Increases in brain activity during social competition predict decreases in working memory performance and later recall
Abstract
In our fMRI experiment, participants completed a learning task in both a noncompetitive and a socially competitive learning environment. Despite reporting a preference for completing the task while competing, participants remembered significantly more during the task and later recalled more from the noncompetitive learning environment. Furthermore, during working memory maintenance, there was performance-related deactivation in the medial prefrontal cortex (mPFC) and the precuneus/PCC. During feedback presentation, there was greater activation in the mPFC and the precuneus/PCC while competing. Differential activation in the precuneus/PCC predicted worse later recall for information learned competitively. Since previous research suggests that the mPFC is involved in social-referencing, while the precuneus/PCC is implicated in off-task thoughts, our results suggest that receiving feedback regarding competition produces more activation in brain regions implicated in social interaction, as well as task distraction. While competition may make a task more enjoyable, the goal of winning may distract from maximizing performance. Hum Brain Mapp 38:457–471, 2017. © 2016 Wiley Periodicals, Inc.
Abbreviations
-
- DMN
-
- Default mode network
-
- GLM
-
- General linear model
-
- mPFC
-
- Medial prefrontal cortex
-
- PCC
-
- Posterior cingulate cortex
-
- PDCAS
-
- Personal development competitive attitude scale
-
- ROI
-
- Region of interest
-
- RUBIC
-
- Rutgers University Brain Imaging Center
-
- SDS
-
- Social desirability scale
-
- STS
-
- Superior temporal sulcus
INTRODUCTION
Although both cooperative and competitive learning situations are often used in educational settings, it remains unclear whether social competition spurs learners to do their best work, or whether social competition causes unnecessary task demands that could hinder performance. Social competition has often been implemented as a means for augmenting effort in a variety of domains, such as in athletic environments when extra effort is required to run faster when a rival is present [Kilduff, 2014], as well as improved practice motivation among competitive cyclists [Frederick-Recascino and Schuster-Smith, 2003]. Social competition has also been shown as a means to augment effort in a laboratory setting [Le Bouc and Pessiglione, 2013]. Although effort is an important part of success, for effort to be effective in a learning environment, it must be aimed at improving performance, which is distinct from winning a competition. Research examining the effects of competition on memory acquisition is sparse, despite the competitiveness of the current state of education [Anderman et al., 1999; Nichols and Berliner, 2007; Roeser and Eccles, 1998; Ryan and Patrick, 2001]. Furthermore, our recent empirical evidence suggests that competition may actually hinder performance on a memory task [DiMenichi and Tricomi, 2015], further emphasizing the need for more examination of the effect of competition on memory acquisition. Specifically, we wanted to investigate the effects of competition on social and performance networks in the brain.
Social Cognition
Incorporating competition during memory acquisition may activate areas associated with reflecting about others. Specifically, the medial prefrontal cortex is an area often associated with social cognition, specifically social comparison [Amodio and Frith, 2006; Mitchell et al., 2005; Mitchell et al., 2006]. We would expect that the addition of a competitor would activate this region, although it is unclear how activation of this region would affect performance. Moreover, the medial prefrontal cortex (mPFC), in conjunction with other midline cortical areas, such as the PCC/precuneus, plays a vital role in the “mentalizing network”—a network of brain regions typically activated when individuals are mentalizing about other's thoughts, or updating social information about others [Atique et al., 2011; Baumgartner et al., 2012; Meyer and Lieberman, 2012; Meyer et al., 2012; Muscatell et al., 2012]. Because the introduction of a competitor in a memory task would require individuals to remember, maintain, and update information about another person's performance, individuals could show increased activation in the mentalizing network when a competitor is introduced to a working memory task. Moreover, social cognition may be a “psychological default”: previous research has suggested that activation in the medial prefrontal cortex and other corresponding regions (such as those within the mentalizing network) is the result of typical self-referential thoughts that occur when participants are scanned during “rest-state” activities [Schilbach et al., 2008]. Indeed, tasks that relate to directing attention towards the self appear to activate similar patterns of brain activity as the mentalizing network, such as resting-state tasks, [Binder et al., 1999; Greicius et al., 2003; Greicius et al., 2009], tasks requiring episodic memory [Hassabis et al., 2007; Hassabis and Maguire, 2007; Shallice et al., 1994], and even tasks that incorporate semantic memory and processing [Binder and Desai, 2011; Binder et al., 2009; Patterson et al., 2007; Skipper and Olson, 2014]. Overall, all of these seemingly contrasting tasks (e.g., self-centered reflection vs. asking an individual to reflect on another's actions in comparison to one's own) may elicit similar patterns of activation because these are all tasks that involve activating an “internal monologue” and directing attention inwardly. Therefore, when an individual completes a memory task in the presence of a competitor—a task that will require self-to-other referencing—there may be similar patterns of activation in comparison to what is typically seen during “resting-state” activities, or other activities that cause an individual to use his or her own thought patterns to direct attention inwardly. Additionally, activation in the mentalizing network may modulate observed performance differences.
Attention
Research has suggested that an important component of successful memory acquisition is attention [Berryhill et al., 2011; Craik et al., 1996; Fernandes and Moscovitch, 2000; Mayer and Moreno, 1998]. A variety of areas are activated during increased attention, including the posterior parietal lobe, posterior cingulate cortex (PCC) and precuneus, middle frontal gyrus, and the cerebellum [Berryhill et al., 2011; Casini and Ivry, 1999; Weissman et al., 2006]. Moreover, the precuneus and PCC have been implicated together as areas associated with task distraction [Castellanos et al., 2008; Lechak and Leber, 2011; Weissman et al., 2006], which may hinder memory acquisition.
