Monocytes in central nervous system remyelination
Funding information: Medical Research Council, Grant/Award Number: MR/V031260/1; Multiple Sclerosis Society, Grant/Award Number: 126
Abstract
Remyelination failure with aging and progression of neurodegenerative disorders contributes to axonal dysfunction, highlighting the importance of understanding the mechanisms underpinning this process to develop regenerative therapies. Central nervous system (CNS) macrophages, encompassing both resident microglia and blood monocyte-derived cells, play a crucial role in driving successful remyelination. Although there has been a focus on the critical roles of microglia in remyelination, the specific contribution of monocyte-derived macrophages is still not fully understood. Until recently, the lack of tools enabling distinction between CNS macrophage populations has hindered our understanding of monocyte influence on remyelination. Recent advances have allowed for identification and characterization of monocyte populations in health, aging and in neurodegenerative conditions like multiple sclerosis, indicating heterogeneity of monocyte subsets impacted by both intrinsic and extrinsic factors. Here, we discuss the new tools enabling distinction between macrophage populations and advancements in understanding the importance of monocytes in remyelination, and reflect on the potential for therapeutic targeting of monocytes to promote remyelination.
1 INTRODUCTION
Myelin ensheathment of central nervous system (CNS) axons by oligodendrocytes provides trophic and metabolic support, and insulation for efficient speed of action potential conduction. The importance of myelin for axonal health and CNS function is evident from the motor, sensory, and cognitive deficits incurred from demyelination in neurological disorders, such as multiple sclerosis (MS) and Alzheimer's disease (AD). The regeneration of myelin, termed remyelination, can occur efficiently in early stages of disease to reduce axonal damage/loss and restore axonal function. This involves the recruitment, proliferation, and maturation of oligodendrocyte progenitor cells (OPCs) into mature oligodendrocytes which make new myelin (Franklin & Ffrench-Constant, 2017). Recent work revealed that a small proportion of mature oligodendrocytes surviving demyelination can also contribute to remyelination (Bacmeister et al., 2020; Duncan et al., 2018; Yeung et al., 2019), although the quality of remyelination is poor with myelin being mistargeted to neuronal cell bodies (Neely et al., 2020). Importantly, remyelination often fails with aging and progression of neurodegenerative disease. This remyelination failure is considered to contribute to axonal dysfunction/loss and clinical decline, for which there is an unmet need for therapeutics (Lubetzki et al., 2020; Stangel et al., 2017). Although pro-remyelination therapies targeting OPC maturation are currently being trialed in MS (Lubetzki et al., 2020; Stangel et al., 2017), it is unclear how effective these will be, whether they will be effective in all affected individuals, and how these will impact the quality of remyelination. Altogether, these findings highlight the importance of understanding the full complement of mechanisms involved in successful remyelination to develop regenerative therapies.
Extensive studies by our lab and others revealed that one of the key determinants of successful remyelination is the macrophage (Lloyd & Miron, 2019). Within a demyelinated lesion, macrophages include CNS-resident microglia and monocytes recruited from the blood which have differentiated into macrophages (‘monocyte-derived macrophages’; MDM). Whereas most macrophage research in remyelination has focused on the microglia, MDMs are also important to consider as they account for up to ~60% of macrophages in MS lesions (Masuda et al., 2019; Zrzavy et al., 2017), and monocytes are known to be dysregulated in both MS and AD. Macrophages, including MDMs, have been suggested to induce demyelination in multiple sclerosis (Yamasaki et al., 2014) and Guillain-Barré Syndrome (Koike et al., 2020), yet it is unclear whether macrophages in these circumstances are furthering demyelination or removing myelin debris/dysfunctional myelin to support repair. Indeed, MDMs have known functions which are relevant for remyelination. However, the paucity of tools to distinguish the contribution of monocytes to remyelination from that of microglia has hampered our complete understanding of how monocytes contribute to remyelination, and how their dysregulation with aging and disease contributes to remyelination failure. In this review, we will summarize existing knowledge on the role of monocytes in remyelination, the gaps in knowledge with regards to their function and dysfunction, and promising therapeutic strategies to restore monocyte-driven remyelination in disease.
