Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: A 15O-H2O PET study
Abstract
We investigated the differences in the resting state corticolimbic blood flow between 20 unmedicated depressed patients and 21 healthy comparisons. Resting state cerebral blood flow (CBF) was measured with H215O PET. Anatomical MRI scans were performed on an Elscint 1.9 T Prestige system for PET-MRI coregistration. Significant changes in cerebral blood flow indicating neural activity were detected using an ROI-free image subtraction strategy. In addition, the resting blood flow in patients was correlated with the severity of depression as measured by HAM-D scores. Depressed patients showed decreases in blood flow in right anterior cingulate (Brodmann areas 24 and 32) and increased blood flow in left and right posterior cingulate (Brodmann areas 23, 29, 30), left parahippocampal gyrus (Brodmann area 36), and right caudate compared with healthy volunteers. The severity of depression was inversely correlated with the left middle and inferior frontal gyri (Brodmann areas 9 and 47) and right medial frontal gyrus (Brodmann area 10) and right anterior cingulate (Brodmann areas 24, 32) blood flow, and directly correlated with the right thalamus blood flow. These findings support previous reports of abnormalities in the resting state blood flow in the limbic-frontal structures in depressed patients compared to healthy volunteers. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Positron emission tomography (PET) studies of cerebral blood flow or glucose metabolism in patients with major depression under resting conditions have shown frontal blood flow and glucose metabolism abnormalities [Fitzgerald et al.,2008; Mayberg,2003]. Decreased prefrontal function is the most reported finding by various research groups [Baxter et al.,1989, Bench et al.,1993; Hurwitz et al.,1990; Martinot et al.,1990, Mayberg,1997], and the area of hypoperfusion is mainly observed in the left dorsolateral prefrontal regions together with the anterior cingulate, with reduced activity in the dorsolateral prefrontal cortex being related to psychomotor retardation or cognitive disturbance [Bench et al.,1993; Dolan et al., 1993; Videbech,2000].
“Hypofrontality” is not a universally replicated finding in PET studies of major depression. Drevets et al. [1992] reported increased blood flow in left ventrolateral prefrontal cortex (Brodmann areas 11, 45, and 47) in a sample of patients with familial major depression using H215O PET. Brody et al. [2001] also reported higher glucose metabolism in the prefrontal cortex (and caudate and thalamus) in unipolar patients, that decreased from pretreatment to posttreatment. Yet, another H215O PET study [Videbech et al.,2001], with the largest sample of depressive patients (42 inpatients with moderate–severe depression), reported significantly increased blood flow to the right hippocampus and the left cerebellum, while there were no significant changes in the dorsolateral prefrontal cortex, cingulate gyrus, or basal ganglia.
Mayberg [1997,2003] proposed a working model of depression with three main compartments, implicating failure of the coordinated interactions of a distributed network of limbic-cortical pathways: cortical (prefrontal cortex, premotor, parietal, anterior cingulate, and posterior cingulate), subcortical (striatum, thalamus, and brainstem), and limbic (subgenual cingulate, hypothalamus, hippocampus, amygdala, anterior and posterior insula). In this model, abnormal chronic activity of the limbic-subcortical structures is thought to result in an exaggerated frontal hyperactivity trying to “over-ride a persistent negative mood generated by abnormal chronic activity of the limbic-subcortical structures.” Failure to initiate or maintain such a frontal compensatory state is thought to cause frontal hypometabolism, with resultant apathy, psychomotor slowness, and impaired executive functioning [Mayberg,1997,2003]. A recent meta-analysis of resting state PET and SPECT studies in depressed subjects further improved our understanding of the specific brain networks related to major depressive disorder [Fitzgerald et al.,2008]. The authors identified eight areas with decreased activation in patients with major depression compared with healthy controls, including pregenual anterior and posterior cingulate, bilateral middle frontal gyri, insula, and left superior temporal gyrus. These areas with decreased activation in resting state also showed a relative lack of activation during induction of negative affect and an increase in activation with antidepressant treatment. The same meta-analysis also identified areas with increased activation, including the thalamus, caudate, medial, and inferior frontal gyri as well as the left superior frontal and right middle frontal gyri. These relatively overactive areas in the resting state were also overactive during induction of negative affect and displayed a reduction in activity with antidepressant treatment, thus supporting the notion that major depressive disorder involves dysfunction of a series of specific networks as put forth by Mayberg [1997,2003].