Research also suggests that PCC/precuneus is activated during off-task thoughts [Castellanos et al., 2008; Lechak and Leber, 2011; Weissman et al., 2006], suggesting that deactivation of the PCC is essential for the engaged attention required for successful memory acquisition. The PCC/precuneus also plays a vital part in the default mode network (DMN)—a network with similar patterns of activation to the mentalizing network, including the mPFC, and the temporoparietal junction. The DMN is activated more during “off-task” thoughts, such as those that occur during mind-wandering [Christoff et al., 2009], levels of conscious awareness [Greicius et al., 2008], and even self-related thought [Qin and Northoff, 2011] and self-projection [Buckner and Carroll, 2007]. Also, previous research has found that increases in task difficulty (such as those required for working memory) lead to decreases in activation of the DMN [Greicius et al., 2003], further supporting the idea that decreases in DMN activity may accompany greater task engagement. However, it is unclear how the introduction of a competitor during memory acquisition may affect attention and activity in PCC/precuneus, as well as other regions in the DMN.
Value
The introduction of a competitor may change the subjective value of performance-related feedback. For example, introducing a competitor to a memory task may change the way an individual subjectively values positive or negative feedback: an individual may subjectively value positive feedback in the presence of a competitor more than the same amount of positive feedback in an independent task setting, or vice versa. Moreover, negative feedback could be seen as subjectively more punishing when it is received against a competitor than when it is received in an independent setting. Because previous research has found that subjective value during memory acquisition has resulted in differential activation of the striatum [Delgado et al., 2005; Elliott et al., 2003; Tricomi and Fiez, 2012], differences in activation in the striatum might occur if subjects value reward earned through competition more than reward in a noncompetitive environment. Therefore, we were interested in investigating if the addition of a competitor affected the processing of performance-related feedback in traditional reward-related regions.
Current Study
Although students are regularly subjected to competitive educational environments, few studies have examined the direct effect of competition on memory in a laboratory setting. The current study examines the effect of competition on working memory performance, as well as later recall. Moreover, we examined brain activation during working memory and feedback processing, as well as how this activation relates to performance differences. Our study examined the direct effects of competition on the neural networks necessary for successful memory, which may shed light on neural mechanisms by which competition influences memory-related performance.
METHOD
Participants
Twenty-one right-handed adult females 18–35 years of age were recruited from Rutgers University's Newark campus and surrounding community. We scanned one gender in order to control for possible gender differences in performance during competition, since previous research suggests that competition may affect males and females differently [Eder, 1995; Gneezy et al., 2009; Herlitz et al., 1997; Niederle and Vesterlund, 2011; Sutter and Rützler, 2010; Voyer et al., 2007]. Furthermore, we decided to scan female participants competing against female confederates because DiMenichi and Tricomi [2015] found a significant difference between the performance of participants who believed they were competing against females and the performance of participants who believed they were competing against males. Sample size was determined based on the results of DiMenichi and Tricomi [2015]. Participants (mean age= 20.6, SD = 2.58) reported to Rutgers University Brain Imaging Center (RUBIC) in Newark, New Jersey, where they were introduced to a fellow “participant” they would later be competing with—a female confederate. The experimenter brought the confederate into a separate testing room and waited for about five minutes, the expected time for the confederate to complete the practice session of the task. Participants then completed a practice version of the task, both the “no competition” condition and the “competition” condition inside the scanner, and following the scan, a surprise recall task and a battery of surveys, including a demographic information survey asking questions pertaining to age, ethnicity, education level, high school and/or college grade point average, etc. After completing the surveys, participants were probed for task believability and debriefed about the confederate and real purpose of the task. Participants received a base rate of $30 for their session, with up to an additional $25 in bonuses based on task performance. One participants was removed from the sample for failing to believe that the confederate was a participant; analyses were conducted on the remaining 20 participants.
Task
We used the same working memory task from DiMenichi and Tricomi [2015]—which was adapted from [Redick et al., 2012]. While inside the scanner, participants decided if a matrix was symmetrical or not, and then were asked to memorize an association between an abnormal shape and a number (1 through 3). Abnormal shapes were taken from Endo, Saiki, and Saito [2001]'s Novel Shape database. All shapes were projected in black and white, with an on-screen resolution of 800 x 450. In each trial, participants saw a symmetry matrix, a shape associated with a number, a second symmetry matrix, a second shape associated with a number, a third symmetry matrix, a third shape associated with a number, and finally, a recall screen with the shapes from the trial, in which participants were asked to recall the numbers associated with the shapes they were just shown. Because symmetry judgements and memory response screens were untimed, no jitter was added since the task had natural variation in timing. The standard deviation of participants' time spent on symmetry judgements and the memory response screen were .84 sec, and 2 sec respectively, indicating that there was some level of natural variation within each subject's performance. The inter-trial interval between each trial was 1–5 sec.
