Development of hippocampal functional connectivity during childhood
Abstract
The hippocampus is a medial temporal lobe structure involved in memory, spatial navigation, and regulation of stress responses, making it a structure critical to daily functioning. However, little is known about the functional development of the hippocampus during childhood due to methodological challenges of acquiring neuroimaging data in young participants. This is a critical gap given evidence that hippocampally-mediated behaviors (e.g., episodic memory) undergo rapid and important changes during childhood. To address this gap, the present investigation collected resting-state fMRI scans in 97, 4- to 10-year-old children. Whole brain seed-based analyses of anterior, posterior, and whole hippocampal connectivity were performed to identify regions demonstrating stable (i.e., age-controlled) connectivity profiles as well as age-related differences in connectivity. Results reveal that the hippocampus is a highly connected structure of the brain and that most of the major components of the adult network are evident during childhood, including both unique and overlapping connectivity between anterior and posterior regions. Despite widespread age-controlled connectivity, the strength of hippocampal connectivity with regions of lateral temporal lobes and the anterior cingulate increased throughout the studied age range. These findings have implications for future investigations of the development of hippocampally-mediated behaviors and methodological applications for the appropriateness of whole versus segmented hippocampal seeds in connectivity analyses. Hum Brain Mapp 38:182–201, 2017. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
The hippocampus is a medial temporal lobe structure important for a number of critical cognitive processes including, but not limited to, episodic memory, stress regulation, and spatial navigation [Eichenbaum and Cohen, 2014; Jacobson and Sapolsky, 1991]. As such, investigations of the hippocampus and its development are important as they may provide unique insights into how neural substrates support cognition, affect, and behavior across the lifespan. For example, understanding the association between the maturation of neural circuitry and developmental changes in cognitive capacities can inform our understanding of how stimuli are represented and processed in the brain [Casey et al., 2005; Nelson et al., 2006]. Additionally, characterizing typical development allows for improvements in the ability to systematically predict, identify, and treat aberrant neural architectures early in life when their impact may have the greatest effects. Despite the broad applications for the investigation of hippocampal development, this area has received little exploration in humans.
To date, most studies investigating hippocampal development during childhood have assessed structural maturation, that is, changes in volume or morphometry. Many studies report ongoing volumetric changes in the hippocampus throughout childhood [Østby et al., 2009; Uematsu et al., 2012] and into adulthood [DeMaster et al., 2014; Giedd et al., 1996; Hu et al., 2013; Wierenga et al., 2014; Yang et al., 2013]. Although there are some inconsistencies regarding the nature and timing of hippocampal structural development across the lifespan, converging evidence demonstrates structural maturation is at least evident throughout childhood. These developmental changes are not homogeneous across the structure, as studies have shown that subregions (i.e., head, body, tail) of the hippocampus undergo differential developmental trajectories [Gogtay et al., 2006; Lin et al., 2013], which may be attributed to the distribution and development of subfields (CA1-4, dentate gyrus) along the longitudinal axis [Lavenex and Banta Lavenex, 2013]. Given the complex reciprocal relations between biological structure and function, ongoing structural changes in the size and shape of the hippocampus may be paralleled by functional changes. However, data that speak directly to functional changes during development are sparse.
The extant literature examining functional development of the hippocampus has predominantly focused on task-based activations [Chiu et al., 2006; Ghetti et al., 2010; Güler and Thomas, 2013; Ofen et al., 2007, 2012; Paz-Alonso et al., 2008, 2013; Qin et al., 2014]. Using task-based fMRI designs, researchers have demonstrated that developmental changes in hippocampal activation [Ghetti et al., 2010] and in the coordinated activity between the hippocampus and other cortical regions (i.e., connectivity) are linked to the emergence of mature episodic memory abilities and other cognitive abilities [Ofen et al., 2012; Qin et al., 2014; for review, see Ghetti and Bunge, 2012]. Mirroring structural development during childhood, there is evidence from adults that hippocampal subregions are functionally distinct [Poppenk et al., 2013; Poppenk and Moscovitch, 2011] and that these subregions show qualitative changes in task-elicited functions during development [DeMaster et al., 2013, 2014].
Although task-based fMRI investigations are effective for studying the neural correlates of specific hippocampally-mediated processes (e.g., memory), they are limited by the nature of the experimental design (e.g., visual vs. auditory stimuli), task difficulty, and the cognitive process being studied (e.g., memory vs. spatial awareness). Thus, these studies alone do not provide sufficient evidence regarding the development of the full hippocampal network, limiting the extent to which conclusions can be drawn about functional development of the hippocampus more broadly. One method for overcoming these limitations is the use of task-independent or resting-state functional connectivity MRI (hereafter rs-fcMRI). Rs-fcMRI, first identified by Biswal et al. [1995], measures spontaneous low-frequency oscillations of brain activity while an individual lies passively in the scanner. Correlations in these low frequency oscillations are thought to be indicative of a history of co-activation, where regions demonstrating temporal coherence at rest are proposed to be the same regions that show coordinated activity during a task [Biswal et al., 1995; Power et al., 2014a, 2014b]. Therefore, rs-fcMRI permits the investigation of complex brain networks unconstrained by experimental paradigms. Moreover, given the lack of cognitive demands during scanning, rs-fcMRI makes these networks identifiable in populations who may find task demands too challenging (e.g., children, clinical patients, and older adults) [Power et al., 2010; Uddin et al., 2010a, 2010b; Vanderwal et al., 2013].
Rs-fcMRI has been successful in characterizing mature hippocampal memory networks in adults. Vincent et al. [2006] evaluated voxelwise whole-brain hippocampal connectivity using an anterior hippocampal seed to identify parietal regions uniquely connected to the hippocampal memory network and the visuo-spatial integration network and documented, across four independent datasets (total N = 47), a hippocampal network including medial prefrontal, posterior cingulate, and bilateral posterior parietal cortices. Others have also supported these findings and further identified hippocampal connectivity with the cerebellum, temporopolar cortex, lateral temporal cortex, striatum, anterior cingulate, angular gyrus, precentral gyrus, middle prefrontal gyrus, and superior frontal gyrus [Poppenk and Moscovitch, 2011; Uddin et al., 2010a, 2010b; Witte et al., 2014; Zhou et al., 2008]. Critically, on-going age-related changes in hippocampal network connectivity has been associated with individual differences in memory ability in aging adults [Salami et al., 2014]. Finally, there is evidence from adults that anterior and posterior segments of the hippocampus show differential functional connectivity throughout the cortex [Poppenk and Moscovitch, 2011]. Despite our knowledge of hippocampal connectivity in adults, little is known about the developmental changes that occur to reach this “mature” state.
Although no study to date has examined the hippocampal resting-state network in children, previous research has used rs-fcMRI to analyze other network architectures during childhood. Pediatric investigations of resting-state connectivity have recently been used to predict performance differences on behavioral tasks [Barber et al., 2013; Langeslag et al., 2013; Zhong et al., 2014], identify aberrant connectivity between patient populations [Alexander-Bloch et al., 2010; Lynch et al., 2013; Yu-Feng et al., 2007], determine general principles of network development [Fair et al., 2009; Supekar et al., 2009], and elucidate age-related differences in connectivity from subcortical regions [i.e., amgdala; Gabard-Durnam et al., 2014].
