Age-related differences in autism: The case of white matter microstructure
Abstract
Autism spectrum disorder (ASD) is typified as a brain connectivity disorder in which white matter abnormalities are already present early on in life. However, it is unknown if and to which extent these abnormalities are hard-wired in (older) adults with ASD and how this interacts with age-related white matter changes as observed in typical aging. The aim of this first cross-sectional study in mid- and late-aged adults with ASD was to characterize white matter microstructure and its relationship with age. We utilized diffusion tensor imaging with head motion control in 48 adults with ASD and 48 age-matched controls (30–74 years), who also completed a Flanker task. Intra-individual variability of reaction times (IIVRT) measures based on performance on the Flanker interference task were used to assess IIVRT-white matter microstructure associations. We observed primarily higher mean and radial diffusivity in white matter microstructure in ASD, particularly in long-range fibers, which persisted after taking head motion into account. Importantly, group-by-age interactions revealed higher age-related mean and radial diffusivity in ASD, in projection and association fiber tracts. Subtle dissociations were observed in IIVRT-white matter microstructure relations between groups, with the IIVRT-white matter association pattern in ASD resembling observations in cognitive aging. The observed white matter microstructure differences are lending support to the structural underconnectivity hypothesis in ASD. These reductions seem to have behavioral percussions given the atypical relationship with IIVRT. Taken together, the current results may indicate different age-related patterns of white matter microstructure in adults with ASD. Hum Brain Mapp 38:82–96, 2017. © 2016 Wiley Periodicals, Inc.
Abbreviations
-
- AD
-
- Axial diffusivity
-
- ASD
-
- Autism spectrum disorder
-
- DTI
-
- Diffusion tensor imaging
-
- DWI
-
- Diffusion-weighted imaging
-
- FA
-
- Fractional anisotropy
-
- IIVRT
-
- Intra-individual variability of reaction time
-
- MD
-
- Mean diffusivity
-
- MRT
-
- Mean reaction times
-
- RD
-
- Radial diffusion
-
- RI
-
- Response incongruent
-
- SI
-
- Stimulus incongruent
-
- TMI
-
- Total motion index
-
- UNC
-
- Uncinate fasciculus
INTRODUCTION
It is becoming widely accepted that autism spectrum disorder [ASD; (American Psychiatric Association, 2000)] is characterized by atypical structural brain connectivity (Vissers et al., 2012). Indeed, numerous diffusion tensor imaging (DTI) studies from childhood to young adulthood noticed disruptions to white matter microstructure in ASD [Aoki et al., 2013; Rane et al., 2015; Travers et al., 2012; Vissers et al., 2012], particularly among long-range fibers (defined as connections between regions across different lobes and/or hemispheres). Together with reduced long-range functional connectivity, disconnectivity of long-range fibers is one of the suggested pathophysiological mechanisms in ASD, and led to the developmental underconnectivity hypothesis of ASD [Courchesne and Pierce, 2005; Just et al., 2004, 2012; Kana et al., 2011]. As with manifestations of autism symptomatology [Mukaetova-Ladinska et al., 2012; Perkins and Berkman, 2012], white matter abnormalities may change across the first half of the lifespan [Travers et al., 2015], but these abnormalities still need to be quantified in (older) adults with ASD. Typical development of white matter microstructure shows a curvilinear pattern plateauing around mid-adulthood and rapidly decreasing in old age [Westlye et al., 2010; Yeatman et al., 2014]. The question is if and how this interacts with ASD-related white matter abnormalities in (late) adulthood.
DTI assesses the orientation and microstructure of white matter tracts in vivo [Basser et al., 1994; Le Bihan, 2003] and aids in understanding the underlying axonal architecture, which may facilitate the communication among brain regions [van den Heuvel et al., 2009]. White matter microstructure is quantified by fractional anisotropy (FA), mean, radial, and axial diffusivity (MD, RD, AD). Lower FA is suggested to indicate low tract coherence, likely due to damage in the tissue structural organization [Pierpaoli and Basser, 1996]. MD reflects overall diffusion magnitude, and could reflect altered cytoarchitecture (differences in cellular size and integrity [Basser et al., 1994; Pierpaoli et al., 1996], with higher MD thought to be indicative for immaturity or tissue degeneration [Sykova, 2004]. Studies in animal models have suggested that AD and RD provide a more specific interpretation of DTI results than FA and MD, and propose that AD is related to axonal integrity (damage or loss), whereas RD is related to myelination processes [Alexander et al., 2007; Kim et al., 2006; Song et al., 2003; Song et al., 2005]. While there's no simplistic one-to-one biological interpretation to these values to human studies, it is essential to include each of these measures to understand white matter microstructure of people with ASD.
White matter abnormalities in early adulthood in ASD appear to converge towards lower FA, and, despite being scarcely reported, also higher MD and RD in various tracts [Bloemen et al., 2010; Catani et al., 2008; Conturo et al., 2008; Gibbard et al., 2013; Itahashi et al., 2015; Langen et al., 2012; Mueller et al., 2013; Thakkar et al., 2008]. However, other studies reported no differences in FA and/or MD [Bakhtiari et al., 2012; Kirkovski et al., 2015; Pugliese et al., 2009; Thomas et al., 2011], or even higher FA [Roine et al., 2013] in young adults with ASD. These mixed results in DTI metrics may be explained by the heterogeneous nature of ASD, different methodological differences (voxel-wise, whole-brain or tractography approaches), small samples and the inclusion of adolescents, hampering interpretation of age-related changes in adulthood [Aoki et al., 2013; Travers et al., 2012]. Furthermore, recent studies have shown that head motion can obscure reliability of diffusion-weighted imaging (DWI) and lead to spurious results [Koldewyn et al., 2014; Yendiki et al., 2014]. To overcome a number of these issues, we used fiber tracking, including stringent data quality and motion control, to examine a relatively large sample of adults with ASD and matched controls (N = 96, aged 30–74 years). Fiber tracking takes into account the diffusion profile orientation to reconstruct major fiber bundle trajectories [Jones, 2008]. Here we used TRACULA [Tracts Constrained by Underlying Anatomy; (Yendiki et al., 2011)] to reliably extract 18 well-known tracts, and quantify the microstructural organization to further the understanding of white matter abnormalities and anatomical connectivity in adults with ASD.