Participants learned number associations for 36 shapes (18 in the no competition condition, and 18 in the competition condition). Shapes were randomly assigned to each condition, and shapes in the no competition condition were not repeated in the competition condition (and vice versa). Furthermore, shapes in each condition were rotated across participants, in case shapes in one condition were somehow more difficult to remember than shapes in another condition. Each trial contained three novel shapes, and each trial was shown twice within each run of each condition during fMRI scanning (12 trials per run). All participants completed two runs of each condition, following an ABAB or BABA design (48 trials total), meaning that participants saw each shape four times in the experiment to best mimic the type of learning that occurs in a classroom setting, where students review the material multiple times. Each run lasted an average of 14 minutes. Conditions were counterbalanced across participants to prevent order effects—participants followed either an ABAB or BABA design. There were no significant order effects. For task illustration, see Figure 1.
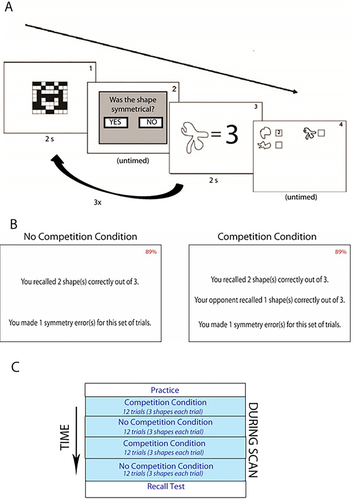
Task Depiction. (a) Participants were shown a matrix for 2 sec (Slide 1) and asked to decide if the shape was symmetrical (Slide 2). Participants were then shown a novel shape paired with a number for 2 sec, and asked to memorize this association (Slide 3). After viewing three shapes (Slide 1–3), participants were asked to recall the numbers associated with the shapes. (b) Subjects were immediately given feedback for 6 sec regarding their performance on the previous trial. In the self condition (left), subjects were informed about how many shapes they recalled correctly, the number of symmetry errors they made on this trial, and the total percentage of symmetry errors made throughout the condition (top right corner—subjects were required to answer at least 85% of symmetry problems correctly in order to receive the monetary bonus). In the competition condition (right) subjects were also given feedback about the number of shapes their “opponent” remembered correctly—a randomly generated number. After a 2 second delay, they were also given feedback about their symmetry performance. (c) Each trial contained three novel shapes, and each trial was shown twice (12 trials per run). All participants completed two runs of each condition, following an ABAB design (shown) or a BABA design (48 trials total), so participants saw each shape a total of four times throughout the experiment. Each run lasted an average of 14 minutes. Conditions were counterbalanced across participants to prevent order effects. [Color figure can be viewed at wileyonlinelibrary.com.]
No competition condition
Participants completed two runs of the task in a noncompetitive environment: in the no competition condition, participants were given feedback about their performance directly after the recall screen. They were told how many shapes they recalled correctly out of three, as well as how many symmetry problems they answered correctly. They were also given the running total percentage of correct symmetry problems for the entire condition. Participants viewed feedback for 6 seconds after each trial, and were told that if they could remember a total average of 66.7% shapes across all trials for each condition, they would be given a $5 bonus, consistent with our reward structure from our previous study with the same task [DiMenichi and Tricomi, 2015]. They were also told that in order to receive the bonus, they were required to complete the task with a symmetry matrix accuracy of at least 85%. Inclusion of the symmetry task also allowed us to examine if general engagement on the task varied across conditions, since this section of the task did not have a memory component. Participants were immediately told at the end of each no competition run if they earned the bonus.
“Competition” Condition
Each participant also completed two runs of a condition in which she believed she was competing against another participant. In the “competition” condition, after each recall screen, participants were given feedback about how many shapes they correctly recalled out of three, as well as feedback about their “competitor's” performance. Competitor performance was randomly generated out of 3, and averaged out to be 2 out of 3 across the entire condition, making task goals equivalent across the no competition and competition conditions. After a 2 second delay, participants were also given feedback about symmetry matrices errors for the trial. This delay was implemented in order to account for load on visual working memory. Total recall viewing time was 6 seconds after each trial. Participants were told if they could remember more total shapes than the other participant during the entire run—as well have a symmetry matrix accuracy of at least 85%—they would get a $5 bonus at the end of each run of the competition condition. Shapes were added to the total score for the run regardless of who “won” the trial, and all participants who remembered an average of 66.7% of shapes for the entire run received an additional $5 bonus. No participants “tied” with their competitor's performance for the entire run.
Recall task
Participants completed a surprise recall task after they exited the scanner and were brought to another room (about 5 minutes). In the surprise recall task, participants were again asked to recall each number associated with each shape. The recall task followed the same format of the learning task: participants saw three shapes at a time from either the competition condition or the no competition condition. Shapes were randomized to prevent order effects. Participants were told if they could recall an overall average of 2 out of 3 number-shape associations correctly in the recall task, they would receive an additional $5 bonus.
Questionnaires
Personal development competitive attitude scale (PDCAS)
The PDCAS examines if individuals regard competition as a means of improving personal development [Ryckman et al., 1996]. The PDCAS reflects on preference for situations in which competition may improve their performance (e.g., “I enjoy competition because it gives me a chance to discover my abilities.”). The PDCAS was selected because it has been previously found to predict competitive performance accuracy on the shape working memory task we used [DiMenichi and Tricomi, 2015].