The majority of the earlier resting state studies, with the exception of two [Drevets et al.,1992; Videbech et al.,2001], have measured glucose metabolism, which is a direct measure of the neuronal activity, whereas the CBF measurement is an epiphenomenon of neuronal activity and is an indirect measure. Also, in these earlier studies, coregistration of PET scans with MRI was not done; rather, PET images were interpreted using standard anatomical maps [Videbech,2000]. The sample sizes ranged from 3 to 18, with the exception of the study by Videbech et al. [2001], where 42 inpatients (29% unmedicated) were assessed. Taking into account the above-mentioned methodological limitations, we designed a resting state H215O PET study with PET-MRI coregistration, with 20 unmedicated depressed patients with major depression and 21 healthy comparisons. We hypothesized that depressed patients would have decreased blood flow in the prefrontal cortex, anterior cingulate and basal ganglia, and that depression severity (as measured by the total score of Hamilton Depression Rating Scale) would correlate with the decreased blood flow in these regions.
SUBJECTS AND METHODS
Subjects
Informed consent was obtained from all subjects, in accordance with the declaration of Helsinki, and under auspices of the Institutional Review Board and Radiation Safety Committee of the University of Texas Health Science Center at San Antonio. The subjects were residents of the San Antonio metropolitan area or surrounding cities and recruited through local media advertisements and flyers posted in the medical center. All the patients were outpatients. No patient was admitted to a psychiatric ward. No patient was taken out of his/her current psychiatric medication for participation in the study. The patients were rapidly evaluated and referred to psychiatric treatment after participation in the study.
Twenty unmedicated patients with major depression and 21 healthy comparisons were included in the analyses. Inclusion criteria for patients were: (1) diagnosis of major depressive disorder, as determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) [Spitzer et al.,2004], administered by experienced research clinicians; (2) a score of ≥18 in the Hamilton Depression Rating Scale—21 items (Ham-D) [Hamilton,1960]; (3) no DSM-IV drug/alcohol abuse/dependence within the past 6 months; (4) free of any psychotropic medications for at least 2 weeks (6 weeks for fluoxetine) before study entry. In order to increase the representativeness of the patient sample, comorbid Axis I diagnoses of anxiety disorders and/or past history of substance abuse (in full remission for at least 6 months before the study—verified by the structured clinical interview and urine drug screening tests) were allowed. We recruited healthy controls by advertisement and assessed them with the Structured Clinical Interview for DSM-IV, nonpatient edition (SCID-NP). Inclusion criteria for healthy controls were: (1) no past or current DSM-IV axis I diagnosis (including drug/alcohol abuse or dependency) and (2) no history of psychiatric disorders or suicide among first-degree relatives. Healthy controls were matched as a group with the unipolar patients for age, gender, and race. Pregnancy, neurologic disorders, including head injury with loss of consciousness, family history of hereditary neurologic disorder, and presence of metallic objects in the body were exclusion criteria for all the subjects. Individuals with clinical conditions, such as high blood pressure, diabetes, severe asthma, and other unstable medical conditions were also excluded. All subjects (except one subject in both patient and control groups) were right handed.
MRI Imaging
An anatomical MR scan was acquired for PET-MRI coregistration, to facilitate precisely determining the structures corresponding to the functional activation foci. Anatomical MRI scans were performed on an Elscint 1.9 T Prestige system (Elscint Ltd., Haifa, Israel). The scans employed 3D Gradient Recalled Acquisitions in the Steady State (3D GRASS), with a repetition time of 33 ms, an echo time of 6 ms, and a flip angle of 35° to obtain a 256 × 256 × 128 volume of data at a spatial resolution of 1 mm3.