The aims of this study were threefold. First we tested if and to what extent white matter microstructure is impaired in adults with ASD, and second if these abnormalities are subject to atypical age-related changes. Based on the available DTI evidence in ASD, albeit incomplete thus far for adults, we hypothesized reduced white matter microstructure primarily in long-range association fibers [Aoki et al., 2013; Rane et al., 2015; Travers et al., 2012]. Furthermore, we expected age-related changes of white matter microstructure to be more pronounced in ASD compared to controls [Kleinhans et al., 2012; Travers et al., 2015]. Given the well-documented associations between white matter microstructure and intra-individual variability of reaction times (IIVRT) in typical development and aging [MacDonald et al., 2009; Nilsson et al., 2014; Tamnes et al., 2012], and as meta-analytical evidence up to age 30 suggests that people with ASD show increased IIVRT compared to controls [Karalunas et al., 2014], we additionally included IIVRT as a behavioral measure to test for the association with white matter microstructure. Thus thirdly, we expected differences in the IIVRT-white matter microstructure association between groups, as disconnectivity in associative pathways can also increase IIVRT [MacDonald et al., 2006].
METHODS AND MATERIALS
Participants
Fifty individuals with ASD and 49 controls between 30–74 years were recruited from a cohort of participants (estimated IQ > 80) of a large-scale behavioral study [Koolschijn and Geurts, 2016; Lever and Geurts, 2016]. Two individuals were excluded due to damaged DTI scans, one due to poor scan quality based on visual inspection. The final sample consisted of 48 participants with ASD (Mean age 51.3 (SD = 12.3), 33 males) and 48 controls (Mean age 50.5 (11.8), 31 males; see Table I). Details on inclusion criteria have been described earlier [Koolschijn and Geurts, 2016]. In short, all individuals with ASD received their clinical ASD diagnosis by a multidisciplinary team with clinicians experienced in the assessment of ASD. To verify the clinical diagnosis we used the following diagnostic inclusion criteria for ASD participants: (1) formal clinical diagnosis of ASD prior to inclusion; (2) confirmation of diagnosis with Autism Diagnostic Observation Schedule module 4 [Lord et al., 1989] and/or Autism-Spectrum quotient, 50-item list [Baron-Cohen et al., 2001]: 31 individuals had a score above the cutoff of the ADOS (≥7) and those not scoring above this cut off did score above the clinical AQ cutoff (≥26) (for similar approaches see [Ecker et al. 2012; Lai et al. 2013]). Please note that, although the evaluation of childhood problems was part of the clinical assessment procedures, obviously, with the age-range of our participants an interview such as the Autism Diagnostic Interview-Revised (ADI-R; [Lord et al. 1994]) is not feasible or reliable; (3) no self-reported history of neurological disorders, chronic illness, learning disabilities or schizophrenia. Controls had to meet criterion #3; with additional exclusion criterion of ASD diagnosis or a first or second-degree family member with ASD.
There were no between-group differences for IQ, age, sex, and handedness (Table 1, additional demographics: Supporting Information Table I). Participants gave written informed consent and received participation fee and travel reimbursement. The university review board approved the study (#2013-PN-2668).
| Description | ASD | CTRL | Statistics |
|---|---|---|---|
| N=48 | N=48 | ||
| #Males (%) | 33 (69%) | 31 (65%) | χ2 = 0.19, P = 0.665 |
| Age (SD)[range] | 51.32 (12.29) [30.04–73.98] | 50.47 (11.83) [30.62–73.77] | F = 0.12, P = 0.733 |
| IQ (SD)[range] | 116.60 (16.04) [86–155] | 110.98 (15.34) [80–139] | F =3.08, P = 0.082 |
| Level of Educational Attainmenta | 1/16/22/9 | 1/11/26/10 | χ2=1.31, P = 0.726 |
| Handedness | χ2=1.23, P = 0.940 | ||
| Left | 5 | 4 | |
| Right | 40 | 41 | |
| Ambidexter | 3 | 3 | |
| Age first diagnosis | 45.70 (13.57) [11.22–68.04] | N.A. | |
| ADOS Total | 7.92 (3.35) [1–19] | N.A. | |
| ADOS cutoff (<7)b | 17 (35%) | N.A. | |
| AQ Total | 35.96 (6.62) | 13.00 (5.94) | F =319.83, P < 0.001 |
| [19–47] | [4–26] | ||
|
AQ-cutoff (<26 ASD, >23 CTRL) |
4 (8%) | 0 | χ2=81.23, P < 0.001 |
| Medication N (%)c | 38 (79%) | 19 (40%) | χ2=15.59, P < 0.001 |
- Note. Numbers in bold reflect significant between-group differences.
- a Number of participants who had pre-vocational education/junior general secondary or vocation education/senior general secondary education or vocation colleges/university education based on the Verhage scale(Verhage, 1964).
