Brain default-mode network abnormalities in hepatic encephalopathy: A resting-state functional MRI study
Abstract
Many neuroimaging investigations focus on hepatic encephalopathy (HE); however, few investigate default-mode network (DMN) in the patients with HE and its underlying physiological relevance using resting-state fMRI. In this study, independent component analysis was used to retrieve components representing the DMN of patients with HE (n = 14) and healthy volunteers (n = 14). Four patients were excluded because of head motion (n = 3) and the artifact from the artificial tooth (n = 1). Comparison results between the two groups revealed significantly reduced functional connectivity in the right middle frontal gyrus and left posterior cingulate cortex in the HE patients. A statistical t-map from the comparison of venous blood ammonia levels and the z-scores of the DMN obtained from independent component analysis was computed in the HE group, which showed negative correlation with the changes in left angular gyrus. In conclusions, resting-state fMRI can be used to examine DMN changes in HE patients. Reduced functional connectivity in the right middle frontal gyrus and left posterior cingulate cortex consisting of the DMN and negative correlation between the functional connectivity changes in left AG and the venous blood ammonia levels support the notion of damages in functional organization of the central nervous system in HE patients. Hum Brain Mapp, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome that develops in patients with severe liver disease and/or portal-systemic shunting, which is characterized by a wide spectrum of clinical manifestations, ranging from alterations of psychometric performance to stupor and coma. Even though many neuroimaging investigations have been performed for the disease, few studies aimed at uncovering the neural mechanism of HE. One author [Schiff et al., 2006] evaluated the efficiency of top-down and bottom-up processes in the extrastriate cortex of cirrhotic patients without overt HE, using event-related potentials (ERP). They found that top-down processes are altered, whereas bottom-up processes are preserved, in the extrastriate cortex of cirrhotic patients without HE. Another author [Zafiris et al., 2004] analyzed pathologically impaired neural mechanisms of cirrhotic patients using functional magnetic resonance imaging (fMRI) with critical flicker frequency as the target task. An early impaired and compensatory neural mechanism was detected during visual judgment in the earliest stages of HE, suggesting an aberrant coupling between cerebral regions in the dysmetabolic brain. In a previous study [Zhang et al., 2007a], we adopted a modified Chinese Stroop test as the target stimulus to observe task-related brain activation in cirrhotic patients using fMRI, in which the abnormal brain network of the anterior cingulate cortex-prefrontal cortex-parietal lobe-temporal fusiform gyrus is found to be the neural basis of cognitive control impairment in cirrhotic patients.
In the past decade, increasing attention has been focused on detecting brain activities in a resting state [Biswal et al., 2010; Buckner et al., 2008; Damoiseaux et al., 2006; Fox et al., 2005; Greicius et al., 2003; Greicius et al., 2004; Raichle et al., 2001; Raichle et al., 2007], with a network consistently existing in healthy subjects and patients. The default-mode network (DMN), consisting of medial prefrontal cortex, rostral anterior cingulate, posterior cingulate, and precuneus, is the most important sub-network in the resting-state networks [Biswal et al., 2010; Buckner et al., 2008; Damoiseaux et al., 2006; Fox et al., 2005; Greicius et al., 2003; Greicius et al., 2004; Raichle et al., 2001; Raichle et al., 2007]. It is known that DMN has high metabolic activity during the rest and suppressed metabolic activity during cognitively demanding tasks, such as visual and auditory attention, language processing, memory and motor activity [Buckner et al., 2008; Greicius et al., 2003]. Abnormal DMN has been shown to be related to the Alzheimer's disease (AD) [Greicius et al., 2003], autism [Paakki et al., 2010], attention deficit hyperactivity disorder [Uddin et al., 2008], schizophrenia [Hoptman et al., 2010], epilepsy [Zhang et al., 2010a, b], and other diseases [Buckner et al., 2008]. Some authors believed that the abnormal default function network can be an early marker for AD [Greicius et al., 2003] or can be used for determining laterality for epilepsy [Zhang et al., 2010a, b]. However, little is known about the connectivity among DMN in HE patients. In a previous study, the DMN of the cirrhotic patients was investigated using a blocked-design fMRI in which a block design was used with modified Chinese Stroop task as the target stimulus [Zhang et al., 2007b]. To the best of our knowledge, there has been no report in the English medical literature, for investigation of DMN activities of cirrhotic patients using a resting-state fMRI (RS-fMRI) method and to quantitatively assess the correlation of DMN with blood ammonia, an important biomarker in HE patients. Our hypothesis is that HE, resulting from neuro-toxic effect of several compounds that are normally metabolized in the liver but enter into the brain as a result of liver dysfunction, likely affects multiple functional systems in DMN, resulting in diffusely decreased functional connectivity in DMN. In this work, we extracted the DMN changes in patients with HE compared with healthy volunteers, and examined the correlation between the DMN changes and blood venous ammonia in these HE patients using a RS-fMRI scheme.
