Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: An in vivo study with TBSS and VBM
Abstract
We investigated the relationship between the damage occurring in the brain normal-appearing white matter (NAWM) and in the gray matter (GM) in patients with early Primary Progressive multiple sclerosis (PPMS), using Tract-Based Spatial Statistics (TBSS) and an optimized voxel-based morphometry (VBM) approach. Thirty-five patients with early PPMS underwent diffusion tensor and conventional imaging and were clinically assessed. TBSS and VBM were employed to localize regions of lower fractional anisotropy (FA) and lower GM volume in patients compared with controls. Areas of anatomical and quantitative correlation between NAWM and GM damage were detected. Multiple regression analyses were performed to investigate whether NAWM FA or GM volume of regions correlated with clinical scores independently from the other and from age and gender. In patients, we found 11 brain regions that showed an anatomical correspondence between reduced NAWM FA and GM atrophy; of these, four showed a quantitative correlation (i.e., the right sensory motor region with the adjacent corticospinal tract, the left and right thalamus with the corresponding thalamic radiations and the left insula with the adjacent WM). Either the NAWM FA or the GM volume in each of these regions correlated with disability. These results demonstrate a link between the pathological processes occurring in the NAWM and in the GM in PPMS in specific, clinically relevant brain areas. Longitudinal studies will determine whether the GM atrophy precedes or follows the NAWM damage. The methodology that we described may be useful to investigate other neurological disorders affecting both the WM and the GM. Hum Brain Mapp 2009. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Structural abnormalities in the normal-appearing white matter (NAWM) have been consistently reported in primary progressive multiple sclerosis (PPMS) using in vivo magnetic resonance imaging (MRI) [Filippi et al.,2004; Leary et al.,1999; Rovaris et al.,2001,2002]. Even in the early stages of PPMS, diffuse NAWM abnormalities are clinically relevant [Ramio-Torrenta et al.,2006; Sastre-Garriga et al.,2005] and predict progression at 1 year [Khaleeli et al.,2007a]. One of the MRI measures most commonly employed to assess the integrity and orientation of WM tracts, is fractional anisotropy (FA), which is derived from diffusion tensor imaging (DTI) [Basser et al.,1994]. Reduced FA, which reflects underlying demyelination and axonal loss [Beaulieu et al.,1996; Schmierer et al.,2007], has been described in the WM of PPMS using both region of interest [Ciccarelli et al.,2001] and histogram analyses [Vrenken et al.,2006]. This is consistent with postmortem studies in PPMS, which have demonstrated widespread demyelination, axonal injury, and microglial activation in the NAWM [Kutzelnigg et al.,2005].
Gray matter (GM) is also affected in PPMS, as shown by irreversible tissue loss (or atrophy) measured with MRI [Sastre-Garriga et al.,2004; Sepulcre et al.,2006]. Since the GM tissue does not have a strong directionality, FA is not a useful measure for the investigation of local GM abnormalities. However, voxel-based morphometry (VBM) [Ashburner and Friston,2000] and histogram analysis of T1-weighted images have demonstrated regions of GM atrophy in patients [De Stefano et al.,2003; Khaleeli et al.,2007b; Pirko et al.,2007; Ramio-Torrenta et al.,2006], which predict clinical evolution over time [Rovaris et al.,2006]. In histological studies, extensive GM demyelination has been described in progressive MS patients [Bo et al.,2007; Kutzelnigg et al.,2005].
One important question is whether the NAWM damage correlates with abnormalities in connected GM or the two compartments are affected independently. Previous histological studies addressing this question have been inconclusive. Kutzelnigg et al. [2005] reported a weak correlation between diffuse WM inflammation and GM demyelination, whereas Bo et al. [2007] found no significant association between abnormalities in the gray and white matter compartments. MRI studies, on the other hand, have focused on the relationship between lesions in the WM and GM damage [Charil et al.,2007; De Stefano et al.,2003; Ramio-Torrenta et al.,2006], suggesting that at least a proportion of cortical pathology may be secondary to WM abnormalities.
