Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: A longitudinal study
Abstract
There are varying, often conflicting, reports with respect to altered striatal volume and morphometry in the major psychoses due to the influences of antipsychotic medications on striatal volume. Thus, disassociating disease effects from those of medication become exceedingly difficult. For the first time, using a longitudinally studied sample of structural magnetic resonance images from patients with childhood onset schizophrenia (COS; neurobiologically contiguous with the adult onset form of schizophrenia), their nonpsychotic siblings (COSSIBs), and novel shape mapping algorithms that are volume independent, we report the familial contribution of striatal morphology in schizophrenia. The results of our volumetric analyses demonstrate age-related increases in overall striatal volumes specific only to COS. However, both COS and COSSIBs showed overlapping shape differences in the striatal head, which normalized in COSSIBs by late adolescence. These results mirror previous studies from our group, demonstrating cortical thickness deficits in COS and COSSIBs as these deficits normalize in COSSIBs in the same age range as our striatal findings. Finally, there is a single region of nonoverlapping outward displacement in the dorsal aspect of the caudate body, potentially indicative of a response to medication. Striatal shape may be considered complimentary to volume as an endophenotype, and, in some cases may provide information that is not detectable using standard volumetric techniques. Our striatal shape findings demonstrate the striking localization of abnormalities in striatal the head. The neuroanatomical localization of these findings suggest the presence of abnormalities in the striatal-prefrontal circuits in schizophrenia and resilience mechanisms in COSSIBs with age dependent normalization. Hum Brain Mapp 36:1458–1469, 2015. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Schizophrenia is a severe and heritable illness with a typical age of onset in late adolescence or early adulthood. Twin and family studies have demonstrated the strong genetic liability for this disease (Cannon et al., ; Saha et al., 2005). Childhood onset schizophrenia (COS), with the onset of psychosis before age 13, is a rare early-onset form of this disorder that is clinically and neurobiologically continuous with the adult onset form (Alaghband-Rad et al., 1995; McKenna et al., 1994; Nicolson and Rapoport, 1999). Despite the rarity of the disorder, early onset patients resemble chronic, severe, and treatment refractory cases of adult onset schizophrenia (AOS) (Rapoport and Gogtay, 2011). COS is phenotypically more homogeneous than AOS, less influenced by environmental causes, and more strongly influenced by genetic factors (Rapoport et al., 2005). To better understand the familial component of COS, the National Institute of Mental Health (NIMH) has been screening and acquiring prospective magnetic resonance imaging (MRI) data from afflicted individuals and their full siblings since 1991 (Rapoport and Gogtay, 2011).
Much of the previous work from the NIMH group has described adolescent psychiatric disorders as being a result of altered courses of neuronatomical developmental trajectories (Greenstein et al., 2006; Rapoport and Gogtay, 2008; Shaw et al., 2008, 2010; Thompson et al., 2001), with COS showing an exaggerated pattern of cortical thinning compared to the normal development trajectory (Thompson et al., 2001). Remarkably, nonpsychotic full siblings of COS (COSSIB) demonstrate similar spatially localized patterns of cortical thinning in the prefrontal and temporal cortices. However, these patterns are far less severe than the COS group, are present only the early ages, and the deficits in healthy siblings normalize by late adolescence (Gogtay et al., 2004, 2007; Greenstein et al., 2006; Mattai et al., 2011). These findings suggest two things; first, some aspects of abnormal brain morphometry observed in COS are heritable. Second, the amelioration of this abnormality in siblings may reflect a resilience phenotype (stemming from either environmental or genetic neuroprotective mechanisms) which safeguards healthy COSSIBs from manifesting psychosis (Bois et al., 2014; Moran et al., 2013).
