates and as a result, show regional specific changes in structure.
Additionally, differences in the relationship of changes in structure to changes in behavior may due to different developmental trajectories for specific cognitive functions as there is yet to be consensus in the literature as to when cognitive control is fully mature. Studies have found that cognitive performance observed during adulthood is typically achieved by late adolescence [Bunge et al., 2002]. However there is also evidence that shows continued improvement in cognitive performance into the twenties and thirties [Clark et al., 2006]. We did not observe improvements in cognition that were attributed to increases in age. The associations we observed between gray matter structure and cognition were also independent of age. The relationship seen here and elsewhere suggest that the relationship between decreasing gray matter volume and improved performance may be reflective of age-independent synaptic pruning occurring as a result of individual differences in genetic factors or due to experience-driven neural plasticity. Indeed both genetic and environmental factors have been shown to underlie both brain structure and cognitive abilities [Finkel et al., 2005].
Further evidence supporting activity-driven mechanisms of network refinement and cognitive control improvement is the observed coordination of CCN development, areas that are known to be functionally connected. Raznahan et al., [2011] reported that anatomical change across different regions of the developing cortex show a highly correlated non-random structure [Raznahan et al., 2011]. These findings support previous neuroimaging studies providing evidence of activity–dependent structural plasticity [Draganski et al., 2004; Hyde et al., 2009]. This suggests that regions sharing coordinated patterns of activation over time will be subject to more similar sets of activity-driven structural change both during and beyond development comparative to cortical regions that are functionally independent of each other. Another finding of particular interest here is that the right mid-frontal node of the DLPFC was an outlier with lack of strong correlation with other regions of the CCN. The right mid-frontal node displayed a linear pattern of decline that had a trending longitudinal relationship with age. This suggests a possible protracted development of this part of the DLPFC. A possible explanation for this comes from previous functional studies that have found age-related lateralization of the DLPFC during development [Bunge et al., 2002; Rubia et al., 2006]. For example during working memory tasks children fail to recruit the right DLPFC to the same extent as that by adolescents and adults [MacDonald et al., 2000; Scherf et al., 2006]. These neurodevelopmental findings provide insight into the knowledge that adolescence and the early twenties is the most at-risk period for development of psychiatric illnesses [Paus et al., 2008]. The age-independent role of structural change and the co-ordination of this change across the network indicate that activity-driven processes have a key role in shaping structural networks and this in turn impacts cognitive control performance.
Understanding the normal trajectory of cognitive control development and how this relates to structural changes in the brain is critical for understanding potential causes for impairment in cognitive control observed in various mental disorders [Phillips et al., 2008; Williams, 2016]. Understanding mental illness through a neural circuitry perspective allows research to better understand and address the complex and heterogeneous nature of these disorders. MRI studies can be used to identify patterns and stereotypes in circuitry dysfunction across various disorders [Williams, 2016]. In the CCN particularly, hypo-activation of the DLPFC and dACC has been identified as a potential cause for a “cognitive dyscontrol” type in depression by failing to successfully tamper rumination and inhibit negative responses [Williams, 2016]. While further research is needed, several studies have also suggested decreased functional activity in areas of CCN may contribute to emotional dysregulation in bipolar disorder [Green et al., 2007; Lagopoulos et al., 2007; Phillips et al., 2008]. By redefining mental disorders using neural rather than symptomatic measures, these studies may impact not only on how mental illness is viewed but also aid in the development of better diagnostic tools and treatments. In particular, an understanding of the mechanisms of cognitive control circuitry neurodevelopment occurring during a period when there is an increased risk for development of mental disorders may lead to a better understanding and subsequent treatment of psychiatric and neurological conditions in which cognitive control circuitry seems to be affected.
The findings in this study were impacted by several limitations. The most substantial limitation for this study is the low numbers of participants under 18 years of age comparative to those over 18 (around 15 under 18, 100 are 18–38). It is likely that the developmental change during this period is underrepresented in the findings. Secondly, the longitudinal data for this study was limited to only two scans. Having more time point measurements per individual increases the power to model longitudinal findings and allows for more sophisticated analysis such as mixed modeling [Mills et al., 2014]. Thirdly, longitudinal processing of structural MRI data has been known to be impacted by choices in preprocessing methodology e.g. bias due to the time point data selected for aligning other time point scans and to standard space templates [Thomas et al., 2009]. We followed the standard recommended longitudinal pipeline for VBM analysis in our study which aligns data to time 1 scans. However we note that although our main findings were robust and remained unchanged when time 2 data was used to align all scans, two of our trend findings were no longer significant in the second analysis. These were observed for the change in emotion identification correlation with change in left middle frontal volume (P value changed from 0.022 to 0.079) &the change in information processing correlation with change in right precuneus volume (P value changed from 0.024 to 0.365). The fourth limitation is that gray matter volume as a measure may be subject to highly individualized fluctuations based on lifestyle and experience. As an example, Ceccarelli et al. demonstrated that gray matter volume can change over two weeks if subjects partake in “cognitive training” [Ceccarelli et al., 2009]. While our sample was screened to include only healthy control participants from the general community, controlling for all individualized factors that could impact gray matter volume is not practically possible. Finally, although our focus was on the CCN, gray matter volume measurements provide only a limited understanding of the relationships between the various nodes of this network. A deeper understanding of these relationships may possibly be revealed by using more sophisticated imaging measures of structural connectivity (Diffusion Tensor Imaging) and functional connectivity (functional MRI) to fully investigate the inter-regional interactions within this network and their links to cognitive control. This will be the focus of our future work.
In summary, these findings adds to the small amount of pre-existing research examining the trajectory of longitudinal structural developmental beyond childhood into the second to fourth decades of life. This data contributes towards the establishment of a normal baseline in late adolescence and early adulthood, a key period of development in which individuals have heightened vulnerability to psychiatric illnesses. These findings may be used in the future to investigate markers for better diagnosis and understanding of the impact of these disorders on neurodevelopment and also to show how improved treatment outcomes may reflect on brain structure. This study provides direct evidence of a correlation between cognitive performance and gray matter volume in key areas of the CCN, independent of age. Additionally, correlation between structural changes over time in the CCN suggests a co-ordination of anatomical change during development that may be influenced by, or in turn influence, the functional connectivity between these areas. Further investigation using a broader scope of neuroimaging measures will aid in gaining a better understanding of the mechanisms driving this important relationship.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study. In cases where individuals where under the age of 18, informed consent was obtained from a parent or legal guardian.