Abbreviations
-
- ACC
-
anterior cingulate cortex
-
- AG
-
angular gyrus
-
- DMN
-
default mode network
-
- fMRI
-
functional magnetic resonance imaging
-
- HE
-
hepatic encephalopathy
-
- ICA
-
independent component analysis
-
- MFG
-
middle frontal gyrus
-
- PCC
-
posterior cingulate cortex
MATERIALS AND METHODS
Participants
This prospective study was approved by our institutional review board and was conducted in compliance with the Health Insurance Portability and Accountability Act. All subjects gave written informed consent before the fMRI study. HE was defined and classified according to a final report of the working party at the 11th World Congresses of Gastroenterology in Vienna in 1998 [Ferenci et al. 2002]. Fourteen patients (nine men, five women; mean ages: 59 years ±10; age range: 44–71 years) with HE were recruited for this study. The inclusion criteria for recruitment of the patients were as following: the patients with clinical proven HE at the Stages 1–3, who could finish the fMRI exam without any MRI contraindication and head motion less than 1 mm, age 18 years or older, and without dental fixtures or other foreign bodies in the head causing significant image artifacts. All the patients had chronic rather than acute liver dysfunction, with complete laboratory tests to evaluate liver function and blood ammonia before MR examination. All patients were right-handed with normal sight. They had no other diseases affecting brain functions, such as drug abuse and trauma.
Fourteen age- and gender-matched normal subjects (12 men, 2 women; mean ages, 52 years ±10; age range, 40–63 years) without history of psychiatric or neurologic diseases were recruited from the local community. All normal subjects had also no diseases of the liver and other systems. Abdominal ultrasound scans revealed no abnormal findings for all normal subjects.
Laboratory Examinations
Blood biochemistry tests, including prothrombin time, protein metabolism tests (including total protein, globulin, albumin, and the ration of albumin and globulin), bilirubin metabolism tests (including total bilirubin, direct bilirubin, and indirect bilirubin), glutamic pyruvic transaminase, and glutamic oxalacetic transaminase, were performed for all patients within one week before MR scanning. All of the above-mentioned tests were used to calculate the Child-Pugh score [Pugh et al., 1973] to assess the severity of liver disease. The score system considered five variables, i.e., ascites, encephalopathy, prothrombin time, and serum levels of bilirubin and albumin, and assigned a score ranging from 1 to 3 to each variable, classifying patients into Class A (score 5–6), B (score 7–9), or C (score 10–15). Venous blood ammonia test was obtained in all patients within 24 h prior to MR scan. No laboratory tests were performed thus unavailable for the 14 normal subjects.
MRI Data Acquisition
MRI data were collected using a 1.5-Tesla scanner (GE Signa, Milwaukee, WI). The participants were instructed to rest with their eyes closed and keep their heads still during MRI scans. Axial anatomical images were acquired using a T1-FLAIR sequence (TR/TE = 2,200 ms/24 ms, matrix = 512 × 512, field of view = 24 × 24 cm, slice thickness/gap = 4.0 mm/0.5 mm, 23 slices covered the whole brain) for image registration and functional localization. The scanning time was 216 s. Functional images were subsequently collected in the same slice orientation with a GRE-EPI sequence (TR/TE = 2,000 ms/40 ms, flip angle = 80°, matrix = 64 × 64, field of view = 24 × 24 cm) to measure 200 brain volumes, which lasted for a total of 400 s.