The striatum is of particular interest in schizophrenia pathology as the structure mediates a range of cognitive and affective functions impaired in the disorder. This includes deficits seen in working memory and executive functions, and anomalies in motivation, emotion, and reward (Arsalidou et al., 2012; Scimeca and Badre, 2012; Tang and Strafella, 2012). It is heavily implicated in many of the cognitive deficits (working memory and executive function (Scimeca and Badre, 2012)), negative symptoms (social withdrawal, lack of motivation), and social interaction deficits (Simpson et al., 2010)) observed in schizophrenia.
Variation in striatal volume in the major psychoses, and in schizophrenia in particular, is still very much an open question. Volumetric analysis cannot disentangle the effects of antipsychotic medication on the neuroanatomy of schizophrenia patients (Chua et al., 2009; Deng et al., 2009; Ettinger et al., 2012; Hannan et al., 2010). In fact, early neuroanatomical analyses of the basal ganglia in COS suggest that volumetric enlargement of the caudate is the result of the administration of typical neuroleptics (Frazier et al., 1996a, 1996b). As a result, our understanding of striatal deficits in major psychosis may be improved through the use of shape mapping rather than more traditional volumetric analyses. Previous studies have not observed differences between the striatal volumes of drug naïve and abstinent schizophrenia patients in comparison to healthy controls (Ballmaier et al., 2008). However, through the use of sophisticated shape mapping techniques, other groups have shown progressive striatal shape alteration in schizophrenia over the course of 2 years (inward pointing deformation over the course of 2 years) (Wang et al., 2008) and also found striatal shape abnormalities in unaffected siblings of schizophrenia patients (Mamah et al., 2008). Given confounds due to medication and the heterogeneous findings in the schiozphrenia, shape may be a complementary neuroimaging endophenotype to volume while studying the striatum in pathophysiology of schizophrenia.
Whether the striatal abnormalities in schizophrenia are state or trait markers is also an open question and the finding thus far have been mixed. For example, a twin-design study observed no differences in striatal volume differences in monozygotic twins discordant for psychosis (Ettinger et al., 2012). Similarly, those at ultra-high risk for psychosis do not demonstrate volumetric differences relative to controls. Caudate volume increases have been demonstrated in studies of unaffected nonpsychotic ultra-high risk (UHR) individuals who are members of a multicomplex family where at least one parent suffers from psychosis (Hajek et al., 2009); but this study showed no striatal volume differences in prodromal UHR individuals and healthy controls. Studies of medication naïve subjects with schizotypal disorders, support the idea of volumetric striatal decreases in females suffering from schizophrenia spectrum disorders (Koo et al., 2006; Levitt et al., 2009). To disambiguate the contributions of antipsychotic medications, structure heritability, and psychosis on striatal morphology the field would greatly benefit from a large-scale and long-term longitudinal examination of COS and their unaffected siblings.
Here, we examine volume and shape of the striatum in a large prospectively scanned sample of COS patients and their nonpsychotic siblings. This is the first study to date to examine the developmental trajectory of striatal shape (along with volume) in schizophrenia at any age. We use an age-centered analysis to demonstrate the developmental trajectories of striatal volume and shape in the COS and COSSIB groups with respect to the NV group. We perform our segmentations of the striatum using a novel multi-atlas label-fusion based methodology (Chakravarty et al., 2013; Raznahan et al., 2014) that relies on the generation of multiple candidate segmentations before using a decision rule to estimate the final segmentation. For shape analyses, we use a local shape metric that is estimated using the nonlinear deformations fields in the segmentation process. The average deformation is then used to estimate displacement of the striatal surface with respect to a reference model. (based on a modification of the metric previously reported in (Lerch et al., 2008)) We hypothesized that there would be clear differences in the striatal development, as measured by volume and shape trajectories, between the COS cohort with respect to NVs. We also hypothesized that the healthy COSSIBs would have an at least partially overlapping pattern of striatal development, homologous to that observed for sibling cortical development (Gogtay et al., 2007), again indicating an age specific endophenotype.
