A voxel-based morphometry study of frontal gray matter correlates of impulsivity†
These findings were presented in part at the annual meeting of the Society of Biological Psychiatry, May 19–21, 2006, Toronto, Canada.
Abstract
Impulsivity is a personality trait exhibited by healthy individuals, but excessive impulsivity is associated with some mental disorders. Lesion and functional neuroimaging studies indicate that the ventromedial prefrontal region (VMPFC), including the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC) and medial prefrontal cortex, and the amygdala may modulate impulsivity and aggression. However, no morphometric study has examined the association between VMPFC and impulsivity. We hypothesized that healthy subjects with high impulsivity would have smaller volumes in these brain regions compared with those with low impulsivity. Sixty-two healthy subjects were studied (age 35.4 ± 12.1 years) using a 1.5-T MRI system. The Barratt impulsiveness scale (BIS) was used to assess impulsivity. Images were processed using an optimized voxel-based morphometry (VBM) protocol. We calculated the correlations between BIS scale scores and the gray matter (GM) and white matter (WM) volumes of VMPFC and amygdala. GM volumes of the left and right OFC were inversely correlated with the BIS total score (P = 0.04 and 0.02, respectively). Left ACC GM volumes had a tendency to be inversely correlated with the BIS total score (P = 0.05). Right OFC GM volumes were inversely correlated with BIS nonplanning impulsivity, and left OFC GM volumes were inversely correlated with motor impulsivity. There were no significant WM volume correlations with impulsivity. The results of this morphometry study indicate that small OFC volume relate to high impulsivity and extend the prior finding that the VMPFC is involved in the circuit modulating impulsivity. Hum Brain Mapp 2009. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Moeller and colleagues defined impulsivity as “a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions to the impulsive individuals or to others” [Moeller et al.,2001a]. Impulsivity is a personality trait that is present in healthy individuals [Barratt,1994], and it may help mold our life roles [af Klinteberg et al.,1992]. However, high impulsivity may also cause problems in social life. For example, high impulsivity is associated with driving errors and traffic violations in the general population [Owsley et al.,2003].
An association between impulsivity and mental illness has also been noted. Patients with mood disorders have a high prevalence of suicide, and their suicidal ideas and behaviors are associated with impulsivity [Swann et al.,2005]. The aggression/impulsivity predicts suicidal acts in patients with mood disorders [Mann et al.,1999; Oquendo et al.,2004]. Impulsivity is also related to the severity of cocaine abuse and the severity of cocaine withdrawal symptoms [Moeller et al.,2001b], and cocaine users show poorer inhibitory control than noncocaine users [Kaufman et al.,2003]. Furthermore, cocaine dependent subjects with high impulsivity are more likely to drop out of treatment [Moeller et al.,2001b]. High impulsivity also is a characteristic of patients with borderline personality disorder (BPD), attention-deficit/hyperactivity disorder (ADHD), and intermittent explosive disorder (DSM-IV-TR) [APA,2000]. These relationships suggest that impulsivity can be an important issue for psychiatric patients and also may be a public health concern. Thus, clarification of the neural underpinnings of impulsivity should be sought for a more complete understanding of impulsivity regulation and the development of new treatments to prevent risky behaviors in subjects with high impulsivity and psychiatric disorders.
Neuroimaging studies provide some evidence that the ventromedial prefrontal (VMPFC) region, including the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC) and medial prefrontal cortex (MPFC), and the amygdala play an important role in modulating impulsivity and aggression [Weiger and Bear,1988]. VMPFC has an anatomically intrinsic cortico-cortical network [Ongur and Price,2000; Ridderinkhof et al.,2004] and an extrinsic connection with striatum, thalamus, brain stem and limbic structures, including amygdala. This circuit is thought to contribute to impulsivity control [Mega and Cummings,1994; Ongur and Price,2000]. Subjects with VMPFC damage show higher impulsivity on inhibition tasks compared with healthy subjects [Bechara et al.,1999]. Other studies also demonstrate that subjects with VMPFC damage exhibit higher impulsivity and aggression compared with subjects with damage to regions of the frontal cortex outside the OFC [Berlin et al.,2004; Grafman et al.,1996]. In addition, patients with amygdala damage exhibit low inhibition in response to an inhibition task similar to that of those with VMPFC damage [Bechara et al.,1999]. Patients with VMPFC and amygdala damage show higher impulsivity compared with healthy subjects [Bechara et al.,1999; Berlin et al.,2004; Grafman et al.,1996]. Abnormal functions of the ACC and VMPFC, including OFC, during impulsivity/inhibition tasks were reported in patients with high impulsivity, such as those with BPD, impulsive-aggressive disorder, and ADHD [Leyton et al.,2001; Siever et al.,1999]. In morphometry studies, child [Carmona et al.,2005] and adult [Hesslinger et al.,2002] patients with ADHD show significantly smaller OFC volumes compared with healthy subjects. However, no morphometry studies have correlated the volumes of these VMPFC regions with impulsivity in healthy individuals, although some functional neuroimaging studies have shown an association between OFC functions and impulsivity/aggression [Horn et al.,2003; Pietrini et al.,2000].