Here, we investigated the relationship between NAWM damage and GM atrophy in vivo in patients with early PPMS, who are in the most clinically active phase of the disease [Thompson,2004], by combining the results of a novel method called Tract-based Spatial Statistics (TBSS) [Smith et al.,2006], which localized areas of reduced FA in the NAWM of patients, with the results of an optimized VBM approach [Good et al.,2001], which detected areas of GM atrophy in patients. Both these techniques test for the whole brain in an unbiased way (i.e., without an a priori hypothesis). A similar methodology of combining TBSS and VBM has been recently applied to patients with adolescent-onset schizophrenia [Douaud et al.,2007] and to patients with Friedreich ataxia [Della Nave et al.,2008]. Here, we further extended this methodology by assessing the quantitative relationship underlying the anatomical correspondence between GM and WM damage. We combined TBSS and VBM for the first time in patients with early PPMS to obtain insights into the disease mechanisms. In particular, we aimed to assess: (i) whether there was an anatomical correspondence between reduced FA in the NAWM and GM atrophy; (ii) whether FA values of abnormal tracts correlated with the GM volume of connected atrophic regions; and (iii) whether NAWM FA or GM volume independently correlated with disability.
METHODS
Subjects
Thirty-six patients with a diagnosis of PPMS [Thompson et al.,2000] within 5 years of symptom onset (15 females, 21 males, mean age 45.3 years, and SD 11.32) underwent an imaging and clinical assessment protocol approved by the Joint Medical Ethics Committee of the National Hospital for Neurology and Neurosurgery and the Institute of Neurology, London. Written and informed consent was obtained from all participants. One patient was later excluded from the analysis (as discussed below), leaving a total of 35 patients (Table I). All patients were assessed on the day of scanning using the Expanded Disability Status Scale (EDSS) [Kurtzke,1983] and the Multiple Sclerosis Functional Composite (MSFC) subtests: Paced Auditory Serial Addition Test (PASAT), Nine-Hole Peg Test (NHPT), and Timed Walk Test (TWT) [Cutter at al.,1999].
| Characteristics | Patients | Controls first group | Controls second group |
|---|---|---|---|
| Number | 36 | 18 | 23 |
| Age, years (SD) | 44.8 (11.13) | 41.5 (12.63) | 35.1 (7.9) |
| Gender, female/male | 15/20 | 8/10 | 12/11 |
| EDSS, median (range) | 4.5 (1.5–7.0) | — | — |
| Disease duration, years (SD) | 3.3 (0.9) | — | — |
| T2 lesion load, ml (SD) | 31.56 (24.79) | — | — |
| PASAT score, mean (SD) | 47.65 (11.24) | — | — |
| NHPT score, mean (SD) | 22.48 (3.69) | — | — |
| TWT score, mean (SD) | 8.63 (8.37) | — | — |
Two different groups of controls were used, as this was a retrospective analysis performed on previously acquired data, which were combined for the purpose of this study. The first group included 18 healthy subjects, who were age-matched to the patient group (8 females, 10 males, mean age 41.5 years, and SD 12.63) and were used for the localization of WM abnormalities. The second group included 23 healthy subjects (12 females, 11 males, mean age 35.1 years, and SD 7.9), who were used for the localization of regions of GM atrophy. The difference in age between this second group of controls and the patient group were adjusted for, at each stage of the analysis.
Image Acquisition and Analysis
All imaging was obtained using a 1.5-T GE Signa scanner (General Electric, Milwaukee, IL), with maximum gradient strength of 22 mT m−1. Images were displayed on a Sun workstation (Sun Microsystems, Mountain View, CA) for analysis.
Dual-echo images, lesion load, and creation of a mean lesion mask
All subjects had a fast spin echo scan that collects proton-density-weighted (PD) and T2-weighted images (repetition time [TR] 2000 ms; echo times [TEs] 17/92 ms; matrix size 256 × 256; field of view [FOV] 240 × 180 mm2; 28 contiguous axial slices of 5 mm thickness). Lesions were delineated by a single observer (who was blinded to the clinical details) on the PD images, with reference to the coregistered T2 images, using a semiautomated contour thresholding technique [Plummer,1992] and used to calculate each subject's lesion load and a lesion mask. Each individual binary lesion mask was obtained by setting the signal within lesion boundaries to one and the remainder of the brain to zero.
Each subject's T2-weighted image was registered to the Montreal Neurological Institute (MNI) template using an affine transformation (FLIRT, part of FSL www.fmrib.ox.ac.uk/fsl) [Jenkinson and Smith,2001]. The same transformation parameters were then applied to the lesion mask. The individual, normalized lesion masks were then averaged to obtain a mean lesion map indicating the proportion of the cohort having a lesion in each voxel. Finally, this map was binarised to retain only voxels with a value higher than 0.1 (indicating that at least 10% of the subjects had a lesion in a given voxel).