These studies highlight the usefulness of rs-fcMRI as a technique to examine functional development of structures, such as the hippocampus, where large gaps remain in our understanding. First, there is minimal research characterizing hippocampal networks in pediatric populations, leaving it unknown whether the network is similar or different than the adult network. Second, no study to date has investigated the early development of these hippocampal networks, especially during early to middle childhood (4–8 years). In fact, few resting-state studies [e.g., de Bie et al., 2012; Gabard-Durnam et al., 2014; Smyser et al., 2010] have examined network properties in awake, non-sedated children under 7 years [for review of resting-state studies in sleeping children, see Graham et al., 2015]. These are significant gaps in the literature given known changes in hippocampal structure [e.g., DeMaster et al., 2014], the rapid development of hippocampally-mediated behaviors during childhood [Bauer and Fivush, 2013; Riggins, 2014; Sluzenski et al., 2006], and evidence of ongoing processes of neural reorganization throughout adolescence and adulthood [Fair et al., 2009; Supekar et al., 2009; Purves and Lichtman, 1985] that has been proposed to influence episodic memory during childhood [Riggins et al., 2015, 2016]. Failure to understand the normal developmental trajectory of the hippocampus and its connections limits our ability to understand the mechanisms driving individual differences and age-related improvements in hippocampally-mediated cognitive and affective capacities and disorders.
The current investigation examined whole-brain seed-based hippocampal connectivity in a sample of 97 4- to 10-year-old children. We sought to identify regions demonstrating stable connectivity profiles (i.e., age-constant connectivity) as well as age-related differences in connectivity. Lastly, given evidence of functional and structural distinctions along the longitudinal axis (i.e., anterior and posterior regions in humans, ventral and dorsal segments in rodents) [DeMaster et al., 2014; Evensmoen et al., 2013; Fanselow and Dong, 2010; Kahn et al., 2008; Poppenk et al., 2013; Poppenk and Moscovitch, 2011; Strange et al., 2014; Zeidman et al., 2014], we also investigated unique connectivity of anterior and posterior regions of the hippocampus.
METHODS
Participants
Participants in the current study were drawn from three investigations of functional and structural brain development during childhood (ages 4–10 years). Inclusionary criteria were as follows: no MR contraindications and no history of developmental disorders or previous brain injury. All studies were approved by the University of Maryland Institutional Review Board. Parents provided informed consent and children over 7 years provided written assent to participate. Participants included in the present report were selected from a larger sample (N = 187) based on: no movement exceeding 3 mm or degrees from the previous volume, no reports of sleeping during the functional scan, greater than 5 minutes of usable scan data after censoring (see below), and no gross structural abnormalities. If an individual participated in more than one study, the scan with the least amount of motion was included (n = 5). When motion was comparable, scans occurring at under-represented ages were included (n = 1). As seen in Figure 1, this resulted in a sample of 97 children (M = 6.68 years, SD = 1.42, range = 4.02–10.81 years; 58.8% female; 48.5% White, 23.7% African American/Black, 12.4% Multi-Racial, 3.1% Other, and 12.4% did not report; 11.3% identified as Hispanic/Latino with 14.4% choosing not to disclose; total household income ranged from <$20,000 to >$100,000 per year). Three participants included in the final sample were born premature at 27, 33, and 36 weeks1. Handedness data were available for 93 individuals, only 13 of which reported being left-handed2. Participants in one contributing study (n = 43) were recruited for an investigation of the effects of maternal depression on childhood development; of the 43 children, 27 were offspring of depressed parents3. Because history of parental depression was not screened for and thus not able to be controlled for in the other contributing studies, these participants were included in the present analyses.
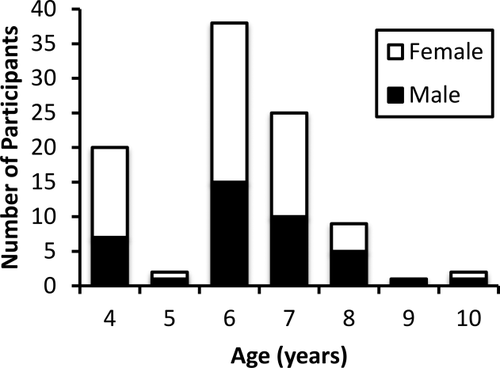
Age and gender distribution of final participant sample (n = 97).
Data Acquisition
All participants completed a 30–60 minute mock scanner training immediately before MR data acquisition in order to become acclimated to the scanner environment and receive motion feedback. Participants were scanned in a Siemens 3.0-T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions, Erlangen, Germany) using a 12-channel coil. Before resting-state data collection, participants watched approximately 2–10 minutes of an animated film of their choice or a slideshow of still color photographs of animals in order to minimize any potential anxiety during scan set-up. All participants completed a 6-minute resting-state scan where they viewed the same video of abstract shapes (similar to a screen saver). In adults, this method does not elicit significant differences in hippocampal connectivity in comparison to a standard fixation resting-state scan, providing preliminary evidence that a non-canonical resting-state scan may be used to tap the hippocampal network without eliciting significantly altered connectivity [see Supporting Information; Greicius et al., 2003; Riggins et al., 2016; Vanderwal et al., 2015]. Functional data were collected with the following scan parameters: 180 EPI volumes consisting of 36 oblique interleaved slices with a 3.0 × 3.0 × 3.0 mm voxel size; 2 s TR; 24 ms TE; 3 mm slice thickness; 90° flip angle; 64 × 64 pixel matrix. Structural data were collected using a high-resolution T1 magnetization-prepared rapid gradient-echo (MPRAGE) sequence consisting of 176 contiguous sagittal slices (1.0 × 1.0 × 1.0 mm voxel dimensions; 1,900 ms TR; 2.52 ms TE; 900 ms inversion time; 9° flip angle; pixel matrix = 256 × 256).
Pre-Processing
Functional data were slice time corrected in the Analysis of Functional Neuroimages (AFNI) software package [Cox, 1996], aligned to the first volume using rigid-body motion correction using Advanced Normalization Tools (ANTs, http://stnava.github.io/ANTs/), coregistered with the skull-stripped anatomical (SPM8; Wellcome Trust Centre for Neuroimaging, London, United Kingdom), and bandpass filtered at 0.009 < f < 0.08. Timepoints where the Euclidean distance of the derivative from the six motion parameters exceeded 1 mm were excluded, along with the previous volume, using censor files. Given convention that resting-state networks can be identified with 5 minutes of useable resting data [Power et al., 2012], participants who had less than 5 minutes of useable resting data (n = 8) after censoring were excluded from analyses (M = 356.56 seconds, SD = 6.85 seconds, 314–360 seconds; average number of volumes censored = 1.72). Nuisance regression included 18 regressors: five CSF and WM timeseries (left/right lateral ventricle, left/right hemisphere white matter, corpus callosum)4, six motion parameters and their six temporal derivatives, as well as baseline, linear, quadratic, and cubic drift. Average hippocampal timeseries were extracted from the nuisance-regressed and filtered data in native space (see below). Data were normalized with a nonlinear transformation algorithm (ANTs) to a 4.5- to 8.5-year-old symmetrical MNI Child Template [Fonov et al., 2011], selected to minimize age-related differences in image registration, then smoothed using a 6mm Gaussian kernel within a whole-brain mask. Whole brain connectivity analyses were run using 3dDeconvolve. The resulting R2 values were converted to Pearson's r and then to z-scores using a Fisher's r-to-z transformation. Individual subjects' z-scored connectivity maps were entered into the group analysis. To control for multiple comparisons, we generated 10,000 Monte Carlo simulations using AFNI's 3dClustSim with an uncorrected voxel-wise threshold of P = 0.001, resulting in cluster extent k > 25 for Pcorrected < 0.05.