Data Preprocessing
SPM2 (Statistical Parametric Mapping, http://www.fil.ion. ucl.ac.uk/spm/) was used to preprocess the fMRI data. After slice-timing adjustment and realignment for head-motion correction, three HE patients whose head motion exceeded 1.0 mm or rotation exceeding 1.0° during scanning were excluded. The standard Montreal Neurological Institute (MNI) template in SPM2 was used for spatial normalization with a resampling voxel size of 3 × 3 × 3 mm3. Spatial smoothing was then performed with an 8-mm FWHM Gaussian kernel.
Region of Interest Extraction
To examine the DMN in both groups, spatial independent component analysis (ICA) was first performed for 10 patients and 14 controls to decompose the data for each individual into 50 independent components with the infomax algorithm using the GIFT software (http://icatb.source forge.net/) in accordance with our previous studies [Zhang et al., 2009, 2010b]. The independent components (ICs) corresponding to the DMN were extracted based on the templates described previously [Mantini et al., 2007; Zhang et al., 2009]. The number (50) of independent components in each session of data was determined by a dimension estimation using the minimum description length criteria modified to account for spatial correlation. For each independent component, its time course corresponds to the waveform of a specific pattern of coherent brain activity, and the intensity of this pattern of brain activity across the voxels is expressed in the associated spatial map. To display the voxels that contributed most strongly to a particular independent component, the intensity values in each spatial map were converted to a z-score. Since the resting state connectivity should only be detected in a very low-frequency range, the components whose time courses showed the maximum power within a high frequency range (>0.1 Hz) were removed. After the ICA separation, DMN templates were then used to select the “best fit” of the remaining low-frequency components in each subject. Briefly, a template-matching procedure was used by taking the average z-score of voxels falling within the template minus the average z-score of voxels outside the template and selecting the component in which this difference (the goodness-of-fit) was the greatest. The z-scores used here reflect the degree to which a given voxel's time series correlates with the time series corresponding to a specific ICA component, scaled by the standard deviation of the error term. Therefore, the z-scores could be used to measure how much of the standard deviation of the signal was from the background noise. The z-scores of each DMN were then gathered in each group for a random-effect analysis using a one-sample t-test. Thresholds were set at P < 0.05 [correction using the false discovery rate (FDR) criterion]. Subsequently, the z-scores of each DMN were compared between the patient and the control groups using two-sample t-tests. To control type I error in this analysis, an integrated threshold at a corrected level of P < 0.05 determined with the AlphaSim program in AFNI (written by D. Ward, http://afni.nih.gov/afni/docpdf/AlphaSim.pdf) was used to generate a statistical map. The threshold was a combination of P < 0.01 for single voxel, a minimum cluster size of 24 voxels, 10,000 simulations, and FWHM = 8 mm.
Statistical Analysis
Statistical analysis was performed using the software SPSS version 13.0 (SPSS Inc. Chicago, IL) and SPM2 (statistical parametric mapping, http://www.fil.ion.ucl.ac.uk/spm/). Independent sample student t-tests were used to compare the average z-scores of each control and HE patient. Box plots were used to graphically display the medians, upper extremes, lower extremes, upper quartiles and lower quartiles of z-scores in the controls and HE patients. We first selected DMN components using DMN mask for each subject in our study. DMN components selected were then combined as a mask for the group, and the group masks were used to observe the brain region changes in DMN mask when two sample student t-test was performed between the patient and control groups. A Pearson correlation analysis was performed to investigate the relationship between average z-scores of the DMN in HE patients and venous blood ammonia, an important biomarker for HE. The data of z-maps of the DMN component (within the mask of the DMN) were also correlated to the venous blood ammonia using a voxel-wise correlation analysis in SPM2 in the patient group. Identical regions of interest derived from the single sample t test were used for all subjects when we used DMN mask at the first and second time. The correlations between the z-scores of the DMN regions in the HE patients and the Child-Pugh scores were also investigated. P values less than 0.05 were regarded as statistically significant.