- b All participants below ADOS threshold scores, had scores above the AQ clinical cut-off.
- c Detailed medication information is available in Supporting Information Table I.
- Abbreviations: ASD, autism spectrum disorder; CTRL, controls; ADOS, Autism Diagnostic Observation Schedule; AQ, Autism-Spectrum quotient; MMSE, Mini-mental state examination; N.A., not applicable.
PROCEDURE AND EXPERIMENTAL DESIGN
Data Acquisition
Participants were trained to lie still in a mock-scanner, and were scanned on a 3-Tesla Philips Achieva MRI-system (Best, The Netherlands). Two high-resolution T1-weighted scans were obtained: 3DFFE, multishot-TFE: TR = 8.2 msec; TE = 3.8 msec, 220slices, voxel-size = 1mm3, FOV = 240 × 188, matrix = 80, 2DSENSE: P(RL)=2.5, S(FH)=2). We collected two DWI measurements [TE(Echo Time)=86ms; TR(Repetition Time)=7542 ms; Flip Angle = 90°; 60 2mm transversal slices; FOV = 2242 mm; voxel-size = 2 mm3, reconstruction matrix 1122, 32 directions, SENSE = 2, b0 = 1,000 s/mm2]. The second DWI set had identical parameters, except that it was acquired with a reversed k-space readout direction, enabling removal of susceptibility artifacts during post-processing [Andersson et al., 2003]. Head motion was restricted using foam inserts around the head.
Tract Reconstruction
Before tract reconstruction, diffusion scans were merged using FSL 5.0.8 [Jenkinson et al., 2012] and processed with a six-stage processing-stream: (1) FreeSurfer 5.3 segmentation and parcellation of T1-weighted scans [Fischl, 2012] (gray matter segmentations will be presented elsewhere); (2) DTI-preprocessing with software developed in-house using the Neuroscience Gateway and the Dutch e-Science Grid [Shahand et al., 2015]. Preprocessing included head motion and eddy current correction with affine registrations (all volumes were registered to the first volume using SPM8), gradient corrections [Leemans and Jones, 2009], Rician noise reduction [Caan et al., 2010] and diffusion tensor calculation using non-linear least squares estimations. Subsequently, FA and MD-maps were computed for each individual; (3) Ball-and-stick-modeling using FSL [Jenkinson et al., 2012; Woolrich et al., 2009]; (4) Probabilistic tracking and track determination using TRACULA [Yendiki et al., 2011] on the Dutch e-science grid through the Neuroscience Gateway [Shahand et al., 2015], including extraction of mean FA, MD, RD and AD for each tract; (5) Quality control. We automatically tested and visually inspected for incomplete or missing tracts using the number of reconstructed streamlines. In case of unsuccessful reconstruction, tract reinitialization was performed, followed by full reconstruction; (6) To account for potential confounding effects of head motion, we computed four head motion measures (average volume-by-volume translation and rotation, percentage of slices with signal dropout, and signal dropout severity; a feature in the TRACULA processing pipeline) and the total motion index (TMI) (a sum variable to estimate slower, between-volume motion captured by the registration-based measures, as well as more rapid, within-volume motion, captured by the intensity-based measures) for each participant as described in Yendiki et al. [2014].
IIVRT
Participants performed an adapted interference control paradigm [Flanker-task; (Eriksen and Eriksen, 1974; van Veen et al., 2001)]. This paradigm is known to be challenging for both people with ASD [Geurts et al., 2014] and older adults [Lustig and Jantz, 2015], and is, therefore, likely to elicit a wide distribution of reaction times. Participants saw five horizontally presented letters (S, M, H, and/or P) and had to respond to the centrally located letter (target) while ignoring the other letters (flankers). There were three trial types: Congruent (CO, the target was flanked by identical letters); Stimulus incongruent (SI, target and flankers were non-identical, but led to the same response); and Response incongruent (RI, target and flankers were mapped onto different responses). Participants were instructed to respond as fast as possible with their left or right index fingers; hand-assignment was counterbalanced across participants (Supporting Information Methods). Mean reaction times (MRT), and accuracy were calculated for each participant overall and per trial type; IIVRT was calculated in terms of standard deviation of MRT (sdRT), and the coefficient of MRT variation (CV = sdRT/MRT), irrespective of performance. Both measures for quantifying IIVRT were included to be able to compare our results with current practices in the ASD literature [Karalunas et al., 2014)].
DATA ANALYSIS
Demographics
Demographics were compared between groups using ANOVAs, and χ2-tests for categorical variables (Table 1).
White Matter Group and Age-Comparisons
Given that we had specific hypotheses about group, and group-by-age interactions, and wanted to examine if these variables explained variance after taking head motion into account, we performed hierarchical linear regression analyses and examined average FA, AD, RD and MD for each of the 18 tracts. TMI was included to control for potential confounding effects of head motion [Koldewyn et al., 2014; Yendiki et al., 2014], followed by group (ASD and CTRL), age and group-by-age interaction as our variables of interest. Next, sex was added to examine possible white matter related sex-differences [Beacher et al., 2012; Sacher et al., 2013]. Data were checked for outliers. All regression analyses were repeated with standardized residuals < −3 and >3 removed. Per outcome measure 0 to 3 cases were excluded to achieve that all residuals fell in the −3 to 3 ranges. If re-analyses didn't change initial outcomes, data including outliers were reported. We considered results to be significant at P < 0.003, Bonferroni correction for 18 tracts. The same procedure was repeated without TMI to demonstrate possible motion-related influences.