Mask Generation
To ensure precise extraction of hippocampal and nuisance timeseries, individual native-space masks were generated from each participant's T1-weighted anatomical scan using an automatic segmentation procedure in Freesurfer (surfer.nmr.mgh.harvard.edu). Resulting segmentations were visually inspected5, aligned with the structural and functional data, resampled to functional resolution, and converted to binary masks. Only resampled voxels which resulted in 100%, 80%, 90%, or 50% inclusion were retained in final masks for bilateral white matter, bilateral lateral ventricles, bilateral hippocampi, and corpus callosum, respectively. Each subject's bilateral hippocampal mask was split into anterior and posterior segments by identifying the last coronal slice that the uncal apex was visible, a standard anatomical landmark [Poppenk and Moscovitch, 2011; Weiss et al., 2005]. Final masks were visually inspected to ensure anatomical precision.
Motion
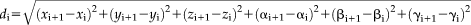
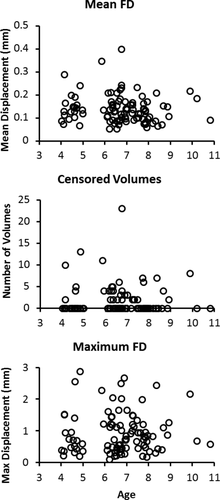
Motion parameters by age. Age (in years) plotted against mean FD, number of censored volumes, and maximum movement to illustrate correlations between age and motion were not a confound in the present analyses.
Despite stringent motion inclusion criteria for such a young, movement-prone sample, our motion inclusion criteria are liberal by standards in the adult literature. Therefore, to identify regions which may have a greater likelihood of displaying false-positives in our primary analyses, we performed follow-up t-tests assessing differences in anterior, posterior, and whole hippocampal connectivity between median-split high and low motion groups (low motion: M = 0.09, SD = 0.02, range = 0.05–0.13; high motion: M = 0.18, SD = 0.06, range = 0.13–0.40; Supporting Information Tables 5–8). The output of these analyses was assessed for overlap with all significant regions reported below.
Age Controlled Analysis
In order to identify regions of hippocampal connectivity that were relatively stable throughout the age range being investigated, we ran an ANCOVA controlling for mean absolute displacement and age (in months), testing against 0, using the 3dttest++ function in AFNI.
Age Dependent Analysis
To identify regions where hippocampal connectivity differed linearly with age, we ran an ANCOVA using the AFNI function 3dttest++ with age (in months) as the predictor, controlling for mean absolute displacement. Age was significantly correlated with whole bilateral hippocampal connectivity with global signal (r = 0.223, P = 0.028), but not white matter (r = −0.056, P = 0.584) or CSF (r = 0.041, P = 0.695) (Fig. 3). Mean FD was not significantly correlated with whole bilateral hippocampal connectivity with global signal (r = 0.101, P = 0.326), white matter (r = 0.018, P = 0.858) or CSF (r = −0.105, P = 0.311) (Fig. 3). Together, this suggests that observed age-related differences in hippocampal connectivity may be driven by meaningful (i.e., non-nuisance) changes in global brain activity. One possibility is that age-related increases in hippocampal-global signal connectivity may reflect ongoing changes in the hippocampus' integration with large-scale brain networks. To explore this possibility and determine how age-related changes in hippocampal connectivity may be associated with developmental changes in the involvement of the hippocampus in global brain networks, separate exploratory whole-brain connectivity analyses were conducted with regions of age-related increases in hippocampal connectivity as seed regions of interest.
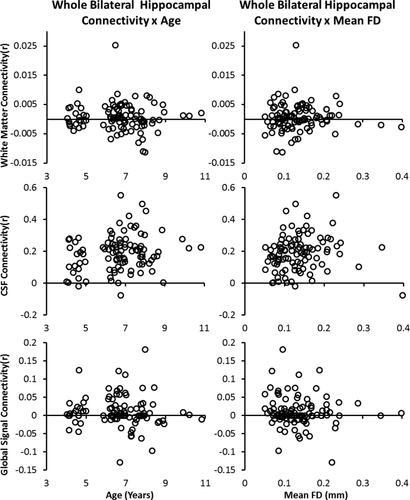
Correlations between whole bilateral hippocampal connectivity with nuisance signals (i.e., white matter, CSF, and global signal) as a function of age and mean framewise displacement.
Hippocampal Subregion Analysis
Given evidence that the hippocampus is a functionally heterogeneous structure [Poppenk et al., 2013; Poppenk and Moscovitch, 2011; Strange et al., 2014], and evidence that subregions show developmental change with age [DeMaster et al., 2013, 2014; Gogtay et al., 2006], we supplemented our bilateral whole hippocampal seed analyses with age-dependent and age-controlled analyses of anterior and posterior hippocampal connectivity, as described above (sections “Age controlled analysis” and “Age dependent analysis”). We employed two complementary methods to qualitatively and quantitatively assess patterns of anterior and posterior hippocampal connectivity [see Gabard-Durnam et al., 2014 for a similar approach]. The first approach, a masking technique, was employed to qualitatively highlight regions that differed in connectivity between anterior and posterior subregions in the primary analyses described above (sections “Age controlled analysis” and “Age dependent analysis”). This method was conducted by masking the thresholded (at P < 10−15 for age-controlled, and P < 0.05 for age-dependent) results of the analyses of separate anterior and posterior connectivity. Regions of map overlap (i.e., regions of connectivity with anterior and posterior connectivity) were removed to highlight regions of overlapping or unique anterior and posterior connectivity. The second approach employed a paired-samples t-test that tested for regions of statistically different anterior versus posterior connectivity. Together these methods provide complementary indices of regionally-specific hippocampal connectivity: the masking method provides a qualitative summary of regions with statistically significant anterior or posterior connectivity without making claims regarding whether or not a region is more highly connected to one subregion or the other whereas the statistical approach provides a direct quantitative comparison to test for regions with statistically different anterior versus posterior connectivity.
RESULTS
Age-controlled analyses for whole hippocampus, anterior, and posterior regions, are presented first, followed by age-dependent analyses.
Age-Controlled Analyses
Whole bilateral hippocampus
At a cluster-corrected threshold of Pcorrected < 0.05, most cortical gray matter was positively correlated with the hippocampal seed, with highest correlations occurring in bilateral hippocampi (k = 59,737). To explore these results in greater depth, the voxel-wise threshold was reduced until regions of the largest cluster segregated into distinct regions (to P < 10−15), which largely resembled the hippocampal–parietal memory network identified in adults at rest by Vincent et al, [2006]. This hippocampal network included, but was not limited to (see Table 1 for entire list of included regions), regions of medial prefrontal cortex (mPFC) extending caudally through the cingulate cortex, angular gyrus extending anteriorly into supramarginal gyrus and through the temporal pole, the precuneus, and left dorsolateral prefrontal cortex (Fig. 4). Additional regions include bilateral precentral gyrus, midline subcortical structures, bilateral cerebellum, bilateral insula, and bilateral orbital cortex. There were no regions of significant negative hippocampal connectivity.