MATERIALS AND METHODS
Recruitment of COS Subjects, their Healthy Unaffected Siblings and Normal Controls
Subject recruitment has been described in detail in previous publications (Greenstein et al., 2006; Rapoport and Gogtay, 2010, Int J Dev Neurosci). For this study, prospective anatomic brain MRI scans were obtained from 86 COS patients who met unmodified DSM-IIIR/IV criteria for schizophrenia with the onset of psychosis before age 13 and 71 of their nonpsychotic full siblings, also rescanned every 2 years. Siblings are considered “nonpsychotic or healthy” if they do not have any schizophrenia spectrum abnormalities either on Axis I or Axis II (Asarnow and Ben-Meir, 1988; Asarnow et al., 2001; Nicolson et al., 2003).
All NVs included in this study were a subset of subjects recruited from the community as part of a larger prospective study of normal brain development (Giedd et al., 1999). Subjects are clear of lifetime medical and psychiatric disorders as determined using structured and standardized interview. First-degree relatives with psychiatric illness were also an exclusion criteria. The NVs used in this study were matched to the COS group based on sex and age. Most COS subjects are on clozapine at the time of discharge from the NIMH and prescribed to remain on it.
The NIMH institutional review board approved the study and informed consents and assents were obtained from the parents and/or participants, respectively.
Acquisition of MRI Data
To study the brain anatomy of these subjects, T1-weighted MRI data were used. All data were acquired on a 1.5T General Electric Signa scanner (Milwaukee, WI, USA) using a 3D spoiled gradient echo with contiguous 1.5 mm slices in the axial plane. The image acquisition parameters were echo time of 5 ms, repetition time of 24 ms, flip angle of 45°, acquisition matrix of 256 × 192, number of excitations equals 1, and 24 cm field of view. Head placement was normalized as described in (Castellanos et al., 2001). In total, data used in this study from the three groups were as follows: 81 NV (54 male, 27 female; 186 scans), 86 COS (50 male, 36 female; 194 scans), and 71 COSSIB (34 male, 37 female; 141 scans).
Automated Identification, Volumetry, and Shape Analysis of the Striatum
The striata were automatically identified using a newly developed segmentation method known as the MAGeT Brain algorithm (Chakravarty et al., 2013). Traditionally, automated model-based segmentation methods rely on the expert identification of a neuroanatomical structure on a single well-define template by single or multiple raters (Bajcsy et al., 1983; Collins et al., 1995; Miller et al., 1997). These labels are then customized to a specific subject's unique neuroanatomy through first estimating a subject-to-template nonlinear transformation and then applying the inverse transformation to the labels (Chakravarty et al., 2009b, 2009c). These types of segmentations may be limited in accuracy by errors in the transformation estimation, irreconcilable neuroanatomical differences between the neuroanatomy of the template and the subject, and resampling errors after the application of the transformation to the labels. MAGeT Brain limits these errors by using a “multi-atlas” based strategy while still relying on a single well-defined template.
To summarize the technique, a single atlas containing the striatum (previously defined using a three-dimensional reconstruction of serial histological data (Chakravarty et al., 2006) and warped to a high-resolution and -contrast MRI based on the average of 27 MRIs of the same individual (Holmes et al., 1998). The atlas is first customized to a subset of the dataset (30 subjects) using a nonlinear transformations estimated in a region-of-interest defined around the subcortical structures (Chakravarty et al., 2008, 2009a, 2009b, 2009c, 2012). This set of subjects now acts as a set of templates and all other subjects are now warped to these templates. This provides 30 candidate segmentations for each subject. The final segmentation is decided on using a voxel-wise majority vote (ie: the label occurring most frequently at a specific location is retained (Collins and Pruessner, 2010)). In our original article, we demonstrate high overlap between our method and gold standards manually derived on MRI data from the NIMH (Dice Kappa of 0.861 when using MAGeT Brain with the ANIMAL algorithm for nonlinear transformation estimation (Chakravarty et al., 2013)).