Diffusion-tensor imaging and TBSS analysis
All patients and the first group of 18 age-matched controls underwent a whole-brain, cardiac-gated Spin Echo Diffusion Weighted Echo Planar Imaging (SE-DW-EPI) sequence [Wheeler-Kingshott et al.,2002]. Full brain coverage was obtained with three separate acquisitions, each collecting 14 axial slices of 3 mm thickness, which were interleaved off-line. The data was acquired with the following parameters: FOV 240 × 240 mm2, matrix size 96 × 96 (reconstructed to 128 × 128), image resolution 2.5 × 2.5 × 3 mm (reconstructed to 1.9 × 1.9 × 3 mm), TE 95 ms, TR 7RRs, and maximum b factor 1000 sm m−2. Diffusion gradients were applied along 25 optimized directions [Jones et al.,1999]. Three images with no diffusion weighting (b0) were also acquired.
After correction for eddy-current-induced distortions [Symms et al.,1997], the diffusion tensor was calculated on a voxel-by-voxel basis [Basser at al.,1994], and FA maps were generated using DTIfit provided by the fMRIB Diffusion Toolbox [part of FSL; www.fmrib.ox.ac.uk/fsl; Smith et al.,2004]. Tract-Based Spatial Statistics (TBSS, also part of FSL) [Smith et al.,2006] was used to create a mean FA skeleton. The skeleton is obtained by aligning every subject's FA image into a common space using nonlinear registration [Rueckert et al.,1999], and then averaging the normalized images to create a mean FA map, which is finally thinned so that the FA skeleton represents the centre of all tracts common to the group. Each subject's FA data was then projected onto the skeleton. The final binary lesion mask was subtracted from the skeleton to produce a skeleton of the NAWM only. Voxelwise differences in projected FA between patients and controls were tested using a two sample t-test. Two contrasts, patients greater than controls' FA and controls greater than patients, were estimated. The resultant statistical maps were thresholded at p < 0.05 corrected at cluster level for multiple comparisons using a permutation-based approach [Nichols and Holmes,2002]. In particular, a cluster-forming threshold t > 2 was used, and the null distribution of maximum values (across the image) of the test statistic was estimated. A similar cluster-forming threshold was used in the only TBSS study of patients with MS published so far [Cader et al.,2007].
T1-weighted volumetric images and SPM2-VBM analysis of GM
All patients and the second group of 23 controls underwent a three-dimensional inversion-prepared fast spoiled gradient recall (3D FSPGR) T1-weighted sequence of the brain [FOV 300 × 225 mm, matrix size 256 × 160 (reconstructed to 256 × 256 for a final in plane resolution of 1.17 mm), TR 13.3 ms, TE 4.2 ms, inversion time 450 ms, 124 contiguous axial slices, slice thickness of 1.5 mm].
Pre-processing of T1-weighted images was performed according to the previously described optimized VBM-style protocol [Good et al.,2001] using the segmentation and registration tools available in the statistical parametric mapping software (SPM2, www.fil.ion.ucl.ac.uk/spm). The protocol was modified to account for the presence of WM lesions in patients, as previously described [Khaleeli et al.,2007b]. We will briefly summarize the procedure. The 3D FSPGRs of all subjects were segmented in native space. Lesions masks were applied to the GM partitions to remove lesions erroneously classified as GM. GM volume was estimated for every subject, and the images normalized onto the SPM GM template. Lesion masks were used to weight the normalization. The normalization parameters were then applied to the T1-volumes, and the normalized images were segmented and masked again to produce GM segments. One patient was excluded from the study because of segmentation failure due to a high lesion load. All data were then smoothed using a 12-mm FWHM Gaussian kernel. To localize areas, where GM volume was significantly lower in patients compared with controls, we performed analysis of covariance adjusted for age and for total GM volume. We used a family-wise error correction at p < 0.05 for multiple comparisons at voxel level across the whole brain. Regions comprising clusters of less than 100 voxels were excluded from the analysis.