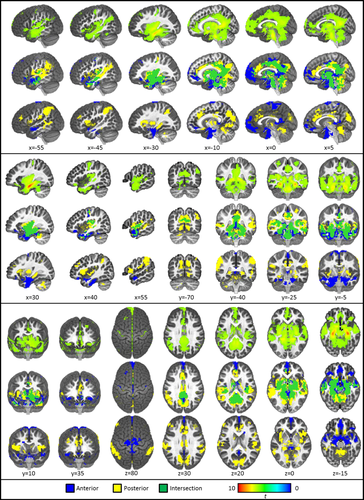
Results of age-constant connectivity analyses. The first line of each panel depicts the results of the age-controlled analysis with the whole hippocampus. The second line of each panel depicts masks of unique and intersecting (green) regions of connectivity with anterior (blue) and posterior (yellow) hippocampal seeds thresholded at Pcorrected < 10 − 15 (i.e., the results of the masking technique). The third row of each panel depicts the results of the quantitative anterior versus posterior analysis revealing regions of significantly greater anterior (blue) or posterior (yellow) connectivity thresholded at Pcorrected < 0.05. Sagittal views are depicted from the left slicing toward the right; Coronal views are depicted with the left hemisphere on the left; Axial views provide an aerial view of the brain with left hemisphere depicted on the right. [Color figure can be viewed at wileyonlinelibrary.com.]
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| Right Hippocampus | 14,563 | 26 | −14 | −21 | 12.61 |
| Bilateral Superior Medial Gyrus | |||||
| Bilateral Amygdala | |||||
| Bilateral Anterior Cingular Cortex | |||||
| Bilateral Caudate | |||||
| Bilateral Cerebellum | |||||
| Bilateral Fusiform Gyrus | |||||
| Bilateral Inferior Frontal Gyrus | |||||
| Bilateral Lingual Gyrus | |||||
| Bilateral Medial Frontal Gyrus | |||||
| Bilateral Middle Cingulate Cortex | |||||
| Bilateral Middle Temporal Gyrus | |||||
| Bilateral Parahippocampal Gyrus | |||||
| Bilateral Posterior Cingulate Cortex | |||||
| Bilateral Putamen | |||||
| Bilateral Rolandic Operculum | |||||
| Bilateral Superior Orbital Gyrus | |||||
| Bilateral Superior Temporal Gyrus | |||||
| Bilateral Temporal Pole | |||||
| Bilateral Thalamus | |||||
| Left Angular Gyrus | |||||
| Pons | |||||
| Left Precentral Gyrus | 136 | −43 | −17 | 60 | 10.6 |
| Right Precentral Gyrus | 134 | 41 | −14 | 54 | 10.44 |
| Left Middle Frontal Gyrus | 56 | −25 | 28 | 45 | 10.52 |
| Right Middle Temporal Gyrus | 32 | 50 | −56 | 21 | 10.36 |
| Left Precentral Gyrus | 26 | −58 | 1 | 21 | 10.23 |
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| Right Hippocampus | 9,553 | 26 | −14 | −21 | 12.63 |
| Bilateral Posterior Cingulate | |||||
| Bilateral Caudate | |||||
| Bilateral Cerebellum | |||||
| Bilateral Fusiform Gyrus | |||||
| Bilateral Lingual Gyrus | |||||
| Bilateral Midbrain | |||||
| Bilateral Middle Temporal Gyrus | |||||
| Bilateral Parahippocampal Gyrus | |||||
| Bilateral Putamen | |||||
| Bilateral Superior Temporal Gyrus | |||||
| Bilateral Thalamus | |||||
| Left Inferior Frontal Gyrus (p. Orbitalis) | |||||
| Pons | |||||
| Left Mid Orbital Gyrus | 651 | −10 | 37 | −12 | 10.76 |
| Right Mid Orbital Gyrus | |||||
| Bilateral Anterior Cingulate Cortex | |||||
| Bilateral Superior Medial Gyrus | |||||
| Right Inferior Frontal Gyrus (p. Orbitalis) | 67 | 38 | 34 | −15 | 10.9 |
| Left Precentral Gyrus | 66 | −43 | −17 | 60 | 10.52 |
| Right Precentral Gyrus | 45 | 41 | −14 | 54 | 10.22 |
| Left Middle Frontal Gyrus | 30 | −25 | 28 | 45 | 10.27 |
| Right Middle Temporal Gyrus | 23 | 44 | −41 | 3 | 10.43 |
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| Right Hippocampus | 12,661 | 29 | −26 | −12 | 12.06 |
| Bilateral Anterior Cingulate Cotex | |||||
| Bilateral Amygala | |||||
| Bilateral Angular Gyrus | |||||
| Bilateral Caudate | |||||
| Bilateral Cuneus | |||||
| Bilateral Fusiform Gyrus | |||||
| Bilateral Inferior Temporal Gyrus | |||||
| Bilateral Insular Cortex | |||||
| Bilateral Lingual Gyrus | |||||
| Bilateral Middle Cingulate Cortex | |||||
| Bilateral Middle Temporal Gyrus | |||||
| Bilateral Olfactory Cortex | |||||
| Bilateral Parahippocampal Gyrus | |||||
| Bilateral Posterior Cingulate Cortex | |||||
| Bilateral Precuneus | |||||
| Bilateral Putamen | |||||
| Bilateral Superior Temporal Gyrus | |||||
| Bilateral SupraMarginal Gyrus | |||||
| Bilateral Thalamus | |||||
| Cerebellum | |||||
| Midbrain | |||||
| Pons | |||||
| Left Precentral Gyrus | 69 | −34 | −20 | 48 | 10.32 |
| Right Precentral Gyrus | 52 | 35 | −20 | 48 | 10.16 |
| Right Angular Gyrus | 31 | 53 | −62 | 24 | 10.31 |
| Left Inferior Frontal Gyrus | 28 | −49 | 19 | −6 | 10.27 |
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| Anterior > Posterior | |||||
| Right Anterior Hippocampus | 1,602 | 23 | −14 | −21 | 5.62 |
| Left Anterior Hippocampus | |||||
| Bilateral Amygdala | |||||
| Bilateral Parahippocampal Gyrus | |||||
| Bilateral Middle Temporal Gyrus | |||||
| Bilateral Olfactory Cortex | |||||
| Bilateral Fusiform Gyrus | |||||
| Left Mid Orbital Gyrus | 291 | −1 | 58 | −12 | 4.21 |
| Right Mid Orbital Gyrus | |||||
| Bilateral Rectal Gyrus | |||||
| Right Posterior Cingulate Cortex | 113 | 5 | −35 | 3 | 3.99 |
| Left Posterior Cingulate Cortex | |||||
| Left Paracentral Lobule | 111 | −1 | −29 | 60 | 4.01 |
| Right Precentral Gyrus | 85 | 14 | −17 | 78 | 4.17 |
| Left Paracentral Lobule | 58 | −10 | −14 | 78 | 4.43 |
| Right Inferior Frontal Gyrus (p. Orbitalis) | 38 | 44 | 34 | −18 | 4.19 |
| White Matter | 23 | 20 | 10 | 24 | 3.99 |
| Posterior > Anterior | |||||
| Right Cuneus | 1631 | 14 | −64 | 39 | −4.21 |
| Left Cuneus | |||||
| Bilateral Lingual Gyrus | |||||
| Bilateral Cerebellum | |||||
| Bilateral Precuneus | |||||
| Left Inferior Parietal Lobule | 736 | −55 | −44 | 48 | −4.5 |
| Left SupraMarginal Gyrus | |||||
| Left Angular Gyrus | |||||
| Right Inferior Frontal Gyrus | 635 | 47 | 10 | 6 | −4.2 |
| Right Insula | |||||
| Right Putamen | |||||
| Left Posterior Hippocampus | 627 | −25 | −35 | −3 | −4.5 |
| Left Putamen | |||||
| Bilateral Thalamus | |||||
| Left Insula | |||||
| Left Inferior Frontal Gyrus | |||||
| Right SupraMarginal Gyrus | 453 | 59 | −41 | 42 | −4.2 |
| Right Inferior Parietal Lobule | |||||
| Right Superior Temporal Gyrus | |||||