White Matter IIVRT Association
First, a partial correlation matrix, controlling for TMI, was built between DTI metrics from all tracts and measures derived from the Flanker task (MRT, sdRT, and CV overall and for each trial type), creating an 84x84 correlation matrix. MRT was included based on earlier associations with white matter microstructure [Gunning-Dixon et al., 2009; Kennedy and Raz, 2009]. Partial correlations were considered significant with P < 0.00006 after Bonferroni correction (18 tracts, 4 DTI metrics, and 12 Flanker measures).
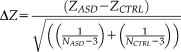
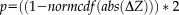
RESULTS
Movement and Tract Reconstruction
There were no differences in translational (MASD = 0.40 vs. MCTRL = 0.26, F = 0.46, P = 0.50) or rotational movement parameters (MASD = 0.19 vs. MCTRL = 0.28, F = 0.25, P = 0.62), or associations with age or group × age interactions (all P's > 0.22).
Out of the 1,728 reconstructed tracts, for 27 participants (18 ASD, 9 CTRL) a total of 44 tracts could not be (fully) reconstructed (2.5%), and were excluded from analyses (Supporting Information Table II).
Are There Differences in White Matter Microstructure Between ASD and CTRLS?
Table 2 shows the results from the hierarchical regression analyses comparing DTI metrics for each tract between groups. Here average values over the entire support of the path distributions are reported (results remained highly similar with weighted averages, Supporting Information Table III).
| Tract | LH/RH | WM | N | Variable | ß | P | adj R2 | F | p-F model | With TMI correctiona |
|---|---|---|---|---|---|---|---|---|---|---|
| fMajor | FA | 90 | 0.104 | 4.43 | 0.006 | |||||
| RD | 89 | 0.108 | 3.44 | 0.020 | ||||||
| fMinor | FA | 89 | Age | −0.354 | 0.010 | 0.24 | 7.94 | 0.001 | ||
| Sex | −0.317 | 0.001 | ||||||||
| MD | 89 | Group | 0.216 | 0.029 | 0.17 | 5.52 | 0.001 | |||
| Group × age | 0.286 | 0.045 | ||||||||
| Sex | 0.210 | 0.035 | ||||||||
| RD | 89 | Group | 0.192 | 0.047 | 0.206 | 6.72 | <0.001 | |||
| Sex | 0.265 | 0.007 | ||||||||
| AD | 89 | Group × age | 0.307 | 0.042 | 0.071 | 3.25 | 0.026 | |||
| ATR | LH | FA | 95 | Group | −0.240 | 0.012 | 0.182 | 6.23 | <0.001 | |
| Sex | −0.222 | 0.021 | ||||||||
| MD | 93 | Group | 0.259 | 0.005 | 0.271 | 9.55 | <0.001 | b | ||
| Group × age | 0.438 | 0.001 | b | |||||||
| RD | 93 | Group | 0.281 | 0.002 | 0.283 | 1.5 | <0.001 | |||
| Group × age | 0.413 | 0.002 | ||||||||
| RH | FA | 95 | Group | −0.213 | 0.020 | 0.235 | 1.62 | <0.001 | ||
| Age | −0.273 | 0.035 | ||||||||
| MD | 95 | Group | 0.227 | 0.012 | 0.269 | 12.51 | <0.001 | |||
| Group × age | 0.390 | 0.002 | ||||||||
| RD | 95 | Group | 0.252 | 0.004 | 0.330 | 16.44 | <0.001 | b | ||
| Group × age | 0.377 | 0.002 | b | |||||||
| CAB | RH | AD | 80 | Group | 0.246 | 0.027 | 0.066 | 2.87 | 0.042 | |
| CCG | LH | FA | 95 | 0.078 | 3.64 | 0.016 | ||||
| RD | 94 | Group | 0.21 | 0.031 | 0.149 | 6.44 | 0.001 | |||
| RH | MD | 96 | Group | 0.242 | 0.017 | 0.064 | 3.15 | 0.029 | ||
| RD | 96 | Group | 0.236 | 0.02 | 0.063 | 3.12 | 0.030 | |||
| CST | LH | FA | 95 | 0.081 | 3.76 | 0.014 | ||||
| RD | 95 | 0.088 | 4 | 0.010 | ||||||
| RH | FA | 95 | 0.087 | 3.97 | 0.010 | b | ||||
| MD | 95 | Group | 0.241 | 0.016 | 0.097 | 4.35 | 0.007 | b | ||
| RD | 95 | Group | 0.236 | 0.015 | 0.147 | 6.39 | 0.001 | b | ||
| ILF | LH | FA | 95 | Age | −0.293 | 0.035 | 0.155 | 6.75 | <0.001 | |
| MD | 95 | Group | 0.201 | 0.032 | 0.2 | 8.85 | <0.001 | b | ||
| Group × age | 0.379 | 0.005 | ||||||||
| RD | 95 | 0.166 | 7.17 | <0.001 | b | |||||
| AD | 95 | Group | 0.199 | 0.047 | 0.069 | 3.33 | 0.023 | |||
| Group × age | 0.346 | 0.018 | ||||||||
| RH | FA | 93 | Age | −0.454 | <0.001 | 0.325 | 15.77 | <0.001 | ||
| MD | 93 | Group | 0.286 | 0.002 | 0.268 | 12.24 | <0.001 | b | ||
| Group × age | 0.311 | 0.018 | b | |||||||
| RD | 93 | Group | 0.233 | 0.007 | 0.335 | 16.42 | <0.001 | b | ||
| Age | 0.313 | 0.013 | b | |||||||
| Group × age | 0.277 | 0.027 | b | |||||||