Here, we report a voxel-based morphometry (VBM) study to elucidate the association in healthy subjects. We hypothesized that healthy subjects with high impulsivity would have smaller VMPFC, including OFC, ACC and MPFC, and amygdala volumes compared to those with low impulsivity.
MATERIALS AND METHODS
Subjects
We recruited subjects who responded to advertisements broadcast on the radio and flyers placed in the community and at hospitals and clinics in the South Texas Medical Center area. Sixty-two healthy subjects were studied. All subjects gave written informed consent after receiving a complete description of the study. The Institutional Review Board of The University of Texas Health Science Center at San Antonio approved this study. A research psychiatrist screened subjects for DSM-IV axis I disorders using the Structural Criteria Interview for Diagnosis (SCID) nonpatient version [First et al.,1996]. The psychiatrist also performed a clinical interview to obtain information including age, gender, handedness [Oldfield,1971], education, and race. Any subject who had current or past Axis I disorders was excluded. Those who had any suicidal history and those with any first-degree relative with any Axis I disorder were excluded. Participants underwent a physical examination and laboratory tests to confirm good general health. Any participant with endocrinological disease, head trauma, neurological disease, family history of hereditary neurological disorder, or a medical condition such as hypertension, diabetes, active liver disease, kidney problems, or respiratory problems was excluded. Left-handed and ambidextrous persons also were excluded from this study.
We used the Barratt impulsiveness scale 11A (BIS) [Barratt,1965,1983,1994] to quantify impulsivity. The BIS is a self-report questionnaire containing 30 4-point Likert-type items [Barratt,1994]. Scoring yields a total score and three subscale scores derived by factor analysis: attention (rapid shifts and impatience with complexity), motor (impetuous action) and nonplanning (lack of future orientation) [Patton et al.,1995]. Higher scores signify higher impulsivity.
MRI Acquisition
Brain images were collected on a Philips 1.5 T MRI system (Philips Medical System, Andover, MA). Images were collected by means of an axial three-dimensional T1-weighted field fast echo sequence (field of view 256-mm, view matrix 256 × 256, repetition time 24 m sec, echo time 5 m sec, flip angle 40°, slice thickness 1-mm).
Image analysis
Preprocessing was performed using SPM2 software (Wellcome Department of Imaging Neuroscience, London, United Kingdom) running under Matlab 7.1.0 (MathWorks, Natick, MA). The images were preprocessed following the optimized VBM protocol [Good et al.,2001]. First, we created a customized anatomical T1 template and prior probability images from the sample of 62 subjects. All original images were manually aligned on the anterior commissure-posterior commissure line. A customized-T1 own template was created by averaging the images of all the participants and prior probability images using an optimized VBM script (http://dbm.neuro.uni-jena.de/vbm.html). For creating a customized template, images were spatially normalized in the same stereotactic space based on the Montreal Neurological Institute template using 12 affine transformation and 16 nonlinear iterations. Normalized images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using an a priori template. Segmented images were smoothed with 8-mm of full-width half-maximum (FWHM) using an isotropic Gaussian kernel. Then the own template was obtained. The original images were analyzed using this own template as follows: the extracted segmented images were normalized with the own GM template, and deformation parameters were applied to the original images, followed by a second segmentation step in stereotactic space. This procedure resulted in removal of nonbrain tissues including scalp, skull, and dural venous sinus automatically. Finally, the segmented images were modulated by Jacobian determinants derived from the spatial normalization. Images were obtained in 1 × 1 × 1 mm resolution by the optimized VBM script. These images were smoothed with 12 mm of FWHM by isotropic Gaussian kernel [Mechelli et al.,2005].