Shape analysis was carried out using a surface-based methodology originally proposed by Lerch et al. (2008), but modified to take advantage of the multiple nonlinear deformation fields that map each subject to the original atlas. We have recently used these techniques in an analysis of local surface area characteristics in subcortical structures through the course of normal development (Raznahan et al., 2014) and in neuropsychiatric disorders (Shaw et al., 2014a, 2014b) and striatal shape characteristics in addictions (Janes et al., 2014). First, surface-based representations of the striata defined on the input atlas were estimated using the marching cubes algorithm (Lorensen and Cline, 1987) and morphologically smoothed using the AMIRA software package (Visage Imaging; San Diego, CA, USA). The resulting surfaces have about 6,300 vertices per striatum. The nonlinear portions of the 30 transformations that map each subject to the input template were concatenated and then averaged across the template library to limit the effects of noise and error and to increase precision and accuracy (Borghammer et al., 2010; Dorr et al., 2008; Frey et al., 2011). The dot product between the nonlinear deformation vector (of the inverse of the averaged atlas-to-subject transformation) and the striatum surface normal (a unit vector describing the direction perpendicular to the surface) is estimated and provides a local measure of inward or outward displacement along the normal. Note that prior to estimating the dot product between the normal to the surface and the deformation field that we explicitly model and remove and global linear effects not originally accounted for in the initial linear transformations. This ensures that the contributions of overall differences in brain volume are minimized in this analysis. The segmentation and morphometric methodology are summarized in the Supporting Information Figure S1.
Statistical Analysis
Demographic characteristics between groups were compared using a general linear model for continuous variables and χ2 tests were used for categorical variables.
Both total volumes and vertex-wise shape measures were analyzed using mixed-model regression as it permits the inclusion of multiple measurements per person at different ages and at irregular intervals between measurements (Pinheiro and Bates, 2000). In the final model (for all volumes or each vertex on the striatum), a random-effects intercept was used. To account for family dependencies random effects included an intercept per family and an intercept for a person nested within a family. To better understand the trajectories of subcortical shape, the model was reused but age-recentred at 2-yr intervals throughout the age range of all study-participants. This approach permits the interpretation of the intercept and group differences using the entire dataset at the centered age instead of with respect to age zero. As a result, this approach does not require that the dataset be split into age ranges. Shape differences at each age-centered analysis can be considered to be a snapshot of the difference between two group's regression lines at a specified age. This is the approach taken by previous studies in our group (Giedd et al., 2014; Gogtay et al., 2007; Greenstein et al., 2006; Raznahan et al., 2011). The age-centered analyses were performed and are only presented for the vertex-wise analyses presented in this manuscript. All vertex-wise analyses were corrected for multiple comparisons using the false discovery rate (FDR) (Genovese et al., 2002).
RESULTS
In general, there were no significant differences between COS and COSSIBs for most of the demographic variables. The COS and COSSIBs had lower socioeconomic status (SES) compared to NVs (P < 0.001 and P = 0.003; respectively) and the COS subjects exhibited lower verbal IQ scores (P < 0.001) compared to controls. See Table 1 for details. Supporting Information Figure S2 demonstrates the distribution of age and number of scans through all study participants.