| AD | 93 | Group | 0.291 | 0.004 | 0.094 | 4.2 | 0.008 | b | ||
| SLFP | LH | FA | 95 | Group | −0.192 | 0.048 | 0.136 | 5.93 | 0.001 | |
| RD | 93 | 0.082 | 3.73 | 0.014 | ||||||
| AD | 91 | Age | −0.516 | <0.001 | 0.102 | 4.42 | 0.006 | b | ||
| Group × age | 0.363 | 0.013 | ||||||||
| RH | FA | 96 | Age | −0.296 | 0.03 | 0.177 | 7.8 | <0.001 | b | |
| MD | 96 | Group × age | 0.394 | 0.003 | 0.214 | 9.61 | <0.001 | |||
| RD | 96 | 0.137 | 5.88 | 0.001 | ||||||
| SLFT | LH | FA | 96 | 0.088 | 4.05 | 0.009 | ||||
| RD | 96 | Group | 0.261 | 0.008 | 0.134 | 5.9 | 0.001 | |||
| RH | FA | 96 | Group | −0.197 | 0.036 | 0.193 | 8.56 | <0.001 | b | |
| Age | −0.273 | 0.043 | ||||||||
| MD | 96 | Group | 0.233 | 0.009 | 0.272 | 12.85 | <0.001 | |||
| Group × age | 0.424 | 0.001 | b | |||||||
| RD | 96 | Group | 0.246 | 0.005 | 0.288 | 13.84 | <0.001 | b | ||
| Group × age | 0.379 | 0.003 | ||||||||
| AD | 96 | Group × age | 0.388 | 0.006 | 0.114 | 5.06 | 0.003 | |||
| UNC | LH | MD | 95 | 0.153 | 6.68 | <0.001 | ||||
| RD | 95 | 0.122 | 5.34 | 0.002 | ||||||
| AD | 95 | 0.121 | 5.33 | 0.002 | ||||||
| RH | FA | 93 | Group | −0.344 | 0.001 | 0.163 | 5.49 | <0.001 | ||
| Sex | −0.219 | 0.025 | ||||||||
| MD | 93 | Group | 0.357 | <0.001 | 0.239 | 10.61 | <0.001 | b | ||
| RD | 93 | Group | 0.406 | <0.001 | 0.283 | 10.06 | <0.001 | b | ||
| Sex | 0.198 | 0.029 | b | |||||||
| RH | AD | 94 | Group × age | 0.297 | 0.036 | 0.085 | 3.89 | 0.012 |
- Note. Stepwise regression analyses were performed with (TMI as correction variable, then) group, age and group × age interaction, followed by sex as predictors. Numbers in bold reflect significant differences after correcting for multiple comparisons with P < 0.003.
- a Supporting Information Table IV provides the ß- and P-values for the TMI-corrected results.
- b Results remained significant after taking TMI into account.
- Abbreviations: fMajor, Corpus callosum – forceps major; fMinor Corpus callosum – forceps minor; ATR, Anterior thalamic radiation; CAB, Cingulum – angular (infracallosal) bundle; CCG, Cingulum – cingulate gyrus (supracallosal) bundle; CST, Corticospinal tract; ILF, Inferior longitudinal fasciculus; SLFP, Superior longitudinal fasciculus – parietal bundle; SLFT, Superior longitudinal fasciculus – temporal bundle; UNC, Uncinate fasciculus; LH, left hemisphere; RH, right hemisphere; AD, axial diffusivity; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity.
Two overall findings emerge: First, including TMI strongly influences significance (Supporting Informaton Table IV provides the β- and p-values for the TMI-corrected results). Second, we found consistently higher MD and RD in ASD compared to CTRLS for nearly all reported tracts.
For exploratory purposes, the between-group analyses were also performed with the ADOS-only group (i.e., those individuals with ADOS-scores above cutoff (>7). Despite the smaller sample size in the ADOS (>7) group, results remained highly similar to the stringent TMI-correct results (Supporting Information Table V).
Is White Matter Microstructure Differently Associated with Age in ASD and CTRLS?
Significant group-by-age interactions were found in bilateral ATR, right ILF, and SLFT (Table 2). The pattern was strikingly similar across the five tracts, showing higher MD and RD with increasing age in ASD relative to CTRLS (Fig. 1).

Group-by-age interactions for mean (MD) and radial diffusivity (RD). Figure 1 displays the significant group-by-age interactions, taking TMI into account, reported in Table II. Abbreviations: MD, mean diffusivity; RD, radial diffusion; ATR, Anterior Thalamic Radiation; ILF, Inferior Longitudinal Fasciculus; SLFT, Superior Longitudinal Fasciculus Temporal. [Color figure can be viewed at wileyonlinelibrary.com.]
Exploratory along-the-tract analyses were performed to appreciate the location of significant between-group results (Supporting Information Fig. 1).
Is the IIVRT-White Matter Microstructure Association Different in ASD and CTRLS?