Statistical analysis
We used SPM2 software that implemented a General Linear Model. We selected four a priori regions of interest (ROIs) based on previous neuroimaging studies of impulsivity: OFC, MPFC, ACC, and amygdala. We identified these regions using Talairach Daemon [Lancaster et al., 2000] and automated anatomical labeling [Tzourio-Mazoyer et al.,2002] via WFU PickAtlas version 2 (www.fmri.wfubmc.edu/download.htm) [Maldjian et al.,2003,2004]. Total brain volumes were calculated by the optimized VBM script.
The negative and positive correlations between the BIS total score and the regional brain volumes were performed using a single subject condition and covariate model in SPM2. Age and sex were treated as nuisance variables because both of them may affect brain structure [Good et al.,2001; Sowell et al.,2003] and men with ventromedial OFC damage reportedly use different strategies to solve similar problems compared to women with OFC damage [Tranel et al.,2005]. The GM and WM images were proportional scaled to total GM and WM volumes with the global means of 50. The resulting set of voxel values for each contrast constituted an SPM of the t statistic (SPM {t}) First, we performed an exploratory whole brain analysis to determine which regions correlated with the BIS total score. For this analysis, we set the threshold for statistical significance at P < 0.005 without correction for multiple comparisons. When this whole brain analysis revealed that some of the ROIs were significantly correlated with the BIS total score, we then applied small volume False Discovery Rate (FDR) correction (SVC) P < 0.05 in SPM to confirm our hypothesis. For the regions where the SVC analysis revealed significant correlations with the BIS total score, we also computed the correlations between the regional volumes and the three BIS subscale scores using SVC. The SVC analysis of the three subscales was corrected by FDR (P < 0.05) as well as the whole brain analysis, and the results obtained were further corrected by applying a Bonferroni correction (P < 0.01; 0.05/3 = 0.016) since the BIS comprises three subscales. All results are presented as Talairach coordinates. Talairach coordinates were obtained by applying Brett's transformation (www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml) to the MNI coordinates output by SPM2. Sex-differences on the total, motor, attention, and nonplanning BIS scores were analyzed by Student's t test with SPSS software for Windows, version 15 (SPSS, Inc., Chicago, IL).
RESULTS
Twenty-four males and 38 females were studied. Mean age of the subjects was 35.4 ± 12.1 years; range 19–65 years). All the subjects were right handed. The mean years of education was 16.3 ± 2.9 years (range 12–25 years). Their ethnic group memberships were 21 Caucasian (34%), 29 Hispanic (47%), 2 African-American (3%), 7 Asian (11%), and 3 others (5%). No participant had a diagnosis of any personality disorder. The mean ± SD and range for BIS scores were 58.5 ± 8.6 and 42–83 for the total score, 23.0 ± 4.0 and 17–31 for Non-planning, 18.8 ± 3.8 and 10–30 for Motor and 16.8 ± 3.3 and 11–27 for Attention subscales. No significant difference due to sex was found for total (male, female; 56.4 ± 6.8, 59.4 ± 9.5, t = −1.44, P = 0.16), nonplanning (22.6 ± 3.7, 23.1 ± 4.4, t = −0.59, P = 0.63), motor (17.7 ± 2.2, 19.3 ± 4.5, t = −1.88, P = 0.07), or attention (16.1 ± 3.2, 17.0 ± 3.2. t = −1.05, P = 0.30) BIS scores.
The mean whole brain GM volume was 682.5 ± 67.0 cm3, and the mean whole brain WM volume was 403.8 ± 42.5 cm3. There was no significant correlation between whole brain GM or WM volumes and the BIS total score (r = −0.11, P = 0.42; r = −0.18, P = 0.16, respectively).
The whole brain analysis revealed that some GM regions, including the ROIs, showed a significant inverse relationship with the BIS total score (see Fig. 1). Left and right OFC and left ACC showed inverse correlations with the BIS total score in the whole brain analysis with the threshold set at P uncorrected <0.005. Table I summarizes the results of the SVC analysis. For the SVC analysis, the GM volumes of the left superior and right middle regions of the OFC were inversely correlated with the BIS total score. The left ACC GM volume showed a tendency toward an inverse correlation with the BIS total score. The right middle OFC GM volume was significantly inversely correlated with the nonplanning BIS score (Table I and Fig. 2), and the left superior OFC GM volume was significantly inversely correlated with the motor BIS score (Table I and Fig. 3) after Bonferroni correction. No OFC GM volume was significantly inversely correlated with the BIS attention score. There were no significant correlations between any of the regional WM volumes and impulsivity, and there were no significant positive correlations between any of the regional GM volumes and impulsivity.