Anatomical correspondence and quantitative relationship between NAWM and GM abnormalities
In patients, areas of anatomical correspondence between tracts of lower FA and adjacent regions of GM atrophy were visually identified by overlaying the results of the two different analyses on the same anatomical image, as previously described [Douaud et al.,2007]. To assess whether there was a quantitative correlation underlying this anatomical relationship, for each area of anatomical correspondence we extracted the mean FA value of the cluster of significantly lower NAWM FA and the mean volume of the cluster of significantly lower GM volume. The relationship between mean FA and mean GM volume of these areas was quantified using the Spearman's correlation coefficient (using SPSS 11.5 for Windows).
Correlation between NAWM and GM abnormalities and disability
The Z-score (z) for each MSFC subtest was calculated using our own sample as reference [Cutter at al,1999]. Patients were also divided into three categories of roughly similar size on the basis of disability as follows: (i) EDSS ≤ 3.5 = Group 1 (patients with minimal disability and fully ambulatory); (ii) EDSS between 4.0 and 5.5 = Group 2 (patients with moderate disability and restricted ambulation); and (iii) EDSS ≥ 6.0 = Group 3 (patients with severe disability who required unilateral or bilateral assistance to walk).
In patients, for each region where a quantitative relationship between NAWM FA and GM volume was found, the association between clinical scores and NAWM and GM changes was assessed using (i) multiple linear regression analysis for the zPASAT, zHPT, zTWT and (ii) multiple ordinal logistic regression analysis for the EDSS categories, as the EDSS is not a linear scale and was not normally distributed. To identify which MRI variable (i.e., GM volume and NAWM FA) was associated with disability, independently from the other and from age and gender, each disability score (i.e., EDSS, zPASAT, zHPT, zTWT) was selected in turn as dependent variable, whilst NAWM FA, GM volume, age, and gender were selected as independent variables. We chose not to analyse the impact of MRI changes on the global MSFC, but rather on the individual MSFC subtests, which can be more informative than the global MSFC score, as this is obtained by calculating the mean of the three subtest z-scores. Results with p values < 0.05 were reported.
RESULTS
Reduced FA of NAWM Tracts
Patients showed reduced FA compared with controls in the NAWM along the corticospinal tracts bilaterally (posterior limb of the internal capsule, corona radiata, and WM adjacent to primary motor cortex), in the WM adjacent to the premotor cortex bilaterally, and in the entire corpus callosum, including rostrum, genu, body, and splenium. FA was also significantly reduced in patients in the following areas bilaterally: (i) thalamic radiation, (ii) optic radiation, (iii) fornix, (iv) fasciculus arcuatus, (v) inferior longitudinal fasciculus, and (vi) WM of the temporal and frontal lobe (Fig. 1).
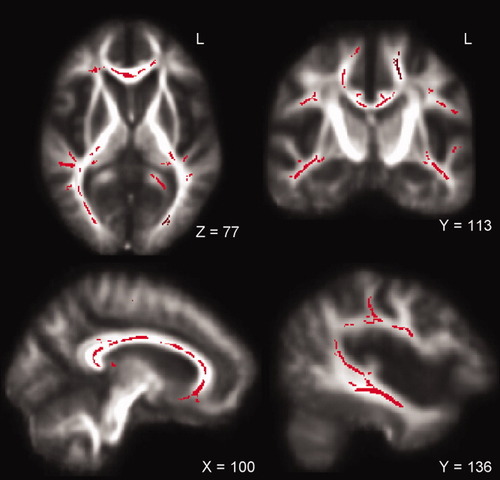
TBSS results: Significant reduction in FA (voxels in red) along the bilateral corticospinal tract, the bilateral optic radiation, the bilateral corona radiate, the corpus callosum, and the left arcuate fasciculus in patients displayed on the mean FA map.
GM Atrophy
The cortical regions which showed the most extensive and significant reduction in volume in patients compared with controls were the sensory motor cortex bilaterally, followed by the insula bilaterally, the GM around the left sylvian fissure, and the right superior temporal gyrus. Patients also showed significantly reduced GM volume in the deep GM regions, including the bilateral thalamus and the left cerebellar hemisphere. Other regions with lower GM volume in patients included the left precuneus and the right angular and cingulate gyrus (Table II, Fig. 2).