| Right Anterior Cingulate Cortex | 184 | 5 | 34 | 15 | −3.89 |
| Left Middle Temporal Gyrus | 139 | −58 | −53 | 3 | −3.83 |
| Left Superior Medial Cortex | 137 | −7 | 34 | 30 | −3.88 |
| Left Anterior Cingulate Cortex | |||||
| Right Posterior Hippocampus | 108 | 29 | −32 | −9 | −6.25 |
| Left Middle Cingulate Cortex | 93 | −7 | −17 | 27 | −4.16 |
| Right Middle Cingulate Cortex | 80 | 8 | −32 | 42 | −4.17 |
| Right Straight Gyrus | 54 | 14 | 19 | −15 | −4.2 |
| Right Inferior Frontal Gyrus | 51 | 38 | 34 | 27 | −3.72 |
| Left Straight Gyrus | 39 | −16 | 25 | −15 | −4.44 |
| Left Middle Frontal Gyrus | 36 | −52 | 40 | 21 | −3.81 |
| Left Caudate Nucleus | 23 | −13 | 1 | 15 | −3.83 |
Anterior bilateral hippocampus
At the reduced voxelwise threshold (P < 10−15) an anterior hippocampal network emerged, similar to previous reports in adults [e.g., Poppenk and Moscovitch, 2011; Vincent et al., 2006] and the whole hippocampal seed described above, including mPFC, bilateral angular gyri, precuneus, anterior and posterior cingulate, bilateral orbital cortex, bilateral temporal poles, midline subcortical structures, and cerebellum. (Table 2; Fig. 4). No clusters of anterior connectivity (k > 10) were absent from the whole hippocampal map, suggesting that regions of whole hippocampal seed connectivity may be largely driven by anterior subfields. No regions demonstrated significant negative connectivity.
Posterior bilateral hippocampus
At the reduced voxelwise threshold, regions demonstrating age-controlled connectivity with the posterior hippocampus included: cingulate cortex, bilateral precentral gyri, angular gyrus extending into supramarginal gyrus, and down through the temporal pole, and cuneus (Table 3; Fig. 4), all regions previously reported in adults [Poppenk et al., 2013; Poppenk and Moscovitch, 2011]. The posterior hippocampus demonstrated connectivity that was not present in analyses with the whole hippocampal seed, including connectivity with bilateral lingual gyrus, a large cluster centered in the left inferior parietal lobe and extending into the superior and middle temporal gyri, bilateral precuneus and superior cuneus, right cerebellum, and isolated regions of anterior and mid-cingulate. There were no regions of significant negative connectivity.
Unique age-controlled connectivity between anterior and posterior seeds
Whereas most regions of age-controlled connectivity overlapped between the anterior and posterior seeds, a number of regions showed unique connectivity with each subregion (Fig. 3B). As described above, two methods were used to characterize regions of unique anterior versus posterior connectivity. Using the masking approach, regions of unique anterior hippocampal connectivity included: ventromedial prefrontal cortex, medial prefrontal cortex, bilateral orbital cortex, left dorsolateral prefrontal cortex, a large cluster extending from inferior temporal gyrus through fusiform gyrus and down through the temporal pole, posterior cingulate, a region of precuneus, (Fig. 4). Regions of unique posterior hippocampal connectivity included a large posterior region extending from precuneus through cuneus, lingual gyrus, and down through the cerebellum, a large temporoparietal region extending from through left inferior parietal lobule, angular gyrus, and supramarginal gyrus down through superior and middle temporal gyri, right middle temporal gyrus, isolated regions of anterior, middle, and posterior cingulate cortex, bilateral regions of superior insular cortex, and regions of cerebellum (Table 4; Fig. 4).
Statistically comparing patterns of anterior and posterior connectivity revealed more localized effects than the masking approach. Regions demonstrating statistically significant greater anterior (vs. posterior) connectivity included bilateral anterior hippocampus extending rostrally to the amygdala and laterally through anterior middle temporal gyrus and fusiform gyrus, ventromedial prefrontal cortex, posterior cingulate cortex, bilateral paracentral lobule, bilateral precentral gyrus, and right orbitofrontal cortex (Table 5; Fig. 4). Regions demonstrating statistically significant greater posterior (vs. anterior) connectivity included: bilateral inferior parietal lobule extending laterally to supramarginal gyrus and angular gyrus; precuneus extending into cuneus, lingual gyrus, and cerebellum; bilateral insular cortex extending into bilateral putamen and thalamus; anterior and middle cingulate cortex; bilateral straight gyrus; bilateral dorsolateral prefrontal cortex; and left caudate (Fig. 4).
Age-Dependent Analyses
Whole bilateral hippocampus
The bilateral whole hippocampal seed showed significant age-related increases in connectivity with bilateral temporal cortex and right piriform area (Fig. 5A; Table 6). No regions demonstrated significant age-related decreases in connectivity. Regions of age-dependent connectivity with the whole hippocampal seed reflect the intersection of age-dependent connectivity in anterior and posterior seeds; therefore, follow-up investigation of the whole-brain connectivity of these seeds is included below.
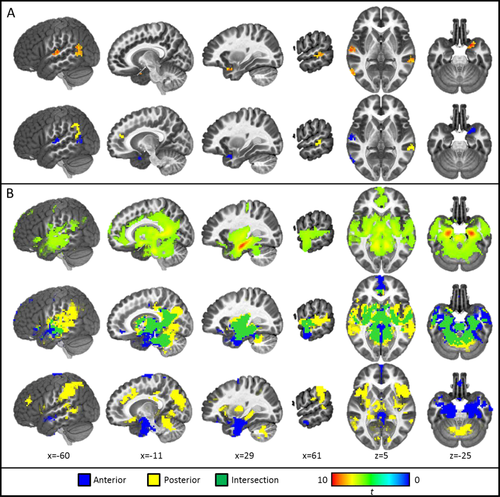
Results of age-dependent connectivity analyses (A) compared with similar views from the age-controlled analysis (B). The first row of Panel A depicts regions of age-related differences in connectivity with a whole bilateral hippocampal seed. The second row of Panel A depicts masks of unique and intersecting regions of age-related connectivity with anterior and posterior hippocampal seeds. Note: All thresholded at Pcorrected < 0.05; Sagittal views are depicted from the left slicing toward the right; Axial views are presented from an aerial perspective with left hemisphere depicted on the right. [Color figure can be viewed at wileyonlinelibrary.com.]