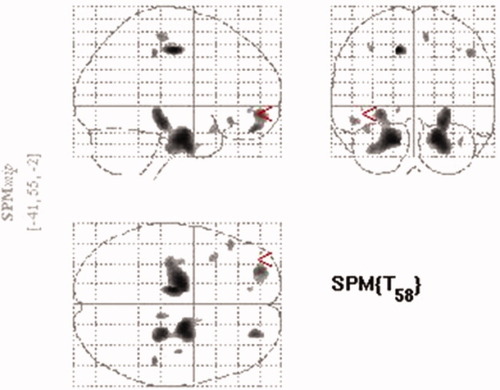
The glass brain map showing the gray matter regions inversely correlated with BIS total score (n = 62) by the whole brain analysis. The significance threshold in the analysis was set at P < 0.005 uncorrected. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
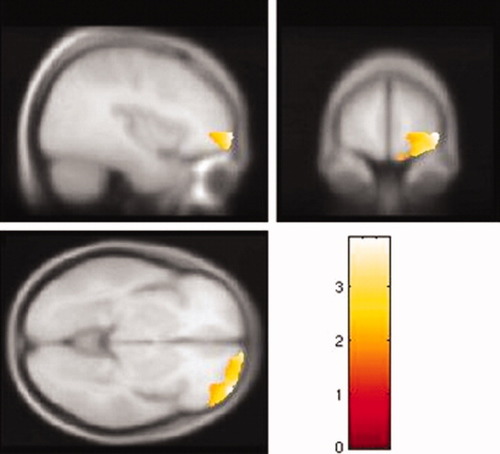
Right middle orbitofrontal cortex gray matter volume is inversely correlated with nonplanning BIS score (n = 62). The color scale represents t scores. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
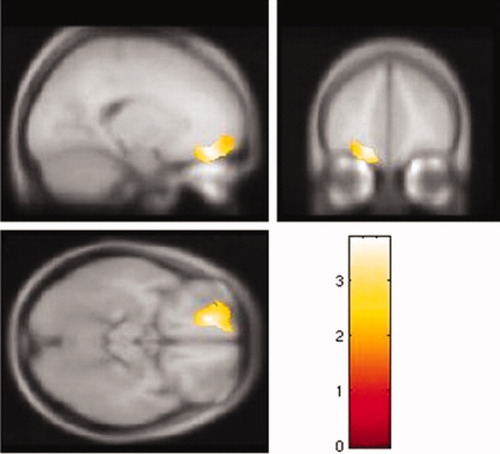
Left superior orbitofrontal cortex of gray matter volume is inversely correlated with motor BIS score (n = 62). The color scale represents t scores. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Talairach coordinate1 | Brodmann area | Cluster size | t | r2 | PFDR-corrected | |
|---|---|---|---|---|---|---|
| Total BIS | ||||||
| Right middle orbitofrontal cortex | (24, 51, −18) | 11 | 364 | 3.30 | 0.13 | 0.039 |
| Left superior orbitofrontal cortex | (−26, 54, −7) | 10 | 1059 | 3.29 | 0.14 | 0.017 |
| Left anterior cingulate | (−14, 43, −5) | 32 | 47 | 2.92 | 0.10 | 0.051 |
| Non-planning | ||||||
| Right middle orbitofrontal cortex | (39, 59, −7) | 10 | 1381 | 3.91 | 0.15 | 0.005 |
| Right middle orbitofrontal cortex | (13, 67, −13) | 11 | 720 | 3.92 | 0.15 | 0.009 |
| Motor | ||||||
| Left superior orbitofrontal cortex | (−18, 42, −16) | 11 | 2623 | 3.77 | 0.24 | 0.004 |
- 1 Talairach and Toronoux (1988) coordinates of the voxel of maximal statistical significance.