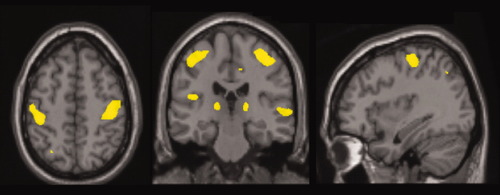
SPM-VBM results: Significant reduction in grey matter in the bilateral sensory-motor cortex, in the bilateral thalamus, in the right superior temporal gyrus, and in the left grey matter around sylvian fissure in patients displayed on the Montreal Neurological Institute template.
| Region | Side | MNI coordinates of local maxima | Number of voxels | Local maximum t-value | ||
|---|---|---|---|---|---|---|
| Sensory-motor cortex | L | −49 | −18 | 56 | 2793 | 6.84 |
| Sensory-motor cortex | R | 42 | −21 | 53 | 4196 | 6.62 |
| Inferior frontal gyrus | L | −61 | −6 | 31 | 184 | 5.58 |
| Inferior frontal gyrus | R | 55 | −15 | 20 | 52 | 5.38 |
| Middle frontal gyrus | R | 43 | −2 | 58 | 73 | 5.52 |
| Superior temporal gyrus | R | 67 | −22 | 2 | 697 | 5.95 |
| Middle temporal gyrus | L | −62 | −4 | −8 | 185 | 5.08 |
| Grey matter around sylvian fissure | L | −42 | −23 | 17 | 858 | 6.30 |
| Precuneus | L | −12 | −69 | 44 | 101 | 5.47 |
| Cerebellar hemisphere | L | −20 | −80 | −38 | 375 | 5.88 |
| Angular gyrus | R | 34 | −63 | 45 | 74 | 5.54 |
| Cingulate gyrus | R | 15 | −65 | 14 | 72 | 5.38 |
| Thalamus | L | −18 | −29 | 5 | 1599 | 6.30 |
| Thalamus | R | −20 | −28 | 5 | 1220 | 6.57 |
| Insula | L | −36 | 5 | 1 | 726 | 6.53 |
| Insula | R | 33 | 9 | 0 | 61 | 5.28 |
Anatomical Correspondence Between NAWM and GM Abnormalities
Anatomical correspondence was seen between the reduced NAWM FA of the corticospinal tract and the GM atrophy in the sensory-motor cortex, bilaterally. Similarly, a topographic correspondence was found between reduced FA in the NAWM adjacent to (i) the left inferior frontal gyrus, (ii) the right superior temporal gyrus, (iii) the left middle temporal gyrus, (iv) the left GM around sylvian fissure, (v) the left precuneus, (vi) the right angular gyrus, (vii) both thalami, and (viii) the left insula, and the atrophy of the same GM regions (Fig. 3A,B).

Examples of regions where anatomical (A and B) or anatomical and quantitative correlations (C) between NAWM damaged tracts (voxels in red) and GM atrophy (areas in blue) have been found in patients. This figure shows: In (A), the left corticospinal tract projecting onto the left sensory-motor cortex (see yellow arrow); in (B), the NAWM immediately adjacent to the GM of the right superior temporal lobe (see yellow arrow); and in (C), the right corticospinal tract and the connected sensory-motor cortex (see yellow arrow).
Quantitative Relationship Between Anatomically Related NAWM and GM Abnormalities
Of the eleven regions showing anatomical correspondence between abnormal NAWM FA and GM volume, four areas showed a quantitative relationship: (1) Reduced FA in the corticospinal tract correlated with greater GM atrophy in the adjacent right sensory-motor cortex; (2) Reduced FA of the thalamic radiations correlated with greater GM atrophy of both thalami; (3) Reduced FA of the tracts immediately adjacent to the left insula correlated with greater GM atrophy of the insula (Table III, Fig. 3C).
| Region | FA, mean (SD) | Volume (ml), mean (SD) | r | p value |
|---|---|---|---|---|
| Right sensory-motor | 0.48 (0.04) | 4.52 (0.30) | 0.53 | 0.001 |
| Left thalamus | 0.43 (0.04) | 5.79 (0.59) | 0.70 | 0.0001 |
| Right thalamus | 0.54 (0.03) | 5.60 (0.53) | 0.64 | 0.0001 |
| Left insula | 0.42 (0.04) | 7.6 (0.21) | 0.51 | 0.002 |
Correlation Between NAWM and GM Abnormalities and Disability
Either the NAWM FA or the GM volume of the four regions showing a quantitative relationship between NAWM and GM damage correlated with disability independently from the other, and from age and gender. In particular, patients with greater GM atrophy in the right sensory-motor cortex had greater upper limb disability, as measured by zNHPT (p = 0.01, coeff. = 1.27, 95% confidence interval (CI) 0.26, 2.28), independently from the NAWM FA of the right corticospinal tract. The NAWM FA of the left and right thalamic radiations was lower in patients with greater cognitive impairment, as measured by zPASAT (p = 0.0001, coeff. = 0.001, 95% CI 0.001, 0.002 and p = 0.0001, coeff. = 0.002, 95% CI 0.001, 0.002, respectively), independently from the GM thalamic volume. We also found that the NAWM FA of the tracts adjacent to the left insula was associated with zPASAT (p = 0.0001, coeff. = 0.001, 95% CI 0.001, 0.002), independently from the insular GM volume.