| Characteristic | Healthy control (n = 81) | COS (n = 86) | COSSIB (n = 71) | Test statistic with df (P-value) | |
|---|---|---|---|---|---|
| COS vs. NV | COSSIB vs. NV | ||||
| Sex | |||||
| Female | 27 | 36 | 37 | Χ2 (df = 2) = 4.74 (P = 0.10) | |
| Male | 54 | 50 | 34 | ||
| Handedness | |||||
| Left | 2 | 9 | 2 | Χ2 (df = 4) = 7.27 (P = 0.12) | |
| Right | 73 | 65 | 64 | ||
| Mixed | 6 | 12 | 5 | ||
| Race | |||||
| Asian | 0 | 6 | 3 | Χ2 (df = 8) = 9.29 (P = 0.32) | |
| Black/African American | 15 | 28 | 15 | ||
| Hispanic | 1 | 0 | 0 | ||
| Other/mixed race | 5 | 3 | 0 | ||
| White | 60 | 59 | 53 | ||
| Socioeconomic status, mean (SD) | 39.97 (20.84) | 60.82 (31.39) | 50.97 (27.25) | t = 7.4 (P < 0.001) | t = 3.58 (P = 0.003) |
| Vocabulary score, mean (SD) | 12.02 (2.46) | 6.85 (3.42) | 11.32 (3.03) | t = −16.17 (P < 0.001) | t = −1.95 (P = 0.502) |
| Age at scan: Overall mean (SD) | 17.11 (4.46) | 17.58 (3.91) | 18.62 (5.07) | t(df = 502) = 1.01 (P = 0.31) | t(df = 502) =2.993 (P = 0.003) |
| Number of scans: | |||||
| 1 total (M; F) | 23 (14;9) | 37 (18; 19) | 36 (18;18) | NA | |
| 2 total (M; F) | 31 (23;8) | 16 (11; 5) | 13 (6;7) | NA | |
| 3 total (M;F) | 14 (11;3) | 19 (13; 6) | 13 (8;5) | NA | |
| 4 total (M;F) | 9 (5;4) | 6 (3; 3) | 5 (1;4) | NA | |
| 5 total (M;F) | 2 (0;2) | 4 (2;2) | 4 (1;3) | NA | |
| 6 total (M;F) | 1 (1;0) | 4 (3;1) | 0 | NA | |
| 7 total (M:F) | 1 (0;1) | 0 (0;0) | 0 | NA | |
Volume Analysis
The analysis of overall striatal volume, using a mixed-model regression, revealed significant age-related increases for COS subjects compared to NVs for both the left (t = 2.48, P = 0.013) and right (t = 2.45, P = 0.015) striatum. Comparison of COSSIBs compared to the COS demonstrated trend-level volume differences (COS > SIB) through the developmental trajectory for the left (t = 1.63, P = 0.10) and right (t = 1.69, P = 0.091) striatum. No significant differences were observed when comparing NVs to COSSIBs for either left (t = 0.27, P = 0.78) or right striata (t = 0.15, P = 0.98). Striatal volumes were quite similar through all three groups up until age 8 followed by an age-related increase only in the COS cohort (Fig. 1).
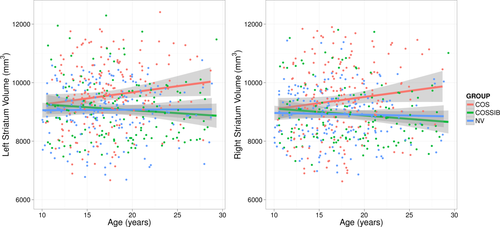
Striatal volumes over the developmental trajectory for COS, COSSIBs, and NV. Note that all three groups start with the same absolute volumes at the beginning of the developmental trajectory, but the COS group volume through the course of maturation. Shaded areas indicate 95% confidence intervals.
Shape Analysis
Vertex-wise shape analyses demonstrate overlapping patterns of shape differences relative to NV in both COS (Fig. 2A,D) and COSSIBS (Fig. 2B,E). Outward displacements were observed in the ventral aspect of the striatum. In COS (Fig. 2A), these displacement are medially focused in the caudate and putamen and along the posterior edge of the putamen and the tail of the caudate. In COSSIBS (Fig. 2B) there is significant overlap with the outward displacements localized in the posterior aspect of the putamen and the tail of the caudate; however, the medial-ventral shape differences are far more medially focused. There is one region of nonoverlapping outward facing displacement (Fig. 2C) localized primarily in the body of the caudate in COS compared to NVs.