Figure 2 and Table 3 represent the IIVRT-white matter microstructure associations between groups. Higher MD and RD in bilateral CAB, ILF, SLFP and SLFT were associated with higher CV in ASD (positive association) compared to controls (negative to no association), but the opposite for CCG. Lower FA in bilateral ILF, and left SLFP were associated with higher CV in ASD, while in controls there was no such association. Finally, higher MD, RD and AD in left CAB were associated with higher overall MRT in controls, but the opposite was found for ASD. In addition, lower MD in left CST and lower AD in bilateral ILF were associated with higher MRT in ASD, while this association was absent in controls. Please note that when accuracy was taken into account, i.e. only correct trials were used for IIVRT-white matter associations, the findings were changed towards larger between-group differences in IIVRT-white matter microstructure associations. Specifically, higher CV (for incongruent trials) was associated with higher MD, RD and AD in several tracts (Supporting Information Table VII and Supporting Information Fig. 2)
| MRT overall | sdRT overall | CV overall | MRT congruent correct | MRT stimulus incongruent correct | CV congruent | CV stimulus incongruent | CV response incongruent | sdRT response incongruent | |
|---|---|---|---|---|---|---|---|---|---|
| LH CAB AD | −2.06 | 2.22 | 2.18 | 2.77 | |||||
| LH CAB RD | −2.30 | 2.20 | |||||||
| LH CAB MD | −2.29 | 1.99 | 2.48 | ||||||
| RH CAB AD | 3.04 | 3.28 | 3.00 | ||||||
| RH CAB RD | 2.50 | 2.75 | 2.53 | ||||||
| RH CAB MD | 2.76 | 3.01 | 2.76 | ||||||
| RH CCG AD | −2.24 | −2.55 | −2.32 | 2.31 | |||||
| RH CCG MD | −2.17 | −2.20 | −2.48 | −2.05 | |||||
| LH CST MD | −1.99 | ||||||||
| LH ILF AD | −1.97 | −2.33 | |||||||
| LH ILF RD | 1.96 | 2.49 | 2.38 | ||||||
| LH ILF MD | 2.44 | 2.34 | |||||||
| LH ILF FA | −2.00 | ||||||||
| RH ILF AD | −2.11 | ||||||||
| RH ILF RD | 2.16 | ||||||||
| RH ILF FA | −2.08 | −1.98 | |||||||
| LH SLFP RD | 1.99 | ||||||||
| LH SLFP FA | −2.55 | ||||||||
| LH SLFT RD | 2.40 | 2.08 | |||||||
| LH SLFT MD | 2.24 | 2.10 | |||||||
| RH SLFT AD | 2.03 | 2.05 |
- Values represent significant ΔZ-scores. Abbreviations: CV, coefficient of variation; MRT, mean reaction time; sdRT, standard deviation MRT; LH/RH, left/right hemisphere; AD, axial diffusion; MD, mean diffusivity; RD, radial diffusion; ATR, Anterior thalamic radiation; CAB, Cingulum – angular (infracallosal) bundle; CCG, Cingulum – cingulate gyrus (supracallosal) bundle; CST, Corticospinal tract.
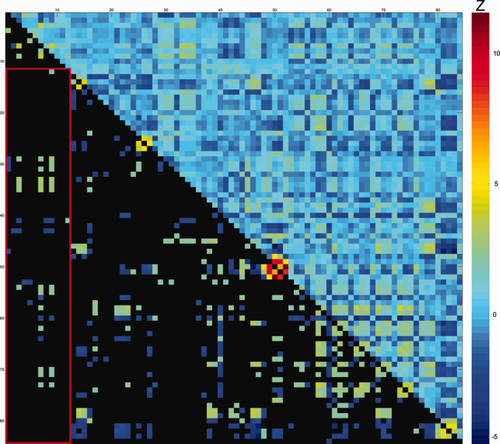
Difference scores of partial correlations between intra-individual variability and white matter microstructure for ASD and controls. Values represent ΔZ-scores, controlled for TMI. Upper diagonal: all ΔZ-scores between ASD and CTRL for DTI parameters and Flanker measures. Lower diagonal: significant ΔZ-scores ΔZ > |1.96|); non-significant values in black. Warm colors indicate more positive partial-correlation in ASD than in controls (e.g. rho-ASD= −0.15 and rho-CTRL = −0.44; or rho-ASD = 0.44 and rho-CTRL = 0.15; both result in positive ΔZ-scores). Cool colors indicate more positive partial-correlation in controls than in ASD (e.g. rho-ASD = −0.44 and rho-CTRL = −0.15; or rho-ASD = 0.15 and rho-CTRL = 0.44; both result in negative ΔZ-scores). See Table 3 for exact values, also in-text explanation and original partial-correlations in Supporting Information Fig. 4. For denotation of rows/columns see Supporting Information Table VI. [Color figure can be viewed at wileyonlinelibrary.com.]
Supporting Information Fig. 3 shows the partial correlation matrix of the IIVRT-white matter association across participants and Supporting Information Fig. 4 shows the partial correlations for each group. Higher IIVRT in terms of CV was associated with higher MD and RD most prominently in left ILF, and bilateral ATR and SLFP. However, after exclusion of incorrect trials, the IIVRT-white matter associations across all participants (i.e. irrespective of diagnosis) were no longer significant across participants (Supporting Information Fig. 5 for partial correlations for each group).
Note that when comparing ASD and CTRLs on behavioral measures of the Flanker task (MRT, accuracy, sdRT, CV) there were no group differences. However, people with ASD were, independently of trial type, slower and more variable (sdRT) than CTRLs (for details see Supporting Information Table VIII).
DISCUSSION
The main objective was to examine white matter microstructure in ASD adulthood. This yielded several new findings. First, widespread disruptions of white matter architecture in long-range fibers were found in ASD. Second, we report higher age-related white matter diffusivity in ASD for bilateral anterior thalamic radiation and right inferior and superior longitudinal fasciculi compared to age-matched controls. Finally, effects of diagnosis on the IIVRT-white matter microstructure association were predominantly accounted for by higher CV and lower white matter microstructure. These findings may indicate different age-related patterns of white matter microstructure in adults with ASD.