PET Imaging
Regional changes in net neuronal activity were assessed by monitoring relative changes in normalized rCBF. Normalized rCBF was measured by recording the regional distribution of radioactivity following the intravenous injection of 15O-labeled water [Fox and Mintun,1989]. PET scans were performed on a GE 4096 camera (Milwaukee, WI) which has a pixel spacing of 2.0 mm, an interplane, center-to-center distance of 6.5 mm, 15 scan planes, and a z-axis field of view of 10 cm. Correction for radiation attenuation was made by means of a transmission scan collected before the first scan using a 68Ge/68Ga pin source. Cerebral blood flow was measured at resting state (eyes closed, quiet room, no task) with H215O administered as an intravenous bolus of 8 to 10 ml of saline containing 50 to 70mCi. At the start of a scanning session, an intravenous cannula was inserted into the subject's left forearm for injection of each tracer bolus. Each subject underwent a series of 10 independent PET blood-flow scans of 50 s. A 10-min interscan interval was sufficient for isotope decay (5 half lives) and return to resting state levels of regional blood flow within activated regions. We did not use all the 10 injections in the analysis presented here. We have only used eyes closed rest condition (one injection per subject). We did acquire two more rest scans with sham TMS, but we have not included this in the present analysis. Also, we acquired six more scans during TMS and one scan during n-back. While all these data were acquired at the same time, they will be presented elsewhere.
Throughout the PET session, subject's head was immobilized with a thermally molded plastic facial mask that was individually made for each subject.
Image Preprocessing
PET images were reconstructed using a Hann filter, resulting in images with a spatial resolution of approximately 7 mm FWHM (full-width at half-maximum). The Brain Extraction Tool method was applied to each individual data to define the MRI volume. PET brain volume was defined by an intensity-thresholding of 30% maximum value in each subject, with values lower than that being considered non brain data. PET images were value normalized to a whole-brain arbitrary mean of 1,000 counts [Fox et al.,1988; Friston et al.,1990]. Following global normalization of each individual PET scan image, interscan, intrasubject movement was assessed and corrected using the Woods' algorithm [Woods et al.,1992]. PET images were normalized to match normalized MR volumes, via the Lancaster et al. [1999] algorithm of convex hulls and implemented in the software Convex Hull Spatial Normalization tool (RIC, UTHSCSA). The Spatial Normalization algorithm [Gao et al.,1995] performed the spatial normalization (nine-parameter), which registered each individual MRI to the target shape provided by the Talairach and Tournox atlas [1988].
Statistical Analysis
Conditional contrast analysis
Significant changes in cerebral blood flow indicating neural activity were detected using a ROI-free image subtraction strategy. The data were analyzed using the Fox et al. [1988] validated algorithm implemented in Medical Image Processing Station (Research Imaging Center, UTHSCSA, San Antonio, TX), where a statistical parametric image (SPI) is computed with like-condition scans averaged across subjects, and the resultant grand-averaged scans from relevant pairs of conditions are subtracted. Here we present the group contrast data of rest condition (patient rest − control rest). In order to further examine the effect of comorbid anxiety disorder on resting CBF, we performed a within group analysis where rest scans of the patients with comorbid anxiety disorder (N = 9) were contrasted with rest scans of patients who had no anxiety disorder (N = 9).
The SPI was smoothed with an isotropic 3 × 3 × 3 Gaussian kernel to yield a final image resolution of 9.9 mm. Control for false positives is provided by the P value criteria used in later stages of the analysis stream employed here. The resulting SPI image data was then analyzed by an omnibus (whole-brain) test.
Local extrema (positive and negative) were identified within each image using a three-dimensional search algorithm [Mintun et al.,1989]. Critical values for beta statistics were chosen at P < 0.00003. In this analysis, a maxima and minima search is conducted to identify local extrema within a search volume of 250 mm3 (P < 0.00003 for a Z score ≥5) [Fitzgerald et al.,2006; Mintun et al.,1989]. Significant changes in cerebral blood flow indicating neural activity were detected using an ROI-free image subtraction strategy (P < 0.00003 for a Z score ≥5, and a cluster size >250 mm3, two-sided). For the Ham-D correlation analyses, we included correlations with R > 0.7 and a Z score >3, with P < 0.001.