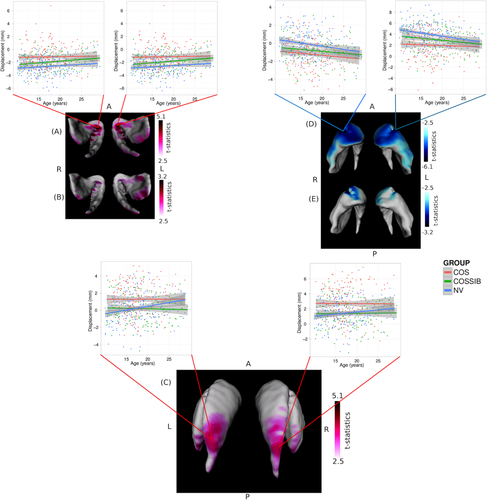
Shape differences estimated as significant outward or inward displacements along the surface representation. In all image orientations are given for anterior, posterior, left, and right as A, P, L, and R, respectively. All outwards displacements are shown in hot colors (red) and all inward displacements are represented in cool colors (blue). Significant outward displacement in overlapping regions for both (A) the COS and (B) COSSIBs (relative to NVs) were observed in the ventral medial portion of the striatum. Striata are oriented such that the view is ventral and the posterior portion of the striata are exiting the page. A shape difference in the COS subjects relative to the NVs was also observed in (C) the body of the caudate. Significant inward displacements were also estimated along the entire ventral anterior portion of the striatum in both (D) the COS and (E) COSSIBs.
Inward displacements were observed in the COS group relative to the NVs along the entire anterior aspects of the head of the striatum and laterally along the head of both the caudate and putamen and the body of the caudate (Fig. 2D). COSSIBs shared a portion of this shape signature along the head of the striatum in both the caudate and the putamen (Fig. 2E).
Age Effects on Shape
Age-centered analyses demonstrated a pattern of overall shape change through the maturation trajectories in both COS and COSSIBs relative to the NV group (see Fig. 3A). Outward displacement differences were colocalized in the ventral regions previously described in the results of the multiple linear regression. In the COS cohort, the differences appeared to be mostly static through the course of adolescence (10–18 years old) and decreased toward the beginning of early adulthood. The COSSIBs shared a portion of this difference only in early ages where most of these shape differences are localized to the left striatum, the abnormalities improved substantially in early adult life (22–24).
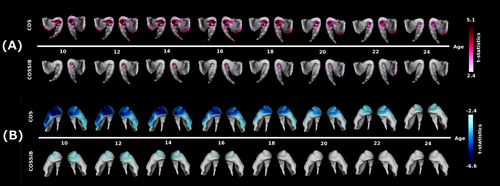
(A) Age-centered analysis for outward facing displacement along the surface. In the COS group, outward facing displacements are seen all along the ventral-medial-posterior aspect of the head of the striatum. This shape difference relative to the NV group is most severe until about age 18 and then begins to dissipate. The COSSIBs demonstrate a similar shape difference relative to NVs, but normalize in the early 20 s. (B) Age-centered analysis for inward facing displacement along the surface. In the COS group, outward facing displacements are seen all along the ventral-medial-anterior aspect of the head the striatum. This shape difference relative to the NV group is most severe until about age 18 and then begins to dissipate. The COSSIBs demonstrate a similar shape difference that normalizes toward age 18.
The age-centered analyses of inward displacement demonstrated a different pattern (see Fig 3B). Shape differences in the striatum in both the COS and COSSIBs relative to the NV group seemed to be largest at the earliest time points (ages 10–12) and the neuroanatomical localization of these differences is consistent with the shape differences described above in the vertex-wise mixed-model regression. However, as the COS group enters later adolescence and early adulthood, the shape difference decreased and was localized to the head of the striatum. In the COSSIBs, the shape differences was mostly localized to the head of the striatum at early age and no longer existed after age 18.