Marlow-crowne social desirability scale (SDS)
We included the SDS [Crowne and Marlowe, 1960] to measure possible bias in responding. This questionnaire examines the extent to which a participant may positively skew their survey responses to represent themselves in a positive manner, and requires a “true or false” response to items such as “I am always courteous, even to people who are disagreeable.” The SDS has been previously used to detect the tendency of participants to have unrealistically positive representations of their own traits [DiMenichi and Richmond, 2015; Hebert et al., 1995; Ones et al., 1996; Zerbe and Paulhus, 1987], and previous research has found that competition may be viewed as a negative trait [DiMenichi and Tricomi, 2015].
Task preference measure
We also probed participants regarding their preference for each condition of the task. A 7-point Likert Scale asked participants to rate if they completely preferred the competition condition (3 on the scale) or completely preferred the no competition condition (−3 on the scale) while completing the task.
FMRI DATA COLLECTION AND ANALYSIS
The study took place at the Rutgers University Brain Imaging Center (RUBIC; Newark, New Jersey) with a 3 Tesla Siemens TRIO scanner and 12 channel head coil. Stimulus presentation was implemented with E-Prime Experimental Software (Psychology Software Tools, Pittsburgh, PA), and fMRI data was preprocessed and analyzed using BrainVoyager QX 2.3.1 Software (Brain Innovation, Maastricht, The Netherlands). Anatomical slices were collected using a T1-weighted protocol of 176 1-mm voxel sagittal slices, while functional slices were collected using a single-shot EPI pulse sequence with a TR of 2500 ms and TE of 25 ms. Forty-one contiguous oblique-axial 3 mm × 3 mm × 3 mm voxel slices were acquired in an oblique orientation of 30° to the anterior commissure-posterior commissure (AC-PC) axis. This orientation has been found to reduce signal dropout in the ventral prefrontal cortex [Deichmann et al., 2003].fMRI data was normalized to the Talairach stereotaxic space [Talaraich and Tournoux, 1988] before preprocessing. Preprocessing included slice-time correction, motion correction, 8 mm spatial smoothing, and high-pass temporal filtering (high pass GLM-Fourier, 3 sines/cosines, 3 seconds). Preprocessed data was then analyzed using a random-effects general linear model (GLM).
For each participant, we modeled the following regressors for the competition condition and for the no competition condition: the working memory portion of the task (i.e., one regressor modeling Slides 1 through 3 of Figure 1a; average duration per trial was 21.32 sec), parametric modulators of the working memory regressors indicating task performance (score of 0–3 for each trial), feedback (6 sec per trial for both conditions), and parametric modulators of the feedback regressors indicating task performance. Both components of the working memory task (i.e., the symmetry matrices and number-shape pairings) were included in the GLM as one regressor to accurately capture brain activation while subjects held working memory information in mind. The regressors were convolved with a canonical hemodynamic response function. A predictor for the response screen (Slide 4 of Fig. 1a; average duration per trial was 11.68 sec) was included in the model as a predictor of no interest, since the feedback screen was presented directly after the responses were entered. Additionally, the six motion parameters were also included in the model as predictors of no interest.
We conducted whole brain-analyses that examined group-level differences. Outcomes of interest included the effect of the task performance parametric modulators of the working memory regressors for the competition and for the no competition conditions, as well as the effect of the task performance parametric modulators of the feedback regressors. Contrasts of interest also included a contrast of the feedback parameter values for the competition condition versus the no competition condition, and a contrast of the working memory parameter values for the competition condition versus the no competition condition. Furthermore, because the PCC/precuneus has been previously implicated in distraction [Castellanos et al., 2008; Lechak and Leber, 2011; Weissman et al., 2006], we correlated behavioral performance scores with individual differences in parameter estimates in this area: a 6 mm radius of peak activation in the PCC/precuneus was extracted from the contrast of the feedback parameter values for the competition condition versus the no competition condition. Behavioral data was collected from the post-test conducted outside the scanner and is therefore independent of our region selection criteria [Kriegeskorte et al., 2009]. Using a continuity-based cluster-level threshold estimator in BrainVoyager, we used an initial significance threshold of P < 0.001, selected to run a series of 1000 Monte Carlo simulations, and corrected each contrast to a contiguity threshold cluster-level false positive alpha rate of 0.05. The resulting minimum cluster level for the analysis examining the effect of the task performance parametric modulators of the working memory regressors for the competition condition was 46 (estimated FWHM: 3.613), and 49 for the no competition condition (estimated FWHM: 3.700). The minimum cluster level for contrast of the working memory parameter values for the competition condition versus the no competition condition was 6 (estimated FWHM: 1.512), while the minimum cluster level value of the contrast of the feedback parameter values for the competition condition versus the no competition condition was 16 (estimated FWHM: 2.338).
Finally, because the introduction of a competitor may change the subjective value of performance-related feedback, and previous research has found that the caudate nucleus within the striatum is especially involved in feedback processing [Delgado et al., 2000; Tricomi et al., 2006; Tricomi et al., 2004], we conducted a region of interest (ROI) analysis of the caudate nucleus of the contrast of competition condition > no competition condition at the time of feedback. We also examined the effect of the task performance parametric modulator in both conditions. The ROI was a 6 mm sphere around Talairach coordinates ± 12, 15, 8 averaged from previous studies that specifically examined feedback-related effects in the caudate nucleus [Delgado et al., 2000; Tricomi et al., 2006; Tricomi et al., 2004].