Gross anatomical labels were applied to the detected local maxima using a volume-occupancy-based, anatomical-labeling strategy as implemented in the Talairach Daemon™ [Lancaster et al.,2000] except for activations in cerebellum which were labeled manually with reference to an atlas of the cerebellum [Schmahmann et al.,1999].
The post hoc regional analysis was restricted to the regions described in the models of depression network [Fitzgerald et al.,2008; Mayberg,1997; Videbech,2000]—dorsolateral prefrontal cortex, anterior and posterior cingulate, thalamus, caudate, striatum, brainstem, subgenual cingulate, hypothalamus, hippocampus, amygdala, anterior and posterior insula, and cerebellum.
HAM-D score correlation analysis
An additional correlation analysis was performed in the patient group. A statistical parametric image of r values (SPI{r}) was computed as a voxel-wise correlation of CBF with the total HAM-D scores using previously described method [Fox et al., 2000]. SPI{r} were analyzed for effects of depressive symptoms first by an omnibus (whole-brain) test and, if omnibus significance was proven, then a post hoc (regional) test was done and local extrema were identified. The SPI{r} were converted to SPI{z}, and P values were assigned from the Z distribution and corrected for the number of positive and negative extrema. We report here correlations with R > 0.7 and a Z score >3, with P < 0.001.
RESULTS
Demographic and clinical characteristics of the sample are summarized in Table I. Mean age of depression onset was 21 ± 14 years. Mean Ham-D score was 22 ± 5 (range 18–38). Fourteen (70%) of the patients had a positive family history for mood disorders. Only one patient had a history of lifetime psychosis and none of the patients had current psychotic symptoms. Ten (50%) patients had a comorbid anxiety disorder and seven (35%) had a past substance abuse diagnosis. The majority (55%) of the patients were Caucasians, whereas the majority (57%) of the healthy controls was of Hispanic origin.
| Characteristics | MDD patients (n = 20) | Healthy controls (n = 21) |
|---|---|---|
| Age (yr) (mean ± SD) | 37.2 ± 13.6 | 34.8 ± 11.0 |
| Males, number (%) | 5 (25) | 7 (35) |
| Ethnicity, number (%) | ||
| Caucasian | 11 (55) | 5 (24) |
| Hispanic | 9 (45) | 12 (57) |
| Asian | 0 | 3 (14) |
| Other | 0 | 1 (5) |
| Education level: number (%) | ||
| Grades 7–12 | 3 (15) | 1 (5) |
| Graduated high school or equivalent | 1 (5) | 1 (5) |
| Part college | 6 (30) | 6 (29) |
| Graduated 2 yr college | 3 (15) | 2 (10) |
| Graduated 4 yr college | 3 (15) | 2 (10) |
| Part graduate/professional | 0 | 3 (14) |
| Completed graduate/ professional school | 4 (20) | 6 (29) |
| Lifetime anxiety disorders (%) | 10 (50) | N/A |
| Panic disorder | 2 | |
| Agoraphobia | 2 | |
| Social phobia | 1 | |
| Specific phobia | 1 | |
| Posttraumatic stress disorder | 6 | |
| Generalized anxiety disorder | 2 | |
| Obsessive-compulsive disorder | 0 | |
| Lifetime substance use disorder (%) | 7 (35) | N/A |
| Alcohol abuse | 4 | |
| Alcohol dependence | 2 | |
| Cannabis abuse | 4 | |
| Cannabis dependence | 0 | |
| Cocaine abuse | 1 | |
| Cocaine dependence | 1 | |
| Other substance abuse/dependence | 1 | |
| Medication history (%) | ||
| Drug-naive | 6 | N/A |
| TCAs | 4 | |
| SSRIs | 11 | |
| Venlafaxine | 2 | |
| MAOIs | 1 | |
| Unknown | 3 |
- TCAs: tricyclic antidepressants, SSRIs: serotonin reuptake inhibitors, MAOIs: monoamine oxidase inhibitiors.