Anterior bilateral hippocampus
Similar to results of whole hippocampal connectivity, the anterior hippocampal seed only demonstrated age-related increases in connectivity in regions isolated to left temporal lobe and right piriform cortex (Fig. 5A; Table 7). Separate exploratory whole-brain connectivity analyses were conducted with regions of age-related increases in anterior hippocampal connectivity as seeds. Results indicated that the region of left superior temporal gyrus is part of the somatomotor network (Yeo et al., 2011; Supporting Information Fig. 2B); the regions of left middle temporal gyrus and the right piriform cortex did not clearly belong to any full network at a threshold of P < 10−15, but displayed some connectivity with regions associated with the default mode network [Raichle, 2015; Supporting Information Fig. 2A and C, respectively).
Posterior bilateral hippocampus
The posterior hippocampal seed showed age-related increases in connectivity with left angular gyrus at the temporoparietal junction, right middle temporal gyrus, and left anterior cingulate cortex (Fig. 5A; Table 8). Separate exploratory connectivity analyses were run for each of these regions to determine brain networks with which they may be associated. All three of these regions demonstrated clear connectivity with the entire extent of the default mode network, but with greatest connectivity with regions adjacent to the seeds. That is, the left angular gyrus demonstrated greatest connectivity to DMN parietal regions (Supporting Information Fig. 3A), the right middle temporal gyrus seed demonstrated greatest connectivity to DMN temporal regions (Supporting Information Fig. 3B), and the anterior cingulate region demonstrated greatest connectivity to frontal regions (Supporting Information Fig. 3C).
Unique age-related differences in connectivity between anterior and posterior seeds
The masking approach revealed no intersecting regions of age-related anterior or posterior hippocampal connectivity. However, there were also no regions of statistically significant age-related differences in connectivity between anterior and posterior subregions. Closer inspection of these results reveals that this apparent contradiction in results is due to similar age-related increases in connectivity with both anterior and posterior segments, with this age-related association only reaching statistical significance for one subregion in the individual statistical tests (i.e., sections “Anterior bilateral hippocampus” and “Posterior bilateral hippocampus”). Taken together, these results indicate considerable anterior/posterior overlap in age-related connectivity (see section “Overlapping connectivity” for discussion of the interpretation of overlapping connectivity).
High versus low motion groups
There were no regions of age-dependent hippocampal connectivity that differed between participants in the high versus low motion groups. Similarly, no regions of age-controlled anterior, whole, or anterior versus posterior connectivity overlapped (k > 15) with regions differing in hippocampal connectivity between participants in the high versus low motion groups (Supporting Information Tables 5–7). However, there were regions of posterior connectivity that differed between participants in the high and low motion groups that overlapped with the age-controlled posterior hippocampal connectivity map (see section “Posterior bilateral hippocampus”). Regions of overlap included: a large region centered in the left thalamus extending laterally into the surrounding white matter and inferiorly to the subhippocampal white matter, a region of white matter superior to the left hippocampus, a region at the junction of the left fusiform gyrus and the cerebellum, a medial region of the right posterior hippocampus, the right thalamus, and the pons (Supporting Information Fig. 4). Age-controlled posterior connectivity in these regions should be interpreted with caution as they may have a particularly high susceptibility to Type I errors induced by participant motion.
DISCUSSION
The present study investigated developmental changes in hippocampal resting-state networks during early to middle childhood (4–10 years). Results revealed that the hippocampus is a highly connected subcortical structure, showing connectivity with diffuse cortical and subcortical regions. Despite widespread connectivity during childhood, age-related increases in the magnitude of connectivity were evident in a number of regions. Moreover, overlapping and unique profiles of connectivity were evident between anterior and posterior segments of the hippocampus, providing converging evidence of functional distinctions along the longitudinal axis. These findings provide some of the first measures of the development of hippocampal functional networks in childhood. The maturation of hippocampal connectivity may signal developmental changes in the efficiency or specificity of neural processing within hippocampal networks and may influence behavioral changes throughout childhood.
Age-Controlled Analyses
Age-controlled analyses, at a conservative threshold, demonstrated a hippocampal network consistent with resting-state findings in adults, including regions of the medial prefrontal cortex, cingulate cortex, and lateral parietal cortex. This pattern of results suggests that the functional connections between the hippocampus and distributed cortical and subcortical regions are apparent early in development (i.e., at least by 4 years of age). Connectivity with the whole hippocampal seed appeared to be an additive map composed of signals generated from anterior and posterior segments. Specifically, using a masking approach, the anterior hippocampus accounted for the observed whole hippocampal connectivity with mPFC and anterolateral middle temporal gyrus, whereas posterior hippocampus accounted for the observed connectivity with the more posterior portions of the middle temporal gyrus extending through the supramarginal and angular gyri.
These effects differed in the statistical approach, which indicated that although significant connectivity may exist, neither anterior nor posterior connectivity was significantly more connected to the most posterior portions of the middle temporal gyrus through the angular gyrus. This may be attributed to a high correlation between anterior and posterior timeseries and/or the current method for selecting statistical thresholds in the masking technique. In fact, the statistical approach suggested much more localized regions of anterior versus posterior connectivity, with anterior hippocampus projecting to medial prefrontal cortex and anterior middle temporal lobes, and the posterior hippocampus projecting to middle cingulate, bilateral insular cortex, cuneus, and inferior parietal lobule. These results are largely consistent with previous reports of the anterior hippocampus projecting to more anterior regions (e.g., mPFC) and posterior hippocampus projecting to cingulate cortex and parietal regions [Poppenk et al., 2013]. However, despite these consistencies, the present investigation provides new evidence that during development the anterior hippocampus demonstrates connectivity with posterior cingulate cortex. This finding may suggest more diffuse or less segregated patterns of connectivity during childhood—an established developmental pattern [e.g., Durston et al., 2006; Fair et al., 2009; Supekar et al., 2009], which has been previously undocumented in the hippocampal network due to the limited research on this network in children.
Overlapping connectivity
Despite the fact that functional specificity is known to exist along the longitudinal axis of the hippocampus [Poppenk et al., 2013], significant overlapping anterior and posterior connectivity was observed in age-controlled analyses in regions not previously reported as overlapping in adults. It is plausible that, in childhood, anterior and posterior regions are functionally connected to overlapping regions of cortex, as similar overlap in subregion connectivity has been demonstrated in the developing amygdala [Gabard-Durnam et al., 2014] and the adult hippocampus [Poppenk et al., 2013]. In contrast, it is also possible that central portions of the hippocampus (i.e., body) may be poorly intrinsically segregated, as has been suggested by proposals by Poppenk et al. [2013] and Moser and Moser [1998], resulting in functional gradations and overlapping connectivity driven by our methodological choice of anterior/posterior seeds. Future research would be needed to decipher between these possibilities.