RESULTS
Behavioral Results
Replicating DiMenichi and Tricomi [2015], a paired-samples t-test revealed that participants remembered significantly more shapes per trial in the no competition condition (M = 2.76, SD=.19) than in the competition condition (M = 2.50, SD=.27; t(19)=4.28, P < 0.001, Cohen's d = 0.96). Furthermore, participants later recalled significantly more shapes learned in the no competition condition (M = 2.72, SD=.32) than in the competition condition (M = 2.23, SD=.42; t(19)=3.87, P = 0.001, Cohen's d = 0.87). There were no significant differences regarding symmetry accuracy across conditions, suggesting that there was no difference in task involvement across conditions. Behavioral results are depicted in Figure 2.

Behavioral Results. Participants remembered significantly more shapes per trial in the self condition than when they believed they were competing against another participant (left graph). Participants also later recalled significantly more shapes learned during the noncompetitive learning condition than those shapes learned when they believed they were competing (right graph). ***Significant at P < 0.001. [Color figure can be viewed at wileyonlinelibrary.com.]
However, we did not find a significant relationship between scores on the Personal Development Competitive Attitude Scale and behavioral performance. Unlike DiMenichi and Tricomi [2015], scores on the PDCAS were marginally negatively correlated with scores on the Social Desirability Scale (SDS) in our sample (r = −0.40, P = 0.079); that is, participants who tended to respond in a socially desirable manner tended to report low levels of competitiveness on the PDCAS. This finding suggests that the participants in our sample may have been biased in responding about their competitive traits and habits. However, task preference scores were not correlated with scores on the SDS. Furthermore, although participants performed better in and remembered significantly more from the no competition condition, 13 of the 20 participants reported preferring the competition condition, with five reporting no preference and only 1 reporting a preference for the no competition condition. We found no significant correlations between condition preference and task or recall performance in either condition. We also found no significant correlations between education level, high school/college grade point average, and performance.
We also conducted an analysis that examined whether scoring better than the competitor significantly predicted later memory recall: we coded each trial as a win, loss, or a tie, and then examined if the average number of wins for a given trial during the competition condition predicted better memory for those shapes during the surprise recall task. However, a linear regression did not reveal a significant effect (β =.075, t(119) =.817, P = 0.415).
FMRI RESULTS
Working memory maintenance analysis
During the working memory maintenance portion of the trial (Slides 1 through 3 of Fig. 1a), participants showed performance-related deactivation in both the medial prefrontal cortex and the PCC/precuneus, in both conditions. As deactivation in these areas increased, performance improved (Fig. 3). Activation peak locations are listed in Table 1. There were no significant differences across conditions on the working memory maintenance portion of the task.

Results of Working Memory fMRI Analysis. (a) Participants showed performance related deactivation of the default mode network in across both conditions. There was no significant difference across conditions. (b) Performance-related deactivation in the PCC/precuneus (6 mm radius of activation peak coordinate −5, 45, 15). (c). Performance-related deactivation in the medial prefrontal cortex (6 mm radius of activation peak coordinate −4, −74, 33). [Color figure can be viewed at wileyonlinelibrary.com.]
| Region of activation | BA | Number of voxels (3 × 3 × 3 mm3) | Peak (Talairach: x,y,z) | t |
|---|---|---|---|---|
| Working memory maintenance | ||||
| Effect of performance parametric modulator on no competition | ||||
| Task activation | ||||
| Right inferior occipital gyrus | 19 | 188 | 44, −74, −6 | 7.54 |
| Left precentral gyrus | 6 | 64 | −7, 4, 51 | 6.44 |
| Left inferior occipital gyrus | 19 | 163 | −61, −74, −12 | 5.52 |
| Task Deactivation | ||||
| Right angular gyrus | 39 | 2500 | 44, −65, 36 | −8.82 |
| Bilateral medial frontal cortex | 45 | 370 | −4, 37, 18 | −9.55 |
| Bilateral PCC/precuneus | 7537 | 8, −44, 30 | −10.68 | |
| Effect of performance parametric modulator on competition | ||||
| Task activation | ||||
| right occipital lobe | 18 | 190 | 26, −80, 6 | 6.79 |
| right precentral gyrus | 6 | 80 | 23, −5, 48 | 7.54 |
| left precentral gyrus | 6 | 116 | −10, 1, 51 | 6.89 |
| Task Deactivation | ||||
| Bilateral medial prefrontal cortex | 45 | 8103 | −5, 45, 13 | −10.42 |
| Right cerebellum | 39 | 56 | 17, −86, −27 | −5.72 |
| Bilateral PCC/precuneus | 3422 | −4, −74, 33 | −11.82 | |
| Left superior temporal gyrus | 1442 | −46, −53, 24 | −8.16 | |
| Event-Related Analysis at Feedback | ||||
| Competition>No competition | ||||
| Right superior temporal sulcus | 39 | 727 | 50, −53, 21 | 7.39 |
| Right dorsolateral prefrontal gyrus | 8 | 651 | 47, 22, 21 | 8.40 |
| Right medial prefrontal cortex | 46 | 810 | 11, 58, 18 | 6.60 |
| Right inferior frontal Gyrus | 47 | 66 | 29, 22, −6 | 5.53 |
| Bilateral PCC/precuneus | 46 | 2746 | 17, −47, −6 | 10.71 |
| Left medial prefrontal cortex | 8 | 44 | −28, 71, 6 | 5.26 |
| Left dorsolateral prefrontal gyrus | 19 | 124 | −37, 16, 45 | 5.93 |
| Left middle temporal gyrus | 45 | 144 | −49, −62, 18 | 5.09 |
| Left ventral lateral prefrontal cortex | 39 | 22 | −40, 58, 3 | 4.64 |
| Left superior temporal sulcus | 351 | −55, −35, −3 | 6.91 | |
| No Competition>Competition | ||||
| Right occipital lobe | 18 | 477 | 29, −92, 12 | −6.80 |
| Right precentral gyrus | 6 | 297 | −22, 1, 57 | −6.61 |
| Left parietal lobe | 39 | 24 | −16, −71, 45 | −6.60 |
| Left insula | 19 | 46 | −22, 10, 15 | −5.19 |
| Left occipital lobe | 40 | 102 | −34, −83, 6 | −6.32 |
| Left postcentral gyrus | 6 | 240 | −40, −35, 42 | −8.55 |
| Left precentral gyrus | 72 | −55, 1, 33 | −6.37 | |
When examining significant correlations with performance and neural activation during the working memory maintenance portion of the task, we found a significant negative correlation between activity in the precuneus/PCC during the working memory phase of the competition condition and performance during the competition condition (r = −0.51, P = 0.022); as activity in the precuneus/PCC during working memory increased, performance decreased. There was no significant relationship between activity during the working memory portion of the no competition condition and performance during the no competition condition (r = −0.30, P = 0.192).