No correlation was found between either NAWM or GM damage and the categorized EDSS.
DISCUSSION
NAWM and GM Abnormalities
We localized tracts of reduced FA in the NAWM of patients with early PPMS using TBSS, which is a recently developed method that overcomes some of the current limitations of the standard voxel-based type of analysis of FA images [Smith at al.,2006,2007]. This method has been previously applied to a cohort of 13 patients with relapsing-remitting and two patients with secondary progressive MS to assess correlations between WM FA and T2 lesion load and EDSS [Cader et al.,2007]. This is the first time that this technique is used to map FA changes in the NAWM of patients with PPMS when compared with controls and to investigate whether they relate to the GM atrophy of connected regions, measured using a VBM approach [Ashburner and Friston,2000]. We were particularly interested in the study of the NAWM, as a correlation between T2 lesion load and GM pathology has already been demonstrated [De Stefano et al.,2003; Ramio-Torrenta et al.,2006]. Moreover, the diffuse NAWM abnormalities in PPMS are known to be only partially explained by axonal destruction in the plaques, followed by secondary Wallerian degeneration [Kutzelnigg et al.,2005; Pelletier et al.,2003]. We found that patients showed reduced FA in several NAWM tracts, suggesting a widespread demyelination and axonal loss [Beaulieu et al.,1996; Schmierer et al.,2007]. This extends previous observations of lower FA in patients with MS, including the PP phenotype, that used a region-of-interest analysis [Ciccarelli et al.,2001,2003; Filippi et al.,2004].
Regions of GM atrophy in patients were localized using VBM [Ashburner and Friston,2000], a widely used method to detect focal GM differences between groups. Our findings indicate extensive and focal atrophy of GM early in the disease course of PPMS, confirming results obtained in relapsing-remitting MS patients [Morgen et al.,2006; Prinster et al.,2006], and in our larger cohort of PPMS patients [Khaleeli et al.,2007b]. It is thought that in vivo MRI measures of GM atrophy reflect neurodegeneration, mainly axonal loss and demyelination [Miller et al.,2002].
Anatomical and Quantitative Relationship Between NAWM Damage and GM Atrophy
Our data on the topographic distribution of reduced NAWM FA and GM atrophy in patients, identified eleven regions of clear anatomical correspondence between the two compartments. For example, we observed a reduced FA in the bilateral corticospinal tract and a reduced GM volume in both connecting sensory-motor cortices. Conversely, other abnormal WM tracts did not show any anatomical correspondence with areas of GM atrophy. Similarly, a number of regions of GM atrophy were not adjacent to any regions of reduced NAWM FA.
Interestingly, of these eleven regions with visible anatomical correspondence, only four areas showed a significant quantitative correlation between GM atrophy and reduced NAWM FA in the connected tracts. These areas were the right sensory-motor cortex and the connected corticospinal tract, both thalami and the adjacent thalamic radiations, and the left insula and the adjacent WM. This suggests that in these regions there may be a link between the pathological processes occurring in the two compartments. For example, it is possible that the GM atrophy is secondary to degeneration of WM tracts resulting from transection of axons in inflammatory WM lesions [Trapp et al.,1998]. Alternatively, the GM may be the primary target of the disease process, resulting in secondary axonal degeneration and demyelination in WM (the “inside out” model of MS) [Tsunoda et al.,2003]. Finally, a combination of the above could also occur. The remaining seven regions, where a quantitative correlation was not found, suggest that the pathological processes occurring in the NAWM and in the GM could to some extent develop independently from each other, at least in some areas of the brain and in the early stages of the disease.