In the resting state, depressed patients showed decreases in blood flow in right anterior cingulate (Brodmann areas 24 and 32) compared with healthy controls (Table II, Fig. 1). Depressed patients also had decreased blood flow in middle frontal gyrus (Brodmann area 9) and inferior frontal gyrus (Brodmann area 10), but the cluster sizes were smaller than 250 mm3 (Table II).
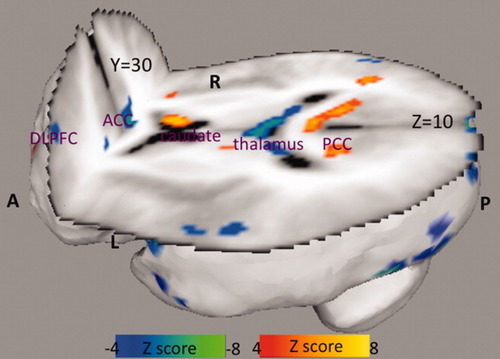
Resting state CBF differences in patients with depression when compared with healthy controls. A: anterior, P: posterior, L: left, R: right, DLPFC: dorsolateral prefrontal cortex, ACC: anterior cingulate cortex, PCC: posterior cingulate cortex.
| Region | Brodmann area | Side | x | y | z | Z score | Cluster size (mm3) |
|---|---|---|---|---|---|---|---|
| Anterior cingulate | 24 | Right | 8 | 28 | 10 | −6.0 | 252 |
| Anterior cingulate | 32 | Right | 12 | 24 | 22 | −5.2 | 262 |
| Middle frontal gyrus | 9 | Left | −36 | 26 | 34 | −5.6 | 182 |
| Inferior frontal gyrus | 10 | Left | −38 | 40 | −2 | −5.6 | 112 |
| Region | Brodmann area | Side | x | y | z | Z score | Cluster size (mm3) |
|---|---|---|---|---|---|---|---|
| Posterior cingulate | 23 | Left | 0 | −22 | 26 | 7.2 | 280 |
| Posterior cingulate | 30 | Right | 14 | −54 | 14 | 6.9 | 278 |
| Posterior cingulate | 29 | Right | 4 | −46 | 12 | 7.1 | 270 |
| Caudate | Right | 10 | 8 | 12 | 7.8 | 280 | |
| Parahippocampal gyrus | 36 | Left | −40 | −30 | −6 | 7.5 | 270 |
Patients had significantly increased blood flow in posterior cingulate (Brodmann areas 23, 29, and 30), in right caudate and left parahippocampal gyrus (Table III, Fig. 1).
The severity of depression was inversely correlated with the blood flow in left middle and inferior frontal gyri (Brodmann areas 9 and 10) and right anterior cingulate (Brodmann areas 24, 32), and directly correlated with the blood flow in right thalamus and left putamen (Table IV, Fig. 2).
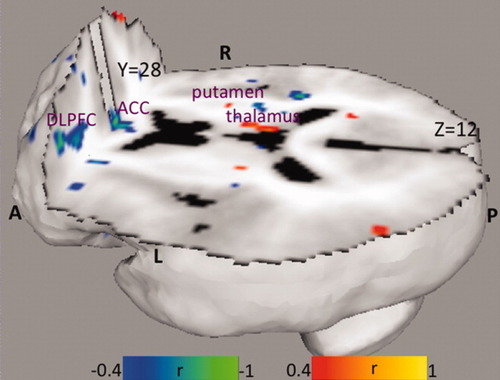
Correlation of resting CBF with HAM-D scores in patients with depression. A: anterior, P: posterior, L: left, R: right, DLPFC: dorsolateral prefrontal cortex, ACC: anterior cingulate cortex.