There was also a significant positive correlation between activity in the precuneus/PCC during the working memory portion of the competition condition and scores on the Personal Development Competitive Attitudes Scale (r = 0.48, P = 0.033), but no significant relationship between activity in the precuneus/PCC during the working memory portion of the no competition condition and scores on the PDCAS (r = 0.34, P = 0.147).
Event-related analysis at time of feedback
An examination of BOLD activation differences across conditions at the time of feedback showed significantly greater activation in the mPFC and the PCC/precuneus, during the competition condition compared to the no competition condition (Fig. 4). We also found significantly greater activation in both superior temporal sulci during the competition condition compared to the no competition condition.
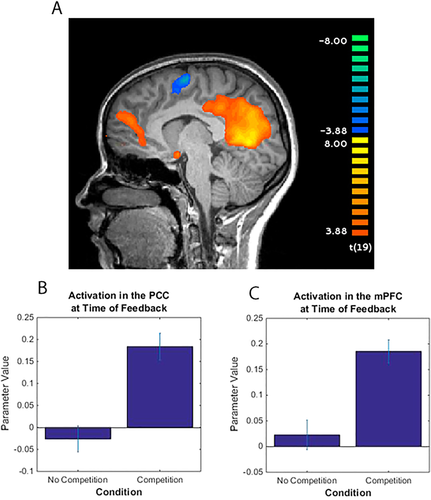
Results of Event-Related Feedback fMRI Analysis. (a) Participants showed greater activation in the medial prefrontal cortex and PCC/precuneus when viewing feedback during the competition condition compared to the no competition condition. Contrast is competition condition > no competition condition. (b) Activation across conditions in the PCC (6 mm radius of activation at peak coordinate 17, −47, 6). (c) Activation across conditions in the medial prefrontal cortex (6 mm radius of activation peak coordinate 11, 58, 18. [Color figure can be viewed at wileyonlinelibrary.com.]
We also calculated individuals' “Competitive Recall Score”: the number of shapes originally learned in the no competition condition correctly recalled at post-test, subtracted from the number of shapes originally learned in the competition condition (allowing us to control from individual differences in memory ability). We found a significant negative correlation between the parameter value for “Competition > No Competition” at the time of feedback and individuals' Competitive Recall Score (r = −0.50, P = 0.026); i.e., we found that the more the PCC/precuneus activation differed at feedback presentation for the competition compared to no competition condition, the less likely the participants were to remember the shapes learned in the competition condition later at the post-test (Fig. 5).
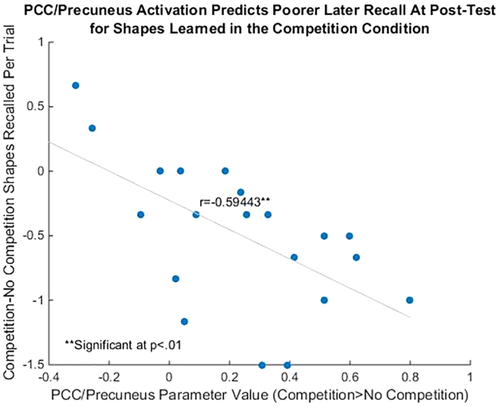
Individual differences in PCC/precuneus activation predict poorer later recall at post-test for shapes learned in the competition condition. Differential activation in the PCC/precuneus (competition condition > no competition condition) was significantly negatively correlated with the number of shapes remembered from the competition condition at post-test. Activation peak coordinate was taken from 11, −62, 3. Beta weights were taken from a 6 mm radius of this peak. [Color figure can be viewed at wileyonlinelibrary.com.]
When examining an ROI of the striatum for the Competition > No Competition contrast at the time of feedback, we found no significant difference when comparing the competition and no competition conditions [t(19) = −1.253, P = 0.225], as well as when examining the parametric modulator of each condition [competition t(19) = 0.34, P = 0.368; no competition t(19)= −0.04, P = 0.483].