White Matter in ASD
Higher MD and RD mainly characterized reduced white matter microstructure in major association fibers in (older) adults with ASD. These results convincingly confirm and build upon the general view of white matter disruptions in ASD, specifically those in long-range tracts, accruing evidence for the underconnectivity hypothesis in ASD. Whereas earlier DWI studies revealed spurious group differences due to head motion in ASD [Koldewyn et al., 2014; Yendiki et al., 2014], these results remained significant after controlling for head motion parameters, hence showing robustness of our findings. Nevertheless, the number of between-group differences failing significance after controlling for head motion, particularly those related to FA, stresses the importance of taking motion parameters into account in ASD research.
Our results indicate that white matter abnormalities in adults with ASD are related to higher directional diffusivities. However it is difficult to pinpoint the origin of these higher diffusivities. For instance, the interpretation for AD varies, with reduced AD found to be associated with axonal damage in animal studies [Kim et al., 2006; Song et al., 2003; Song et al., 2005], but the opposite in human studies [Bastin et al., 2009; Klawiter et al., 2011; Salat et al., 2010; Sullivan et al., 2010; Vernooij et al., 2008; Vernooij et al., 2009]. Thus, DTI metrics are sensitive for white matter microstructure, but the relationship may be indirect and not a one-to-one association with the actual physical properties [Walhovd et al., 2014]. Nonetheless, evidence from scant post-mortem studies confirms myelin-related abnormalities to be associated with disconnection of long-distance pathways and neighboring connectivity [Broek et al., 2014; Zikopoulos and Barbas, 2010]. Regardless of deciphering the direct physical associations, our findings of higher diffusivity are consistent with the notion that underconnectivity in ASD is related to altered axonal microstructure [McFadden and Minshew, 2013].
When specifically focusing on long-range fibers and their relationship with ASD, we see that reduced fiber coherence in bilateral Anterior Thalamic Radiation (ATR; left higher MD, right higher RD) expands earlier ASD reports showing reduced ATR fiber microstructure from infancy to young adulthood [Bloemen et al., 2010; Cheng et al., 2010; Itahashi et al., 2015; Jou et al., 2011; Keller et al., 2007; Kleinhans et al., 2012; Lazar et al., 2014; Shukla et al., 2011; Wolff et al., 2012]. ATR is a projection fiber connecting thalamic nuclei with prefrontal and anterior cingulate cortices [Schmahmann and Pandya, 2006] and abnormalities contribute to cognitive [Mamah et al., 2010; Van der Werf et al., 2003] and affective [Coenen et al., 2012; Spalletta et al., 2013; Torso et al., 2015] dysfunctions, but no studies to date explicitly tested these behavioral associations in ASD. The ILF is a long-range tract connecting the occipital cortex with temporal structures, and the observed reduced fiber microstructure confirms earlier ASD reports in childhood and young adulthood [Kleinhans et al., 2008; Koldewyn et al., 2014; Pugliese et al., 2009], and extends those to older ages. ILF disconnectivity has been associated with impaired face and emotion recognition [Philippi et al., 2009], visual perception, and language functions [Catani and Thiebaut de Schotten, 2008], which are affected in ASD [Weigelt et al., 2012]. The SLF findings are partly in line with earlier reports in young adults [Bloemen et al., 2010; Mueller et al., 2013] and build upon meta-analytical evidence of reduced SLF microstructure in ASD [Aoki et al., 2013]. This major cortical association fiber pathway facilitates a network essential for language processing [Makris et al., 2005; Schmahmann et al., 2008], and other core processes such as attention, memory, and emotion [Urger et al., 2015], all of which have historically been associated with ASD. Finally, we showed higher MD and RD in the right uncinate fasciculus (UNC), a prominent white matter tract connecting inferior frontal and temporal lobe regions [Catani et al., 2002; Catani and Thiebaut de Schotten, 2008; Ebeling and von Cramon, 1992]. Although the exact functions of the UNC remain matter of debate, its functions lie at the intersection of memory and social–emotional processes [Von Der Heide et al., 2013] and may contribute to social impairments in ASD [Ameis and Catani, 2015].
Age-Related White Matter Differences
Age-related patterns were strikingly similar to the group-analyses, showing higher diffusivity primarily in right fiber bundles, confirming our second hypothesis of higher age-related white matter microstructure decline in ASD. Again, these alterations were limited to projection and association fibers (ATR, ILF, SLF). Importantly, the age-related findings of the healthy controls were similar to the trajectories reported by two large DTI lifespan studies in healthy individuals [Westlye et al., 2010; Yeatman et al., 2014], with MD showing minor increases within our age-range. The moderate increase of RD with advancing age in ILF was similar to these large studies, whereas the age-related trajectories for RD in ATR were less steep compared to typical aging patterns [Westlye et al., 2010].
Prior white matter cross-sectional studies in ASD have revealed rather mixed results with increasing age [Cheng et al., 2010; Keller et al., 2007; Kleinhans et al., 2012; Lazar et al., 2011; Lee et al., 2007; Lisiecka et al., 2015; Pugliese et al., 2009; Shukla et al., 2011]. Sparse evidence from longitudinal studies suggests atypical postnatal development of fiber microstructure, followed by blunted development leading to lower coherence around age two [Wolff et al., 2012]. Similarly, in an older sample, atypical high FA characterized the corpus callosum before age 10, after which group differences stabilized up to age 40 [Travers et al., 2015]. In an animal model of ASD, atypical growth trajectories in FA (low) and MD (high) were found in limbic, motor, and frontal brain regions suggestive for impaired connectivity [Kumar et al., 2012]. This might suggest that there are at least three marked postnatal developmental stages of WM in ASD: (1) early infancy; (2) transition from childhood to adolescence/young adulthood; (3) mid adulthood, which may be the period of a more premature transition into aging as compared to the onset of typical aging [Giorgio et al., 2010; Yap et al., 2013].