RESULTS
Four patients were excluded because of head motion (n = 3) and image artifacts from artificial tooth (n = 1), and the data sets from other remaining 10 patients were included into final data analysis. Of the remaining available 10 patients, 5 patients had a previous history of hepatic encephalopathy (HE) or overt clinical symptoms of HE; the remaining 5 patients were encountering HE for the first time. The patient demographics and clinical data were summarized in Table I.
| Patient No. | Age (Y)/Gender | Etiology/Duration (Mo) | Episode | Child-Pugh scale | Ammonia (μmol/L) | Stage of HE |
|---|---|---|---|---|---|---|
| 1 | 67/M | Schistosome cirrhosis/110 | Multiple | C | 234 | 3 |
| 2 | 56/F | Post-TIPS/1 | First | B | 50 | 1 |
| 3 | 51/M | UA/1 | First | C | 104 | 1 |
| 4 | 70/F | Autoimmune cirrhosis/48 | Multiple | C | 69 | 3 |
| 5 | 68/M | Post-TIPS /11 | Multiple | C | 76 | 3 |
| 6 | 57/F | HCC/1 | First | B | 74 | 2 |
| 7 | 65/F | HCC/2 | First | B | 122 | 2 |
| 8 | 63/F | UA/96 | Multiple | B | 64 | 2 |
| 9 | 48/M | HCC/5; BC/48 | First | B | 92 | 2 |
| 10 | 46/M | UA/36 | Multiple | C | 181 | 2 |
- Y, year; Mo, month; TIPS, Transjugular intrahepatic porto-systemic stent shunt; UA, unknown etiology; BC, b-hepatitis cirrhosis; HCC, hepatic cellular carcinoma.
DMN was successfully extracted from each patient and control included. The results from one-sample t-test showed a strong functional connectivity for the controls at the rest state in bilateral posterior cingulate cortex (PCC) and adjacent precuneus, angular gyri, anterior cingulate cortex (ACC), middle frontal cortex, and medial temporal cortex (P < 0.05, FDR corrected). Figure 1A illustrates the DMN of the controls overlaying on the standard MNI template.
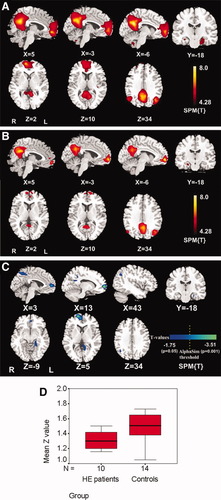
DMN of the controls and HE patients. A: DMN of the controls consists of bilateral precuneus/posterior cingulate cortex, medial prefrontal cortex, anterior cingulate cortex, angular gyri, and temporal pole (P < 0.05, FDR corrected). B: DMN of the HE patients consists of bilateral precuneus/posterior cingulate cortex, medial prefrontal cortex, anterior cingulate cortex, angular gyri, and temporal pole (P < 0.05, FDR corrected). Patterns in the HE patients are similar but with reduced size compared to the ones of normal subjects in (A). C: Differences between the DMN of the HE patients and the controls were in the left posterior cingulate cortex and bilateral precuneus, right angular gyrus, bilateral middle frontal cortex, and left parahippocampus (P < 0.05 for all, uncorrected) and in the right middle frontal gyrus and left posterior cingulate cortex (P < 0.05 corrected, combined height threshold P < 0.01 and a minimum cluster size of 24 voxels). D: Independent sample student t test of z-scores of the DMN of the controls and the HE patients retrieved by independent component analysis showed decreased z-scores in the HE patients (P = 0.029).
A similar DMN but with some diffuse impairment of brain areas was observed in the HE patients (Fig. 1B) compared to those in controls. The results from one-sample t-test showed decreased functional connectivity in bilateral PCC and adjacent precuneus, angular gyri, ACC, middle frontal cortex, and medial temporal cortex for the HE patients at the rest state (P < 0.05, FDR corrected). Comparison results from an independent sample student t test revealed that there was significantly reduced functional connectivity in the HE patients compared with the controls in the left PCC and bilateral precuneus, right angular gyrus, bilateral middle frontal cortex, and left parahippocampus (P < 0.05, uncorrected; Fig. 1C and Table II); functional connectivity in the HE patients in the right middle frontal gyrus (MFG) and left PCC was significantly reduced after corrected (Fig. 1C and Table II). Although left precuneus and left parahippocampus have no statistically reduced functional connectivity in the HE patients compared with controls after correction, the trend of reduced functional connectivity in these regions can be observed (Fig. 1C and Table II).