Age-Dependent Analyses
Although widespread hippocampal connectivity appears to be present by age 4, the strength of some connections increased in older children. Because the results using a whole hippocampal seed mirrored the unique connectivity of anterior and posterior segments, for the sake of brevity, we will focus our discussion on the results of anterior and posterior seeds.
All regions demonstrating age-related increases in hippocampal connectivity have been previously linked to cognitive processes involving the hippocampus. For example, many temporal lobe regions have been identified as relay stations that project multimodal cortical inputs to the hippocampus for rich memory encoding [Lavenex and Amaral, 2000]. Additionally, the strength of posterior hippocampal connectivity with the right middle temporal gyrus is associated with episodic memory performance during early childhood (4–6 years) [Riggins et al., 2016]. Increasing hippocampal connectivity in more posterior regions, including the temporoparietal junction, may reflect ongoing age-related improvements in a number of cognitive processes that are supported by both of these regions, such as: autobiographical memory retrieval, prospection, navigation, and theory of mind [Maguire and Frith, 2003; Spreng et al., 2009; Svoboda et al., 2007]. The piriform cortex and the peri-amygdaloid areas have known reciprocal connections with the ventral hippocampus in rodents (anterior hippocampus, in humans) [Eichenbaum et al., 1996] and have been implicated in the cognitive aspects of olfactory perception and odor memory [Bensafi, 2012]. The emergence of olfactory memory is established very early in life, with the majority of adult odor-cued memories generated from the childhood years (<10 years) [for review, see Larsson and Willander, 2009; Mouly and Sullivan, 2010]. Thus, the observed age-related increases in hippocampal-piriform connectivity may be linked to the importance of olfactory cues in autobiographical memory formation during childhood [Chu and Downes, 2000] versus adulthood (see Supporting Information). As a proposed site of long-term memory storage [Ross and Eichenbaum, 2006], the observed increase in anterior cingulate-hippocampal connectivity may play an important role in developmental improvements in long-term memory encoding, consolidation, and storage [Poppenk and Moscovitch, 2011; Ross and Eichenbaum, 2006] and may provide insight into the neural basis of well-documented changes in long-term memory performance during middle childhood [Ghetti and Bunge, 2012].
Follow-up connectivity analyses revealed which large-scale brain networks the regions of age-dependent connectivity belonged. Both anterior and posterior seeds were connected to the default mode network, with only the region of anterior connectivity to the left superior temporal gyrus being linked to the somatomotor network. Hippocampal involvement in the default mode network is contentious, with some studies reporting inclusion [James et al., 2013; Kaplan et al., 2016] and others reporting only the surrounding parahippocampal cortex as part of the network [Fair et al., 2008; Uddin et al., 2009; Ward et al., 2014]. The present results suggest that the hippocampus may be becoming increasingly connected to regions of the default mode network during childhood. Interestingly, regions of increased posterior hippocampal connectivity demonstrated more robust inclusion in the default mode network in comparison to regions of increased anterior hippocampal connectivity, a trend which has been documented in adults [Kim, 2015]. It is possible that the posterior hippocampus becomes more functionally integrated with the default mode network earlier than the anterior subregion. To the best of our knowledge, this is the first evidence to suggest hippocampal connectivity with the somatomotor network; studies in adults suggest the hippocampus, and medial temporal lobe more generally, operate in isolation of this network [e.g., Hayes, 2012; Kaplan et al., 2016]. The possibility of developmental changes in and relevance of hippocampal connectivity with these networks should be addressed by future research.
Due to a predominance of research investigating the memory functions of the hippocampus, less is known about how age-related increases in hippocampal connectivity may support the maturation of other known or suggested hippocampally-mediated behaviors [e.g., theory of mind; Spreng et al., 2009] or the functionality of the default mode and somatomotor networks. Future investigations are necessary for systematic identification of the behavioral relevance of the ongoing functional integration of the hippocampus with these distributed regions and broader large-scale networks.
Interestingly, not all regions of age-dependent increase in connectivity were evident in the age-controlled analyses. Specifically, the anterior hippocampus did not demonstrate connectivity with the right piriform cortex or the left middle temporal gyrus in either age-controlled analysis, suggesting these functional connections may emerge during the studied age range. In contrast, all regions of posterior hippocampal connectivity were evident in at least one of the age-controlled analysis, suggesting these connections may exist early in life and increase in strength with age. Together, these differing patterns of results from age-controlled and age-dependent analyses reveal the possible emergence and refinement of both anterior and posterior functional connections during childhood.
Lack of Negative Associations
The current investigation found no evidence of significant negative hippocampal connectivity or decreasing connectivity with age. Previous studies that demonstrate age-constant or age-related decreases in connectivity have used a global signal regressor [Barber et al., 2013; Fair et al., 2009; Gabard-Durnam et al., 2014; Kelly et al., 2009] or had less stringent motion control [Fair et al., 2009; Gabard-Durnam et al., 2014; Power et al., 2015; Supekar et al., 2009]. The global signal regressor is, however, a contentious tool in resting-state analyses, with clear benefits to controlling for noise [Power et al., 2015], but also widely acknowledged to induce difficult-to-interpret negative correlations [Murphy et al., 2009] and evidence that removal of the global signal eliminates meaningful functional connections [Schölvinck et al., 2010]. Additionally, it is possible that there are no developmental decreases in connectivity during the narrow age-range in the current study (4–10 years) or decreases may be more variable and therefore not easily measured in terms of age-related differences.
Limitations and Future Directions
Despite strengths in examining hippocampal connectivity in a young, unexplored age range using whole and segmented hippocampal seeds, the present investigation had several limitations. First, the present investigation used a resting-state scan in which children passively viewed abstract shapes. This method was utilized as it was devoid of any overt task yet was engaging enough to minimize motion in the young sample [see Vanderwal et al., 2015 for similar approach]. Previous studies have used a similar approach in order to obtain task-independent fMRI data in young children. For example, Emerson and Cantlon [2012] examined functional connectivity from scans during which children passively viewed an educational video on “letters, numbers, and other concepts.” Critically, we report that a non-traditional low-level visual stimulation abstract shapes screen saver did not elicit significantly different hippocampal network connectivity in comparison to a fixation in a sample of adults, providing preliminary validation for this technique in collecting resting-state data from young children. Despite no differences in the hippocampal connectivity between abstract versus fixation scans in adults, it is possible that this methodological technique (as opposed to eyes open viewing fixation or eyes closed) played a role in the present findings in children. For instance, increased attention during video viewing may have elevated hippocampal network activity, resulting in the observed whole-brain correlations or obscuring negative connectivity which may have been evident in a classic resting-state paradigm (i.e., fixation). However, the abstract shapes are not enough to explain the present results in light of the converging evidence of widespread connectivity found with the amygdala using a standard fixation [Gabard-Durnam et al., 2014], overlap of our findings with the existing rodent and adult neuroimaging literature on hippocampal networks, as well as, recent and ongoing validation of non-traditional resting-state scans [e.g., Emerson and Cantlon, 2012; Vanderwal et al., 2015].