IIVRT-White Matter Associations
The observed association of higher IIVRT (CV), with higher MD and RD in prominent major fiber bundles, irrespective of diagnosis, is consistent with reports on typical development and aging [Fjell et al., 2011; Grydeland et al., 2013; Tamnes et al., 2012]. Moreover, the tracts associated with IIVRT were identical to those reported in older adults [Mella et al., 2013]. However, when including only trials with correct responses, the associations were no longer significant, which has been reported previously [Mazerolle et al., 2013]. Thus, these findings provide limited evidence for the hypothesis that lower white matter microstructure generates performance variability [MacDonald et al., 2006, 20092009].
Subtle dissociations in IIVRT-white matter microstructure associations were observed between groups, even when incorrect trials were excluded from the analyses. The patterns in people with ASD converge towards those associations often reported in cognitive aging [Fjell et al., 2011; MacDonald et al., 2009; Mella et al., 2013]. For example, reduced fiber coherence in callosal fibers was associated with higher CV in ASD, but not in controls. These results in ASD are in line with the general idea that IIVRT reflects neural noise and disruptions of action potentials associated with reduced structural connectivity [MacDonald et al., 2009; Russell et al., 2006; Walhovd and Fjell, 2007]. Although at first sight this might not fit our observations in the controls, recent evidence from a lifespan study suggested that IIVRT-white matter microstructure relations are more prominent with advancing age, and that aging-related altered axonal microstructure contributes to higher IIVRT [Grydeland et al., 2013]. Thus, the strength of these associations might indeed be stronger in older ages, but less strong or absent within the age-range of our controls. Hence, we provide evidence, although speculative, for different age-related patterns of white matter microstructure in ASD.
Strengths and Limitations
The study has some potential limitations. While the sample is relatively large compared to the current DWI-literature in ASD, a question can be raised concerning the extent to which the sample represents the general ASD population, as this is first DWI study to include older adults. Nevertheless, our reported widespread white matter microstructural differences resemble previous meta-analytical findings [Aoki et al., 2013; Travers et al., 2012] and thus indicate robustness of white matter abnormalities in ASD across the spectrum. Some may argue that our sample is mildly affected based on relatively low AQ and ADOS-scores in some participants and IQ-scores above 80. Prior studies have shown that ASD symptoms reduce with increasing age, but ASD-related impairments do remain pervasive across the lifespan [Perkins and Berkman, 2012]. Moreover, adults with an established ASD diagnosis tend to have lower scores on self-report questionnaires [Bishop and Seltzer, 2012], though our mean AQ-scores match those from a large recent study [Ruzich et al., 2015], and, if reported, from most adult DTI studies [Bakhtiari et al., 2012; Gibbard et al., 2013; Itahashi et al., 2015; Kirkovski et al., 2015; Mueller et al., 2013; Roine et al., 2013]. In addition, it is known that those with a formal diagnosis of ASD in childhood are not always meeting an ADOS cutoff in adulthood [Anderson et al. 2014; Fein et al. 2013; McGovern and Sigman 2005] and it seems that diagnoses based on a combination of history/caregiver information and clinical observation are significantly more stable over time than results from any single instrument such as the ADOS [Bastiaansen et al. 2011; Jones and Lord 2013; Lord et al. 2006]).
Psychotropic medication exposure, such as antipsychotics, may be an important potential confounder in adult ASD research as it is rather commonly prescribed (see for example Starkstein et al., 2015 where 78% of the adult ASD sample was using some form of neuroleptics) and might impact white matter microstructure (e.g. in schizophrenia: Szesko et al. 2014, but see [Kanaan et al. 2009; Tamnes & Agartz et al. 2016], and Bipolar disorder [Hafeman et al. 2012] for contrasting findings). As also a part of our ASD group was on psychotropic medication this might have had an effect on the observed pattern of findings. However, when excluding those on antipsychotic treatment the majority of our findings remained comparable (Supporting Information Table IX), despite a smaller sample size and exclusion of a relatively large number of adults with ADOS-scores >7. From an IQ-perspective, the current sample does not represent the entire ASD population (i.e., including those with intellectual disability), given that IQ-scores were above 80. However, in MRI-studies, and particularly current adult DTI studies, IQ-scores are often above 80 to ensure full participation in the study, and to be able to follow procedures and understand cognitive tasks. Thus, even if our sample has “lower” symptom severity, this not only affects the autism community investigating adults in general, but it also demonstrates the robustness of structural white matter abnormalities reported in ASD.
CONCLUSION
Taken together, our results provide substantial evidence for widespread reductions in white matter microstructure in (older) adults with ASD, lending support to the structural underconnectivity hypothesis. Most importantly, group-by-age interactions revealed higher age-related white matter diffusivity in ASD, and the IIVRT-white matter association pattern in ASD resembles observations in cognitive aging. Thus speculatively, our cross-sectional findings may indicate different age-related patterns of white matter microstructure in ASD. However, whether this will already have repercussions in daily life within this age-range remains to be tested in a longitudinal study, as earlier cross-sectional reports on neuropsychological behavioral tasks did not reveal premature aging patterns [Lever and Geurts, 2016; Lever et al., 2015; Ring et al., 2015].
ACKNOWLEDGMENTS
The funding body had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors acknowledge all involved with data acquisition, and the support of SURF Foundation for the computational resources of the Dutch e-science Grid.