| Brain areas | MNI coordinates (mm) | Brodmann area | Uncorrected Voxela | Corrected Voxel | Corrected P value |
|---|---|---|---|---|---|
| X, Y, Z | |||||
| Right MFG | 9, 60, 0 | 10 (R) | 98 | 74 | <0.001 |
| Left PCC | −12, −54, 6 | 30 (L) | 61 | 26 | 0.036 |
| Right precuneus | 6, −62, 48 | 7 (R) | 56 | >0.05 | |
| Left parahippocampus | −24, −39, −6 | 30 (L) | 40 | >0.05 | |
| Left precuneus | −3, −60, 51 | 7 (L) | 20 | >0.05 | |
| Left MFG | −6, 45, −12 | 10 (L) | 17 | >0.05 | |
| Right AG | 42, −60, 33 | 39 (R) | 15 | >0.05 |
- MNI, Montreal Neurological Institute; PCC, posterior cingulate cortex; MFG, middle frontal gyrus; AG, angular gyrus; R, right; L, left.
- a Uncorrected P values were less than 0.05 for all data.
Independent sample student t test of z-scores of the DMN of the controls and the HE patients retrieved by ICA showed a statistically lower z-scores in the HE patients than one in the controls (P = 0.029) (Fig. 1D). No statistical difference was found for the z-scores of the above-mentioned DMN brain regions in HE patients, with all P values larger than 0.05.
An average z-score from the whole DMN of each patient showed no significant correlation with blood ammonia levels (r = 0.606, P = 0.063); however, a negative correlation was found between diseased brain areas of the DMN in HE patients and venous blood ammonia levels. Figure 2 is a statistical t-map of venous blood ammonia levels against z-scores in ICA in the HE group, which showed a negative correlation in right MFG, left PCC, left parahippocampus, bilateral angular gyri color coded dark blue (uncorrected, P < 0.05 for all) and in the left angular gyrus color coded bright blue (corrected, P = 0.048; Table III). No positive correlation between brain areas of the DMN and blood venous ammonia levels was observed in HE patients. The results indicated the effect of venous blood ammonia levels on the brain function of DMN in the HE patients, consistent with the clinical observations of the patients with HE.
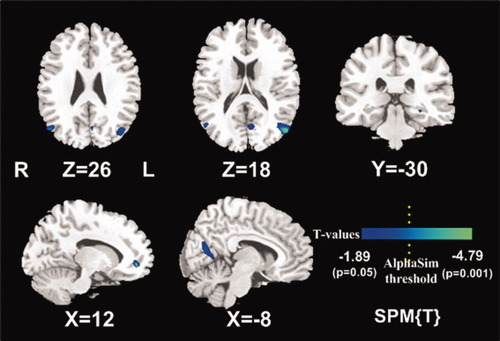
Statistical t-maps of the vein blood ammonia against z-scores in ICA in the HE group. The right middle frontal gyrus, left posterior cingulate cortex, left parahippocampus, bilateral angular gyri (P < 0.05 for all, uncorrected), and the left angular gyrus color coded bright blue (P < 0.05 corrected, combined height threshold P < 0.01 and a minimum cluster size of 24 voxels) had a negative correlation with venous blood ammonia.
| Brain areas | MNI coordinates (mm) | Brodmann area | Uncorrected Voxela | Corrected voxel | Corrected P values |
|---|---|---|---|---|---|
| X, Y, Z | |||||
| Left AG | −54, −75, 18 | 39 (R) | 58 | 24 | 0.048 |
| Left PCC | −6, −60, 6 | 30 (L) | 24 | >0.05 | |
| Right AG | 48, −72, 24 | 39 (R) | 22 | >0.05 | |
| Left parahippocampus | −18, −39, −15 | 30 (L) | 11 | >0.05 | |
| Right MFG | 12, 48, −6 | 10 (R) | 10 | >0.05 |
- MNI, Montreal Neurological Institute; PCC, posterior cingulate cortex; MFG, middle frontal gyrus; AG, angular gyrus; R, right; L, left.