DISCUSSION
Consistent with our prediction, subjects with high impulsivity had smaller GM volumes of the left and right OFC compared with those with low impulsivity, although total brain GM volume was not significantly correlated with impulsivity. Higher impulsivity also tended to be associated with smaller ACC GM volumes. These results suggest that small OFC volume relates to high impulsivity in healthy individuals and extends previous findings suggesting that VMPFC is involved in the circuit modulating impulsivity. Notably, since our sample comprised healthy individuals, and their BIS scores fell within the normal range for the general population (700 subjects, total BIS, mean ± SD 64.2 ± 10.7, range 39–103)[Spinella,2007], our results can elucidate the association between impulsivity and OFC volume among mentally healthy subjects, but they do not indicate that the subjects with high impulsivity will engage in violent or risky behaviors or that they will potentially develop psychiatric disorders with abnormal impulsivity.
This is the first morphometry study in healthy individuals to indicate an association between small OFC volume and high impulsivity. Previous functional neuroimaging studies in healthy subjects reported a relationship between OFC function and impulsivity [Horn et al.,2003; Pietrini et al.,2000]. A positron emission tomography study demonstrated that healthy subjects show decreased regional cerebral blood flow (rCBF) in the medial OFC and increased rCBF in the cingulate gyrus during the imagination of aggressive behaviors compared with a neutral control condition [Pietrini et al.,2000]. A functional MRI study also demonstrated significant activation in the anterior lateral OFC and medial cingulate gyrus during an inhibition task, but the activation was not correlated with BIS score [Horn et al.,2003]. The results of our study support the findings of the functional neuroimaging studies, although these studies had some methodological differences from ours such as an activation paradigm vs. a morphometry study and use of an inhibition task vs. the BIS self-report scale for assessment of impulsivity/inhibition control.
In a morphometry study of psychiatric patients, those with major depressive disorder (MDD), alcoholism, ADHD, PTSD, antisocial personality disorder or bipolar disorder showed positive correlations between left, right, and total OFC gray matter volumes and BIS motor impulsivity scores [Antonucci et al.,2006]. Antonucci et al. (2006] also identified a positive relationship between aggression scores and asymmetry of left and right OFC volumes, i.e., right larger than left in patients with mood disorders and between impulsivity scores and total OFC volumes. We discussed the possibility that the OFC may play a regulatory role in aggression and a generative role in impulsivity. In that study, the patients with these diagnoses showed abnormally high levels of impulsivity. However, these findings could explain not only impulsivity but also some other characteristics of the patients because other symptoms of the patients may be confounded. Nonetheless, combined with our results, these findings suggest that OFC volumes are associated with impulsivity. We also may speculate that the OFC may play a different role in impulsivity regulation in psychiatric and healthy subjects. Morphometric studies in psychiatric patients with abnormal impulsivity are warranted to clarify this speculation.
The results of our study also showed that small left OFC volumes related to high motor impulsivity and small right OFC volumes related to high nonplanning impulsivity. Since BIS motor impulsivity relates to violence behaviors [Monahan et al.,2000], our results suggest that the left OFC may control risky behaviors. However, the roles of the left and right OFC in impulsivity regulation has been controversial. Patients with right hemisphere damage had disinhibition syndrome with aggressive outbursts, euphoria, hyperphagia, and irritability [Shulman,1997]. Patients with right OFC damage also showed impairment of real-life decision making and exhibited poor performance on a gambling task compared with those with left OFC damage [Bechara,2004]. Another study showed that subjects with OFC damage had similar scores on decision-making tasks to healthy subjects and those with left OFC damage showed similar scores on the tasks compared with those with right OFC damage [Manes et al.,2002]. In schizophrenia patients, reduced WM integrity of the right ventromedial frontal cortex is associated with BIS motor impulsivity [Hoptman et al.,2002], whereas in MDD patients a history of suicide attempt is associated with smaller OFC volumes in both hemispheres compared with healthy subjects [Monkul et al.,2007]. Nevertheless, the findings of our study may help in understanding the different roles of left and right OFC.