DISCUSSION
This is the first study to analyze the subregion specific longitudinal development of the striatum using a novel shape detection algorithm in a group of subjects suffering from COS, their full non psychotic siblings, and matched controls (Gogtay et al., 2007; Rapoport and Gogtay, 2010, Int J Dev Neurosci). Our results demonstrate that the COS, COSSIB, and NV groups all had similar striatal volumes at younger ages (approximately, 8–9 years old). However, the COS group showed a steady increase in striatal volume with age compared to the NV group whereas the striatal volume in COSSIBs remained unchanged from controls with age. The results of our shape analyses demonstrate there were significant subregional shape differences between COS and NVs which were at least partially shared by COSSIBs, suggesting a more subtle, age-specific endophenotypic nature of sub regional striatal development.
Historically, understanding striatal volume in major psychosis has been fraught with confounding factors due to medication. For example, there are several reports in the literature that demonstrate a relationship between chronic haloperidol (a typical antipsychotic) treatment and increase in striatal, specifically caudate nucleus, volume (Chakos et al., 1994; Dazzan et al., 2005; Ho et al. 2011; Keshavan et al., 1994; Tost et al., 2010) while others report conflicting data regarding increased (Glenthoj et al., 2007), unchanged (McClure et al., 2008), or decreased striatal volume (van Haren et al., 2007) in response to treatment with atypical antipsychotics such as rispiridone. Therefore, unmedicated nonpsychotic full siblings present a unique opportunity to analyze the relationship between psychosis and striatal volume. For example, larger caudate nucleus volumes were demonstrated in a study of nonpsychotic ultrahigh risk individuals who are members of a multicomplex family where at least one parent suffers from psychosis (Hajek et al., 2009). Similarly, studies of patients with schizotypal personality disorders, where medication confounds are typically not present, also support alterations of striatal volumes in schizophrenia spectrum disorders (Koo et al., 2006; Levitt et al., 2009). However, other groups have demonstrated no differences in striatal volume in ultrahigh risk individuals that convert to psychosis compared to those who do not (Hannan et al., 2010) nor in monozygotic twins discordant for psychosis (Ettinger et al., 2012).
In addition to the heterogeneity of these findings, it is difficult to draw inferences from AOS studies as these individuals have a much later age-of-onset. Further, the heterogeneity from the volumetric studies suggests that the adult onset phenotype may not be particularly robust in understanding the role of striatal alterations in schizophrenia. Our results demonstrate that shape may be capturing aspects of the striatal structure that may serve as a complement to volumetric analyses. This is evidenced by the heterogeneity of the findings reviewed in the Introduction of the manuscript.
The shape phenotype that we demonstrate show an inward displacement in the COS and COSSIBs at the anterior portion of the striatal head and the outward displacement at the posterior portion of the striatal head. It is known from probabilistic tractography studies the tracts from the striatal head project extensively to the prefrontal cortex (Leh et al., 2007) which shows cortical thickness deficits in both COS and, to a lesser extent, in COSSIBs (Gogtay et al., 2007; Greenstein et al., 2006; Rapoport and Gogtay, 2011). Both these regions are heavily implicated in the pathophysiology of schizophrenia relevant to the dopamine hypothesis (Davis et al., 1991; Weinberger, 1987). The overlapping improvement with age in the striatal head between COS and COSSIBS may be related to the improvement in cortical thickness deficits probably suggesting a common mechanism for the synchronized structural plasticity (Gogtay et al., 2008, 2012).