- a Uncorrected P values were less than 0.05 for all data.
An average z-score from the whole DMN of each individual patient showed no significant correlation with the Child-Pugh score (r = 0.477, P = 0.163). All the above-mentioned DMN brain regions in HE patients showed no statistical correlation (all P values larger than 0.05) with the Child-Pugh score, except for the right angular gyrus (r = 0.646, P = 0.044).
DISCUSSION
Our study showed the impairment of DMN in HE patients using a resting state fMRI method, and left angular gyrus of DMN in HE patients were found to be negatively correlated with venous blood ammonia. To the best of our knowledge, this is the first report on the brain activities of DMN in the cirrhotic patients using ICA-based resting fMRI method. In this study, we found the functional connectivity of right MFG and left PCC of the DMN was decreased in the HE patients compared with controls. The trend of reduced functional connectivity in the left precuneus and left parahippocampus can be observed, which indicated a strong trend toward a larger percentage of patients having reduced functional connectivity in these regions. Thus, further studies in a larger population are needed to verify the finding. These findings are supported by other neuroimaging studies, such as magnetic resonance spectroscopy [Miese et al., 2006; Zhang et al., 2010a], magnetization transfer imaging [Miese et al., 2006] and diffusion tensor imaging [Kale et al., 2006; Rai et al., 2008]. Many authors have demonstrated multiple brain regions had metabolic abnormalities, such as increased glutamine-glutamate (Glx), decreased myo-inositol (mIns), and choline (Cho) levels [Miese et al., 2006; Zhang et al., 2010a]. Magnetization transfer imaging and diffusion tensor imaging studies which can reflect integrity of white mater tract also supported mild and diffuse brain edema in cirrhotic brain [Kale et al., 2006; Miese et al., 2006; Rai et al., 2008]. The brain damage of multiple DMN regions in HE patients can be contributed to ammonia induced neuro-toxicity, which is different from other diseases, such as AD. Unlike HE, AD pathology arises from neuron and synapse loss that begins in the entorhinal cortex'then spreads throughout the limbic regions of the temporal lobe, including the hippocampal formation. Subsequently, AD pathology is observed in temporal, parietal, and frontal lobes [Hollanda et al. 2009]. Thus, decreased resting-state activity of the hippocampus can be observed in the RS-fMRI study [Greicius et al. 2004]. We noted the main difference between the previous [Zhang et al., 2007b] and the present studies in HE is that the task-induced deactivation of the PCC and precuneus was absent when patients with hepatic cirrhosis performed the word-reading task rather than color-naming task in the previous studies, indicating that the PCC and precuneus are vulnerable for cirrhotic patients or that the difficulty degree of the task has an effect on the activity of brain DMN. We speculated that the difference between the two studies can be resulted from differences in fMRI scheme and data processing, patient selection, and task delivered to the patients. The difficult degree of the task will have an effect on the DMN because the DMN and other systems sustain “dynamic equilibrium” and “anticorrelations” [Buckner et al., 2008, Park et al., 2010; Fox et al., 2005].