| Region | Brodmann area | Side | x | y | z | Z score | Cluster size (mm3) | Correlation coefficient |
|---|---|---|---|---|---|---|---|---|
| Middle frontal gyrus | 9 | Left | −32 | 32 | 24 | −3.3 | 93 | −0.751 |
| Inferior frontal gyrus | 47 | Left | −36 | 34 | −1 | −3.4 | 55 | −0.770 |
| Medial frontal gyrus | 10 | Right | 12 | 55 | 8 | −3.8 | 83 | −0.841 |
| Anterior cingulate | 24 | Right | 2 | 24 | 0 | −3.3 | 80 | −0.734 |
| Anterior cingulate | 32 | Right | 14 | 40 | 2 | −3.2 | 32 | −0.713 |
| Thalamus | Right | 10 | −18 | 13 | 3.39 | 61 | 0.756 | |
| Putamen | Left | −18 | 6 | −4 | 3.09 | 38 | 0.688 |
No significant differences in rCBF were seen in the sub analysis of conditional contrast between patients with anxiety disorder and patients without anxiety disorder.
DISCUSSION
Depressed patients had decreased blood flow to the right anterior cingulate (Brodmann areas 24 and 32) and in middle and inferior frontal gyri (with small cluster sizes) compared with healthy subjects. These resting state rCBF findings in the prefrontal cortex and anterior cingulate are in accordance with what has been previously reported in depression [Fitzgerald et al.,2008; Mayberg,2003; Videbech,2000]. In contrast, bilateral posterior cingulate (Brodmann areas 23, 29, and 30), as well as right caudate and left parahippocampal gyrus had increased blood flow in the resting state.
An extensive literature review of PET studies of depressed patients in the resting state [Videbech,2000] revealed that patients with major depression have reduced blood flow and glucose metabolism in the prefrontal cortex, anterior cingulate, and caudate nucleus. Differences in sample sizes, patient selection, image protocol, and image analyses make direct comparisons among our study and the prior PET studies difficult. However, two of the earlier PET studies utilized the radiotracer H215O with depressed mostly unmedicated unipolar patient samples, which renders comparisons with our findings more plausible [Drevets et al.,1992; Videbech et al.,2001]. Of these, Drevets et al. [1992] study evaluated a group of 13 currently depressed unmedicated patients (54% female) with “familial pure depressive disease” (primary major depressive disorder in an individual with a first-degree relative, i.e., parent, sibling, or offspring, who has major depressive disorder, but no first-degree relatives with mania, alcoholism, or sociopathy). The measures were obtained from relatively low-resolution PET images using a stereotaxic method based upon skull X-ray landmarks (no coregistration to MRI). Depressed patients had increased blood flow in the left ventrolateral prefrontal cortex and left amygdala, with a positive correlation between total Ham-D scores and amygdala activity and a negative correlation between total Ham-D scores and activity in the left prefrontal cortex. In a later study with 10 currently depressed unmedicated patients (seven females) using [18F]fluodeoxyglucose (FDG), the same group of investigators reported decreased subgenual prefrontal cortex metabolism relative to healthy controls, a finding possibly reflecting the noted 48% reduction in the gray matter volume of the corresponding cortex in the unipolar depressives compared with the healthy controls [Drevets et al.,1997]. We did not measure the gray matter volumes of the corresponding anterior cingulate areas with decreased blood flow (Brodmann areas 24 and 32), so it is not possible to know whether the decreased blood flow reflects a volume change. The other H215O PET study, by Videbech et al. [2001], mapped cerebral blood flow in 42 inpatients (30 females, three with bipolar disorders) with moderate–severe depression. The patients had increased blood flow to the right hippocampus and the left cerebellum (controlled for age, gender, and medication), and in contrast to earlier studies, no significant increase or decrease of blood flow was found in dorsolateral prefrontal cortex, cingulate gyrus, or basal ganglia. Contrary to our findings of depression severity being inversely correlated with the blood flow in left middle and inferior frontal gyri (Brodmann areas 9 and 10) and right anterior cingulate (Brodmann area 24, 32), and directly correlated with the blood flow in right thalamus and left putamen, there were no significant correlations between any region and the Ham-D scores in the study by Videbech et al. [2001]. A limitation in this study was that patients were scanned while on antidepressant medications. Brody et