It should be noted here, for clarity, that we use the term “anatomical correspondence” to indicate areas which appear contiguous on visual inspection, while more sophisticated approaches, such as DT tractography, would be needed to confirm the connection between the NAWM and GM areas identified in this study. Tractography is able to reconstruct WM tracts starting from a region or “seed point,” selected a priori. Therefore, it would be interesting, for example, to use the abnormal areas identified by TBSS and VBM as seed points to assess the presence of a real connection between them.
From a methodological point of view, our quantitative approach has proved valuable in providing additional information not available from a purely qualitative and anatomical analyses previously published [Douaud et al.,2007]. For example, the finding that only a minority of anatomically associated areas showed a quantitative correlation between the MRI measures, may help to explain the minimal or absent correlations between the two compartments reported in histological studies [Bo et al.,2007; Kutzelnigg et al.,2005].
This study has a major limitation, which is that the analysis of the differences in NAWM FA and GM volume between patients and controls was performed using two different groups of controls, with differing mean age. Further investigations based on data from the same control group are warranted to confirm our findings. However, the age of the two control groups was not significantly different from each other (p = 0.07, using the two-sample t-test), and the difference in age between patients and the group of 23 controls was corrected for, at each stage of the analysis. Furthermore, the mapping of regions of reduced FA and increased atrophy is consistent with what has been reported in previous studies, as discussed above. Another methodological limitation of this study lies in the coregistration of DTI and FSPGR images, which have different slice thicknesses, onto the same anatomical image, during post-processing procedures. These aspects, together with the smoothing of the images, which is needed in the VBM analysis to render the data normal for statistical analysis, may have influenced our results by compromising accurate localization of abnormal regions and subsequent identification of areas of anatomical correspondence.
Contribution of NAWM and GM Abnormalities to Disability
Interestingly, all four areas of visually identified and quantitatively confirmed correlation between NAWM damage and GM atrophy were clinically eloquent, and in each area we observed an independent contribution from one of the two compartments to disability. In particular, the GM atrophy in the right sensory-motor cortex correlated with the upper limb function, as measured by the NHPT, independently from the NAWM damage in the right corticospinal tract. These findings indicate that, in this area, GM damage has a role in determining motor disability, which is greater than, and independent from, the injury of the connected corticospinal tract. This extends previous findings of a relationship between atrophy in the motor network and disability in MS [Filippi et al.,2004; Khaleeli et al.,2007b; Pirko et al.,2007].
We also found that reduced FA in the thalamic radiations and in the tracts adjacent to the left insula was independently associated with poor performance on the PASAT test in patients; this association was independent of the GM atrophy of the thalamus and insula, respectively. These data suggest an important contribution from these tracts to cognitive function, as the PASAT test is a complex working memory task involving several distinct brain areas that interact simultaneously. This is in line with previous reports of a correlation between NAWM injury and PASAT score in patients at the earliest stage of MS [Audoin et al.,2005].
The lack of correlation with EDSS, on the other hand, could be explained by the limitations of this scale [Hobart et al.,2000]. However, it is important to maintain that NAWM and GM damage in the brain are not the only contributors to clinical disability in PPMS. WM lesions and spinal cord atrophy (which were not included in this investigation) are also known to make a significant contribution [Ingle et al.,2003], and the interpretation of our results should not disregard these important aspects of MS pathology.
In conclusion, NAWM damage and GM atrophy in early PPMS are strictly interdependent in specific, clinically relevant brain regions. This suggests that that there may be a link between the mechanisms of damage occurring in the two compartments in these areas in the early phase of progression, and it is noteworthy that damage in these regions was associated with disability. Our results suggest that the future application of TBSS and VBM in longitudinal studies on patients with PPMS has the potential to spatially and temporally characterize the changes occurring in the NAWM and in the GM, offering unique insights into the complex relationship between pathological changes in these two compartments. In particular, the pathological relationship between the damage occurring in the NAWM and in the GM might be crucially dependent on disease stage, and future work will aim at assessing its development over time. Moreover, the combination of TBSS and VBM could be applied to investigate whether this relationship varies according to MS phenotype. This is an exciting new field of research aimed to clarify this critical aspect of the pathogenesis of MS. Moreover, our findings indicate that the application of these promising novel techniques could be extended to the study of the pathogenesis of other neurological diseases affecting both the WM and the GM.
Acknowledgements
The authors thank C. Wheeler-Kingshott and J. Jackson for their important help and advice. They thank all the subjects for agreeing to take part in this study.