No positive correlation between the average z-score and ammonia level was found in HE patients. However, the decreased functional connectivity in multiple brain areas of DMN was found in this study. It reflected the brain damage in HE patients, which may arise from an excess of ammonia in the blood (hyperammonemia) [Bosoi et al., 2009; Córdoba et al., 2008; Ferenci et al., 2002; Lockwood et al., 2004]. No previous reports have been published to evaluate the correlation between blood venous ammonia and functional connectivity of brain areas in HE patients using the fMRI method. In this study, average ammonia of HE patients was 108.6 ± 61.5 μmol/L; however, the normal reference value was 8–54 μmol/L. Our study found the correlation between the average z-scores from the whole DMN of each patient and blood ammonia was 0.606 (P = 0.063), which indicated a strong trend toward possibly a significantly high correlation if sample size was large enough. But, the present study also showed the z-scores of the decreased functional connectivity in the left angular gyrus in the resting state correlated with venous blood ammonia. This may be explained by the role of ammonia in the pathogenesis of HE. In the brain, ammonia is converted to glutamine and glutamate inside the astrocyte, leading to increased cellular osmolarity. Consequently, water shifts from the extracellular fluid space to the intracellular fluid space resulting in edema of the astrocytes [Lee et al., 1999; Miese et al., 2006; Rovira et al., 2008]. The clinical manifestations of HE are thought to be secondary to this edema [Hazell et al., 1999]. This edema has been proposed by diffusion tensor imaging [Rai et al., 2008], magnetization transfer imaging [Miese et al., 2006], and magnetic resonance spectroscopy studies [Miese et al., 2006; Rovira et al., 2008]. Other studies using PET and 13NH3 showed accumulation of toxic levels of ammonia in human HE [Ahl et al., 2004; Lockwood et al., 1991]. Increased brain ammonia has been suggested to be correlated with the brain edema in the experimental and human HE [Hazell et al., 1999; Master et al., 1999]. Thus, the correlation of the functional impairment of the DMN in patients with blood venous ammonia is quite plausible. In addition, alterations of the cerebral metabolic rate of O2 consumption and cerebral blood flow may explain the blood oxygen level dependent (BOLD) signal reduction in the brain DMN, since the BOLD signal is correlated with the amount of regional deoxyhemoglobin in the brain and is determined by cerebral metabolic rate of O2 consumption and cerebral blood volume as well as the cerebral blood flow [Zafiris et al., 2004].
Child-Pugh score, developed in 1960s and modified in 1970s, is believed to reflecting liver functional reserve [Pugh et al., 1973]. In this study, no z-scores of DMN brain regions but of right angular gyrus showed positive correlation with Child-Pugh score. Previous MR spectroscopy studies also found the metabolic findings in MR spectroscopy had a negative correlation with Child-Pugh score [Lee et al., 1999; Zhang et al., 2010a], indicating chronic metabolic derangement of the brain is associated with hepatic functional reserve. One study found myoinositol (mIns), as osmolytes, levels were inversely related to the Child-Pugh score [Lee et al., 1999]. In another study, mIns, the most sensitive biomarker for detection of abnormality in the cirrhotic patients, was correlated with Child-Pugh scale and HE severity [Zhang et al., 2010a]. The present findings indicated that severity of the hepatic diseases can have an effect on brain functional activities because of disability to intoxicate in the late stage of liver diseases.
Some limitations in the current study can be identified. First, this study is preliminary and our results are limited by a small heterogeneous sample size for HE patients with variations in the age and disease on-set time, which can have an effect on the statistical analysis of this study. A large-cohort study is needed. However, in this study, a standard statistical processing pipeline was followed with accepted software and procedures. We believe most findings therefore are validated based on these analyses. Second, not of all patients have the first episode of HE or the same stage of HE when the RS-fMRI was performed. Heterogeneity of patient etiology can decrease the statistical power of a study. Further studies with more patients at the same stage of HE, such as minimal HE, are warranted to investigate the correlation of ammonia and Child-Pugh score with the z-scores of the abnormal DMN in HE patients. Third, the ICA method used in the study has its own limitations. The correct selection of the target components is a general problem in ICA as a data-driven approach. In the present study, the templates used in the “best-fit” procedure were obtained from the results of a published work [Greicius et al., 2004], and the utilization of random-effect analyses could reduce the false-positive rate more effectively than the fixed-effect analysis. Last, our results are confined to analyses under the framework of default network interactions and DMN regions. In reality, the effects of hepatic encephalopathy may be more global. An exploration of connectivity based on the whole brain connectivity matrix may be most useful and warrants future studies.
In conclusion, RS-fMRI can be used to examine the DMN changes in patients with HE. In patients with HE, reduced functional connectivity was found in the right MFG and left PCC within the DMN. Negative correlation was found between the functional connectivity changes in left AG and the venous blood ammonia levels. The z-scores of the right AG showed a positive correlation with Child-Pugh score. These findings provide imaging evidence of damages in functional organization of the central nervous system in HE.
Acknowledgements
The authors thank Dr. Wei Liao, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu, China, for his helpful comments.