al. [2001], using [18F]fluodeoxyglucose PET, reported higher glucose metabolism in right dorsolateral prefrontal cortex, left ventrolateral prefrontal cortex, right dorsal caudate, and bilateral thalamus at baseline that decreased from pretreatment to posttreatment (with either paroxetine or interpersonal psychotherapy). Another [18F]fluodeoxyglucose PET study by Drevets et al. [2002] in depressed unmedicated patients also demonstrated increased metabolism in the left and right lateral orbital cortex/ventrolateral prefrontal cortex, left amygdala and posterior cingulate, and decreased metabolism in the subgenual cingulate and dorsal medial/dorsal anterolateral prefrontal cortex relative to healthy subjects. Metabolism significantly decreased in the left amygdala and left subgenual cingulate with corresponding changes in the orbital and posterior cingulate approaching significance following treatment. In line with our finding of increased blood flow in the posterior cingulate, several other groups in addition to Drevets et al. [2002] also reported elevated posterior cingulate metabolism in depression [Bench et al.,1993; Buchsbaum et al.,1997], and that posterior cingulate blood flow correlated positively with anxiety [Bench et al.,1993].
The severity of depression, as measured by Ham-D, was inversely correlated with the blood flow in left middle and inferior frontal gyri (Brodmann areas 9 and 47) and right medial frontal gyrus (Brodmann area 10) and right anterior cingulate (Brodmann areas 24, 32), and directly correlated with the blood flow in right thalamus and left putamen. In other words, all the regions we identified with decreased blood flow at resting state showed a negative correlation with the total Ham-D scores. Videbech et al. [2002] demonstrated a similar strong negative correlation between degree of psychomotor retardation (as measured by the Depressive Retardation Rating Scale) and the blood flow to bilateral dorsolateral and supraorbital prefrontal cortices (Brodmann areas 11 and 47). They also reported increased blood flow to hippocampus in depressed patients, which was directly correlated with the total Ham-D score in female patients, suggesting that hyperperfusion in this structure may be pathophysiologically important in depression. We did not find such a direct relationship between areas with increased blood flow (i.e. posterior cingulate, caudate and parahippocampal gyrus) and total Ham-D scores. An earlier study by Baxter et al. [1989] also showed a significant correlation between left dorsolateral prefrontal cortex glucose metabolism and the Ham-D score, where percentage change in the Ham-D score correlated with the percentage change in left dorsolateral prefrontal cortex glucose metabolism increase with medication. We did not attempt a correlation analysis among different Ham-D symptom clusters and regional blood flow; however, earlier PET data [Bench et al.,1993] indicate that symptomatic specificity may be ascribed to regional functional deficits in major depressive illness. In this study, the authors made a factor analysis on patients' symptom ratings and then correlated the scores for these factors with rCBF. The first factor had high loadings for anxiety and correlated positively with rCBF in the posterior cingulate cortex and inferior parietal lobule bilaterally. The second factor had high loadings for psychomotor retardation and depressed mood and correlated negatively with rCBF in the left dorsolateral prefrontal cortex and left angular gyrus. The third factor had a high loading for cognitive performance and correlated positively with rCBF in the left medial prefrontal cortex.
To the best of our knowledge, this is the first resting state H215O PET study with MRI coregistration in a sizeable number of unmedicated depressed patients. Yet, a larger sample would have allowed improved detection of changes not reaching significance. Also, we included subjects with other Axis I comorbidities. While these confounding illnesses might have affected rCBF, it might have made our results more generalizable as major depression is a highly comorbid illness. In summary, we found decreased blood flow in the emotion-cognition integration areas (i.e. Brodmann areas 9, 10, and 24) that has inverse correlation with disease severity, and increased blood flow in the sensory-cognitive integration areas (i.e. posterior cingulate and parahippocampal gyrus) of the limbic-cortical dysregulation model of depression [Mayberg,1997,2003].