Second, although the present investigation is the first of its kind to investigate hippocampal network development in a large sample of young children (4–10 years), the final sample includes many more children in younger age ranges (4–6 years, n = 60) than older ages (7–10 years, n = 37). This is a consequence of data compilation across three studies, each designed to examine unique research questions, but may obscure important age-related differences due to low power in the older age groups. Future investigations with wider age ranges and longitudinal designs would be beneficial to advance our current understanding of hippocampal networks.
Third, despite heeding methodological recommendations for ameliorating the effects of motion in pediatric resting-state data [see Power et al., 2012, 2014a, 2014b 2015; Satterthwaite et al., 2012; Van Dijk et al., 2012], it is possible motion contributed to the present results. For instance, at standard statistical thresholds (i.e., voxelwise threshold P < 0.005, Pcorrected < 0.05), age-controlled analyses revealed significant hippocampal connectivity with the whole brain, which may indicate inadequate control of nuisance signals induced by motion. At stricter thresholds, a hippocampal network reminiscent of that reported in adults [Vincent et al., 2006] emerged. The ability to test these effects was likely at least partially afforded by our large original sample (N = 187) which enabled exclusion based on relatively conservative motion criteria while maintaining a large sample size (n = 97). Ongoing consideration of the appropriate motion thresholds in pediatric neuroimaging is necessary.
Despite these limitations, the present study analyzed anterior, posterior, and whole hippocampal seeds which enabled the examination of regionally-specific developmental differences in hippocampal connectivity. Although the whole hippocampus seed was largely useful in tapping connectivity of its component parts, use of the whole hippocampal seed did obscure regions of unique age-related differences in connectivity. For instance, use of the whole hippocampal seed did not reveal age-related differences in connectivity with the left putamen; however, age-related differences in connectivity with the left putamen were evident when using the anterior seed. Future investigations of hippocampal connectivity should keep the functional heterogeneity of the hippocampus in mind when determining whether whole or segmented regions are more appropriate for examining the process of interest.
Finally, and critically, it is necessary for future investigations to examine the cognitive, affective, and behavioral relevance of the observed maturation of hippocampal connections. Specifically, a developmental perspective may provide insight into on-going debates regarding the nature of the neural computations carried out by anterior and posterior hippocampi [for extended discussion, see Poppenk and Moscovitch, 2011].
CONCLUSION
In sum, the present study was the first to investigate functional hippocampal networks in a young pediatric population. Results revealed that even in childhood the hippocampus is a highly connected subcortical structure that demonstrates functional distinctions along the longitudinal axis. In addition, both stable and age-related differences in connectivity were apparent throughout early to late childhood. Demonstration of both age-dependent and age-controlled changes in hippocampal connectivity are relevant to ongoing investigations of hippocampally-mediated cognitions and behaviors in health and disease.
| Region | k | x | y | z |
|---|---|---|---|---|
| Anterior | ||||
| Pons | 1,458 | 6 | −13 | −21 |
| Bilateral Temporal Pole | ||||
| Bilateral Fusiform Gyrus | ||||
| Bilateral parahippocampal gyrus | ||||
| Bilateral Amygdala | ||||
| Bilateral Thalamus | ||||
| Left Superior Medial Gyrus | 571 | −1 | 54 | 2 |
| Bilateral Rectal Gyrus | ||||
| Right Superior Medial Gyrus | ||||
| Bilateral Mid Orbital Gyrus | ||||
| Bilateral Olfactory Cortex | ||||
| Left Supplementary Motor Area | 95 | 1 | −18 | 51 |
| Right Supplementary Motor Area | ||||
| Left Inferior Frontal Gyrus (p. Orbitalis) | 84 | −40 | 30 | −13 |
| Right Inferior Frontal Gyrus (p. Orbitalis) | 67 | 41 | 32 | −14 |
| Left Precuneus | 42 | −1 | −58 | 32 |
| Right Precentral Gyrus | 21 | 40 | −15 | 42 |
| Posterior | ||||
| Right Lingual Gyrus | 2864 | 15 | −46 | 4 |
| Bilateral Posterior Cingulate Cortex | ||||
| Bilateral Cuneus | ||||
| Left Lingual Gyrus | ||||
| Bilateral Cerebellum | ||||
| Right Middle Temporal Gyrus | ||||
| Bilateral Fusiform Gyrus | ||||
| Bilateral Rolandic Operculum | ||||
| Left Superior Temporal Gyrus | 1424 | −48 | −29 | 10 |
| Left Angular Gyrus | ||||
| Left SupraMarginal Gyrus | ||||
| Left Rolandic Operculum | ||||
| Let Middle Temporal Gyrus | ||||
| Left Inferior Temporal Gyrus | ||||
| Left Insula | ||||
| Left Putamen | ||||
| Right Anterior Cingultae Cortex | 275 | −2 | 28 | 23 |
| Right Putamen | 58 | 17 | 13 | −11 |
| Left Inferior Temporal Gyrus | 45 | −41 | 7 | −37 |
| Right Postcentral Gyrus | 33 | 40 | −20 | 49 |
| Right Caudate Nucleus | 30 | 17 | −3 | 16 |
| Left Postcentral Gyrus | 30 | −32 | −25 | 53 |
| Left Thalamus | 22 | 0 | −14 | −1 |
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| Left Middle Temporal Gyrus | 56 | −64 | −59 | 18 | 3.84 |
| Right Middle Temporal Gyrus | 33 | 68 | −35 | 6 | 3.8 |
| Left Superior Temporal Gyrus | 31 | −64 | −20 | 9 | 3.84 |
| Right Piriform Cortex | 26 | 23 | 10 | −24 | 3.97 |
| Right Periamygdaloid Cortex |
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| Right Piriform Cortex | 29 | 23 | 10 | −24 | 3.96 |
| Right Periamygdaloid Cortex | |||||
| Left Superior Temporal Gyrus | 29 | −64 | −17 | 9 | 3.95 |
| Left Middle Temporal Gyrus | 21 | −58 | −65 | 9 | 3.76 |
| Region | k | x | y | z | t |
|---|---|---|---|---|---|
| Left Angular Gyrus | 47 | −61 | −59 | 42 | 3.79 |
| Left Inferior Parietal Lobe | |||||
| Left Middle Temporal Gyrus | |||||
| Right Middle Temporal Gyrus | 34 | 68 | −35 | 6 | 3.84 |
| Left Anterior Cingulate Gyrus | 25 | 2 | 40 | 24 | 3.61 |
ACKNOWLEDGMENTS
The authors would like to thank members of the Neurocognitive Development Lab, Developmental Social Cognitive Neuroscience Lab, Child Stress and Emotions Lab, the Laboratory of Cognition and Emotion, and the Maryland Neuroimaging Center for their contributions to this work, especially, Dr. Luiz Pessoa, Srikanth Padmala, Jason Smith, Lauren Weiss, Alan Siegel, Louis Marti, Katherine Rice, Brieana Viscomi, Daniel O'Young, Amna Zehra, Jonathan Segars, Jennifer Sloane, Heather Clark, Katherine Leppert, Josh Kinnison, Mahshid Najafi, and Jennifer Stark, and the families who participated in this study.