Small left ACC volumes tended to be associated with high impulsivity in this study. ACC has reciprocal connections with OFC [Ridderinkhof et al.,2004] and contributes to executive functions including attention, inhibition, and resolution of cognitive conflict in executive processes [Devinsky et al.,1995; Fletcher and Henson,2001; Luks et al.,2002]. Healthy subjects reportedly show left ACC activation during an inhibition control task [Horn et al.,2003]. Psychiatric patients who have impulsive temperament and behaviors show abnormal ACC function and volumes compared with healthy control subjects [Carmona et al.,2005; Leyton et al.,2001; Siever et al.,1999; Tebartz van Elst et al.,2003]. Functional neuroimaging studies also demonstrate that ACC and OFC activations are associated with anger expression more than impulsivity [Dougherty et al.,1999; Kimbrell et al.,1999]. Although, to our knowledge, no study has found a relationship between ACC volume and impulsivity, these prior findings provide some evidence that ACC function is involved in the circuit of impulsivity regulation. The reasons for the lack of an association between ACC volume and impulsivity in this study are not clear, but a methodological issue is suggested, i.e., the BIS is related to impulsivity mediated in the OFC rather than the ACC. This is suggested because the BIS scores are associated with other neuropsychological measurements of impulsivity [Spinella,2004]. Further studies into the association between OFC and ACC with tasks that evoke emotional expression in healthy and psychiatric subjects are warranted to illuminate the brain underpinnings of impulsivity.
We found no statistically significant correlation between amygdala volume and impulsivity. Amygdala is connected with the OFC, striatum, and thalamus in rats and primates [Schoenbaum et al.,2006]. Amygdala cooperates with OFC to encode the information that may be used to guide goal-directed behavior [Schoenbaum et al.,1998]. However, amygdala is also reported to have a different role in emotional regulation from that of OFC [Winstanley et al.,2004]. Amygdala is activated in response to tasks that evoke fear and negative affect [Hariri et al.,2002; Morris et al.,1996]. Patients with amygdala damage show low inhibition on an inhibition task that requires decision making. Since the decision-making test may measure a more complex process including impulsivity regulation than the BIS, one explanation for the lack of a relationship between amygdala volume and impulsivity in this study is that amygdala may play other roles in the regulation of the impulsivity that the BIS cannot measure.
Some studies suggest differences in impulsivity control and expression in males and females [McGirr et al.,2006; Zalsman et al.,2006]. Consecutive females who committed suicide had lower BIS scores than males who committed suicide [McGirr et al.,2006]. Male patients with bipolar disorder had higher risk for a lethal suicide attempt compared to females [Zalsman et al.,2006]. In contrast, no sex-difference was demonstrated in the general population [Spinella,2007]. In this study, we treated sex as a nuisance variable for VBM analysis, although our sample did not show any sex difference on the BIS scores. Since the functional brain mechanism regulating impulsivity may be different between males and females [Walderhaug et al.,2007], sex-related morphometric differences in impulsivity regulation should be studied.
Some methodological limitations of this study should be noted. We used the BIS to measure impulsivity. The BIS is one of the most useful and widely used assessments of trait impulsivity. However, the answers to the BIS questions may not fully reflect the impulsivity of the subjects because it is a self-report scale, and subjects may not answer the questions honestly. Furthermore, since impulsivity is a complex process, various brain regions probably contribute to impulsivity regulation, and the BIS may not assesses the full spectrum of trait impulsivity. Our results should be interpreted cautiously as the OFC may represent only one region of the brain relevant to impulsivity regulation. Thus, future studies should include some tasks to measure various forms of impulsivity/inhibition to better elucidate the association between impulsivity and the roles of these regions, e.g., the Impulsivity Rating Scale [Lecrubier et al.,1995], the Go/No-go task and the Iowa gambling task. Also, we did not administer the SCID-II for the assessment of Axis II disorders, although impulsivity is a characteristic of patients with Axis II disorders such as BPD. However, no participant displayed symptoms of any personality disorder clinically, and none had received such a diagnosis. Since there is a strong relationship between impulsivity and personality disorders, future research will benefit from the assessment of Axis II disorders more formally. Another limitation is that we did not evaluate and control other mood or cognitive factors relevant to the VMPFC and ACC, i.e., fear, anxiety, cognition, and attention. These potential traits in the participants may affect the results. Finally, we did not analyze the potential effects of genetic factors in the participants. Some genotypes relevant to the serotonergic system such as monoamine oxidase A and B genes [Meyer-Lindenberg et al.,2006] and a polymorphism in the promoter region of the serotonin transporter (5-HTT) gene [Sakado et al.,2003) reportedly play a role in the neural mechanisms of impulsivity or aggression. Unexplored variations in these genotypes may confound our results.
Nonetheless, our results indicate that small OFC volumes are associated with high impulsivity in healthy subjects and further support the theory that VMPFC, including OFC and ACC, is involved in the circuit modulating impulsivity. Further studies of psychiatric patients with high impulsivity would help clarify this association and advance our understanding of the pathophysiology of the patients.