DISCUSSION
Students are regularly subjected to competitive educational environments. For example, standardized test scores give students normative feedback about their percentile rank, which can often determine acceptance into honors tracks, colleges, and even graduate programs. At higher levels of education, tests are often graded on curves that pit students against each other, and many competitive law, engineering, and health science programs only offer continued admittance to a certain number of students in the program, creating fierce competition in the classroom. Yet, research has yet to examine the direct effect of competitive environments on working memory and related brain activity. In our study, we found that participants performed worse in and remembered less from a competitive task environment, replicating previous research [DiMenichi and Tricomi, 2015]. However, participants reported preferring our competitive environment over a noncompetitive setting, despite these performance differences. Since reward can have up-regulating effects on performance [Shohamy and Adcock, 2010], our finding that participants prefer the competitive context despite performing worse is surprising. However, previous research has suggested that high-pressure situations—such as important sports win, a good score on an important exam, or large monetary outcomes—can lead to decrements in performance [Baumeister, 1984; Beilock and Carr, 2001, 2005; Ramirez et al., 2013]. Since participants reported preferring the competition condition over the no competition condition, it may be that subjects found succeeding in the competition condition a high-pressure situation, and previous research has suggested that the prospect of high-pressure situations can lead to decrements in performance [Beilock and Carr, 2001, 2005; Chib et al., 2012].
Competition and Working Memory Maintenance
During working memory maintenance, activation in the mPFC and PCC/precuneus was consistent with behavioral performance: participants showed performance-related decreases in these areas across both conditions. Since previous research has suggested that activity in the PCC/precuneus and the mPFC tends to decrease as task difficulty increases [Greicius et al., 2003; Hampson et al., 2006], this finding provides further support that deactivation in the PCC/precuneus and mPFC is associated with successful memory performance.
Interestingly, activation in the PCC/precuneus during the working memory maintenance portion of the competition condition was positively correlated with scores on the Personal Development Competitive Attitudes Scale, a scale that measures individuals' preference for competition during performance. It may be that participants highly invested in “winning” against the competitor may have been distracted from successful memory performance, since decreases in activation in the PCC/precuneus are usually related to improvements in memory performance [Fransson and Marrelec, 2008].
Competition and Memory Acquisition
During feedback processing, participants showed greater activation in the PCC/precuneus during our competitive environment compared to our noncompetitive environment. New research has suggested that during instrumental learning, the PCC/precuneus is involved in other-referencing and updating social information about others [Hackel et al., 2015], a necessary component of the shape memory task utilized in our experiment. Participants also showed greater activation in the mPFC during our competitive task environment, an area which has also consistently shown increased activation during social comparison across a number of studies [Amodio and Frith, 2006; Bault et al., 2011; Decety et al., 2004; Dvash et al., 2010; Mitchell et al., 2005; Mitchell et al., 2006]. Northoff et al. [2006] reviewed a large body of research that suggested that midline structures—including the PCC/precuneus and mPFC—are activated during a self-reference or self-in-comparison-to-others reference. Therefore, increased activation in the mPFC and PCC/precuneus during a competitive learning environment may reflect the social nature of competition, as well as the social referencing and social-information updating required for competitive memory acquisition.
We also observed significantly greater activation bilaterally in the superior temporal sulcus (STS) at time of feedback during the competition condition as compared to feedback during the no competition condition. Given the social nature of the competition condition in our study, this finding is consistent with previous work that has found that the STS [Blakemore et al., 2003; Choudhury et al., 2006; Pelphrey et al., 2004] is more active during tasks that ask an individual to reflect on another person's actions.
The “mentalizing network”—a network of brain areas that includes the mPFC, STS, and precuneus/PCC—refers to a network of regions typically activated while mentalizing about others, as well as during the online maintenance and manipulation of numerous elements of social information [Atique et al., 2011; Baumgartner et al., 2012; Meyer et al., 2012; Muscatell et al., 2012]. Increases in load on “social working memory”—changing information about another person—have been found to lead to increases in brain regions believed to be involved in metalizing [Meyer and Lieberman, 2012; Meyer et al., 2012]. This finding also supports our interpretation that increases in activation in the mPFC, STS and PCC are a result of the introduction of a competitor.
When examining how our neural findings related to behavioral memory measures, we found that increases in the precuneus/PCC during the working memory maintenance portion of the competition condition predicted worse performance during the competition condition. We also found that increases in activation in the precuneus/PCC at time of feedback predicted worse later memory for shapes learned in a competitive setting. Since deactivation of midline structures like the precuneus/PCC leads to better later memory [Chai et al., 2014], our finding suggests that individual differences in how much the presence of a competitor produces activation in brain areas thought to be involved in mentalizing and social working memory may also reflect how likely an individual is to be distracted by a competitor in a learning context.
Furthermore, since previous research has suggested that the PCC/precuneus is also more likely to be activated during off-task thoughts [Castellanos et al., 2008; Lechak and Leber, 2011; Weissman et al., 2006], the finding that differential activation of the PCC/precuneus predicts reduced later recall suggests that increased activation in this area at feedback may reflect task distraction caused by thoughts about one's performance in comparison to a competitor. Other attention research pertaining to “dual-tasking” suggests that attempting to complete two tasks concurrently results in decrements in performance [Pashler, 1994; Strayer and Johnston, 2001]. In relation to the memory acquisition task that we employed, perhaps adding a competitor to a learning environment creates a secondary goal of beating a competitor in addition to the primary goal of mastering the task, and this secondary goal distracts individuals from that primary goal. This assertion may explain why we saw decrements in performance and later memory in a competitive environment.