Our knowledge of neuroanatomical fronto-striatal connectivity is derived largely from experiments in nonhuman primates (Alexander and Crutcher, 1990; Nambu et al., 1997; Takada et al., 1998a, 1998b; Zaborszky, 2002); although there is some exploration of this connectivity based on diffusion tensor imaging studies (Gutman et al., 2009; Leh et al., 2007). The orbitofrontal cortex is known to project to the ventro-medial part of the caudate nucleus (Cummings, 1993) while the nucleus accumbens and ventral striatum project to the frontal poles and to the medial frontal cortex (Cummings, 1993). Generally speaking fronto-striatal connections are thought to form several parallel circuits that connect the frontal lobes, striatum, pallidum, and thalamus (specifically the ventral anterior and medio-dorsal subnuclei) (Cummings, 1993). It is likely that many specialized circuits and subcircuits exist within these connections that permit concurrent multilevel processing (Zaborszky, 2002) related executive function and flexibly to the maintenance or shifting of sets (Cummings, 1993); functions that are known to be compromised in schizophrenia (Freedman and Brown, 2011). Detailed tract tracing studies in nonhuman primates also demonstrate that there are two types of projections from the dorsolateral prefrontal cortex (DLPFC) to the head of the striatum: focal, targeted projections, and diffuse, widespread projections (Eblen and Graybiel, 1995). This region at the head of the striatum extending posterior to the dorsal striatum is involved in the integration of information contained in the DLPFC (Calzavara et al., 2007; Graybiel, 1998; Reep et al., 2003), thus making it integral in working memory and executive function and the subsequent ability to derive novel actions and processes from this integration process (Calzavara et al., 2007). Taken together, along with previous reports of executive function deficits reported in unaffected siblings of COS patients (Gochman et al., 2004) and recent tensor-based analysis of the white matter structure showed slowing in white matter growth rates, particularly in the frontal lobes in COS (Gogtay et al., 2008) as well as in the young healthy siblings (Gogtay et al., 2012), these findings strongly suggest impaired fronto-striatal circuitry in COS as an age specific endophenotype.
In adolescence, the projections between subcortical regions and frontal lobe structures are amongst the last to develop and are the sites of potential neuroanatomical deficits implicated in the pathophysiology of schizophrenia (Gogtay et al., 2004, 2012; Paus et al., 2008). However, due to the timing of COS (prior to the initiation of puberty, therefore prior to the frontal lobe maturation); the fronto-striatal circuits may also show deficits both due to lack of maturity as well as genetic liability indicated by the shared shape abnormalities in the COSSIBs. These abnormalities may also suggest altered maturation of the DLPFC-striatal circuitry (unlikely to be the effect of medication because also shared by siblings) which should be explored further with connectivity analyses.
It is tempting to speculate about the nature of nonoverlapping endophenotype in the body of the caudate. Is it possible that this shape difference may, at least partially, be the product of medication effects? Although we did not explicitly test this hypothesis it is in line with some of the previous studies of medication effects on striatal volume (Chakos et al., 1994; Dazzan et al., 2005; Ho et al., 2011; Keshavan et al., 1994; Tost et al., 2010) and the lack of overlapping deficits in healthy siblings further support the medication influence in probands. However, testing this in the current dataset would require a disentanglement of the altered developmental trajectory in COS and the potentially differential effects of typical and atypical antipsychotics on striatal morphology. The severity of the COS phenotype makes such analyses difficult and medication naïve COS subjects are impossible to obtain.
Other limitations should be considered. The differences between the vocabulary and the SES scores of the NV and COS groups could influence the findings. Although there are established correlates between intelligence, cognitive ability, and SES and neuroanatomy (Shaw et al., 2006), these differences are also likely to be intrinsic to schizophrenia phenotype (Barch and Ceaser, 2012). It should also be noted that there were no significant volumetric differences between NVs and COSSIBs, therefore the sibling shape differences found were unaccompanied by cognitive differences. As a result, the shape phenotype that we demonstrate in both the COS and COSSIBs represents a complex trait marker.
ACKNOWLEDGMENTS
The authors would like to thank SciNet for being able to use Canada's largest supercomputer platform, which is funded by the Canada Foundation for Innovation, NSERC, the Government of Ontario, Fed Dev Ontario, and the University of Toronto.