Limitations
One possible criticism of our working memory task is that task goals are more specific in the no competition condition (i.e., participants must get 66.7% of shapes correct to win the bonus), while task goals in the competition condition are less specific and depend on confederate performance scores. Although some research has suggested increased specificity of task goals can lead to more activation in areas of the brain typically associated with motor planning and execution, such as the precentral gyrus [Majdandžić et al., 2007; Rizzolatti et al., 1988], these findings do not fully explain why performing our working memory task in a competitive environment produced increased activation in areas associated with task disengagement. However, future research incorporating similar goal types across conditions would be useful to examine this perspective more closely.
In our study, we only included female participants who believed they were competing against females. This selection criterion was implemented because previous studies have found that gender may have an effect on competitive performance [Eder, 1995; Gneezy et al., 2009; Herlitz et al., 1997; Niederle and Vesterlund, 2011; Sutter and Rützler, 2010; Voyer et al., 2007]. Furthermore, we conducted a previous study that found significant differences between the performance of participants who believed they were competing against males and the performance of participants who thought they were competing against females [DiMenichi and Tricomi, 2015]. However, our results may be difficult to generalize to other competitive settings involving males and/or inter-gender interactions.
Unexpectedly, our task did not elicit significant differences across conditions in classic reward regions, such as the striatum. This finding was surprising, especially since most participants reported preferring the competition condition over the no competition condition, and since acts of social comparison can affect activity in the ventral striatum [Bault et al., 2011; Dvash et al., 2010; Fliessbach et al., 2007]. However, this finding (or lack thereof) could have occurred for a variety of reasons. Firstly, most reward tasks that elicit significant activation in the striatum incorporate feedback in the form of an absolute reward (e.g., “correct”) or absolute loss (e.g., “incorrect”) [Delgado et al., 2000; Tricomi et al., 2006; Tricomi et al., 2004]. However, our task offered performance feedback in a parametric format (e.g., 2 shapes out of 3). Secondly, although participants were given immediate feedback about their performance, participants were not told whether or not they beat the competitor until the end of each run; therefore, striatal response at the time of feedback for the individual trials may have been reduced. Alternatively, because the striatum has been found to be associated with both feedback-based and social learning [Hackel et al., 2015], the striatum could have been equally involved across both conditions of the task, therefore failing to produce significant differences across conditions. Furthermore, although participants reported preferring the competition condition over the no competition condition, participants could have subjectively valued winning across conditions equally, and thus activation was not significantly different across conditions. On the other hand, literature from counterfactual thinking suggests that one's subjective experience after making a choice that results in loss in a private setting—i.e., regret—differs significantly from one's subjective experience after making a choice that results in loss in a social comparison setting—i.e., envy (see Coricelli and Rustichini [2010] for a comprehensive review). Therefore, perhaps differences in counterfactual thinking across conditions could have affected the saliency of rewards/losses, resulting in a lack of significant activation differences across conditions in the striatum.
Although we found significantly greater activation in the mentalizing network for the competition condition during the feedback portion of the task, we did not observe any significant differences in activation across conditions during the working memory portion of the task. Furthermore, PCC/precuneus activation at feedback during the competition condition was significantly negatively correlated with later memory for those shapes, while there was no significant relationship between PCC/precuneus activation during the working memory portion of the task and later memory performance. Perhaps the relationship between increased activation in the PCC/precuneus at feedback while competing and poorer memory recall represents a disruption of consolidation rather than a disruption at initial encoding, where one might observe a difference in activation during the working memory phase of our task. Further research would be needed to clarify this issue.
There may also be other factors that mediate the relationship between competition and working memory, such as stress. For example, acute stress can be harmful to performance in tasks requiring working memory [Beilock, 2008; Kirschbaum et al., 1996] Therefore, perhaps performing our memory task in a competitive environment was more likely to induce stress, therefore resulting in decrements in performance. Although examining the effect of stress as a mediator between our observed relationships between competition, performance, memory, and brain activity was beyond the scope of the current study, future research could include physiological measures of stress, such as cortisol measurements, during competitive performance and learning.
Conclusions
Broadly speaking, our findings suggest that adding a competitor during memory acquisition may be distracting in certain short- and long-term memory contexts, and the effects of this distraction on performance are predicted by activation in the mPFC and the precuneus/PCC, areas typically believed to be a part of the “mentalizing network”—a network that typically shows increases in activation when an individual is mentalizing about another person. Furthermore, increased activation in the mPFC and precuneus/PCC may be related to decreased working memory performance, and increased task-distracting self-reference thoughts caused by competition. Moreover, individual differences in differential PCC/precuneus activation may reflect the degree of distraction caused by competition, leading to decrements in later recall. Our findings have implications for understanding why educational strategies aimed at harnessing competition to improve performance may sometimes be counterproductive.
ACKNOWLEDGMENTS
The authors thank Christina Bejjani, Ahmet Ceceli, Samantha DePasque, David Amodio, and David V. Smith for their helpful comments, as well as Holly Sullivan Toole, Rebecca Williams, Onaisa Rizki, Kiranmayee Kurimella, and Stuti Prajapati with their help as confederates.