2 MONOCYTE SUBSETS IN HEALTH, AGING AND DISEASE
2.1 Monocyte subsets
Monocytes originate from hematopoietic stem and progenitor cells in the bone marrow, from which they emigrate to circulate in the blood before entering tissues and differentiating into either MDMs or monocyte-derived dendritic cells. Here, they can either aid in maintenance of tissue homeostasis or respond as part of mounting innate immune reaction following injury, infection or in disease. In addition to the bone marrow, the spleen holds a large pool of monocytes enabling immediate monocyte migration during inflammation or injury (Swirski et al., 2009). The recruitment of monocytes into tissues is governed by locally secreted chemokine ligands (CC motif chemokine ligands; CCLs) that attract monocytes expressing the corresponding chemokine receptors (CC motif chemokine receptors; CCRs). For example, in the injured mouse CNS, locally secreted CCL2 promotes the migration of CCR2-expressing monocytes from the peripheral blood into the brain or spinal cord to aid in a localized immune response (Dzenko et al., 2005; Howe et al., 2017; Ma et al., 2002).
Although monocytes largely differentiate into macrophages following migration into inflamed tissues, the specific tissue environment in which monocytes reside (e.g., blood, lymph tissue, homeostatic, or inflamed organs) can alter the monocyte differentiation pathway. For example, during infection monocytes can differentiate into dendritic cells (DCs), specifically tumor necrosis factor (TNF)- and inducible nitric oxide synthase (iNOS)-producing dendritic cells (Tip-DCs) to aid bacterial clearance (Serbina et al., 2003), or remain as undifferentiated monocytes that have antigen presenting capabilities (as reviewed in Jakubzick et al., 2017). This distinct ability to respond to different environmental cues enables monocytes to be highly plastic and adaptable to mediate an appropriate immune response.
Monocytes exist as different subsets. In mice, these are the classical (or ‘inflammatory’) and non-classical (or ‘patrolling’) monocytes. These monocyte subpopulations also exist in humans, in addition to an ‘intermediate’ phenotype (Passlick et al., 1989). This intermediate subset may represent a transition between classical to non-classical monocytes, as is known to occur in mouse and human (Patel et al., 2017; Yona et al., 2013). Monocyte subsets can be distinguished by expression of distinct markers. In mice, classical monocytes are CCR2+ Ly6Chi CX3CR1lo and Nr4a1lo, whereas non-classical monocytes are CCR2lo Ly6Clo CX3CR1hi and Nr4a1hi. In humans, classical monocytes are CD14+ CD16−, non-classical monocytes are CD14lo CD16+ and the intermediate subset is CD14+ CD16+. Each subset has distinct transcriptomic profiles (Anbazhagan et al., 2014; Schmidl et al., 2014; Wong et al., 2011) which can translate into differing functional capabilities (Auffray et al., 2009). Classical monocytes express high levels of chemokine receptors allowing them to respond to injury and have high phagocytic capacity which contributes to wound healing (Weber et al., 2000; Wong et al., 2011), whilst non-classical monocytes act to patrol tissue endothelium and promote neutrophil adhesion (Auffray et al., 2007; Chimen et al., 2017).
2.2 Monocytes in aging and disease
It is conceivable that changes in monocyte subsets in aging and disease may have an impact on remyelination. Changes in the immune system with age, termed ‘inflammaging’ (Franceschi et al., 2018), includes dysregulation of monocyte subsets and their function (Damasceno et al., 2019; Hearps et al., 2012; Nyugen et al., 2010). For example, with aging, non-classical monocytes significantly increase in human blood yet have reduced surface expression of CX3CR1 and HLA-DR (MHC-II) (Seidler et al., 2010), and are known to become less phagocytic (Hearps et al., 2012). In aged mice, levels of both circulating non-classical and classical monocytes increase and display differential patterns of expression cell surface markers (Strohacker et al., 2012). For instance, aged non-classical monocytes have reduced expression of MHCII and increased expression of TLR2, whilst aged classical monocytes have reduced expression of TLR4 and CD80 (Strohacker et al., 2012). Monocyte changes are also apparent in diseases including AD and MS (Fani Maleki & Rivest, 2019; Munawara et al., 2021; Saresella et al., 2014; Zhang et al., 2013). In AD, there is a higher proportion of classical monocytes (Saresella et al., 2014), and monocyte hyperactivation is associated with disease progression (Munawara et al., 2021). In MS, there is a suggested association of monocyte subsets with clinical outcome, yet with conflicting results. Whereas some studies indicate an increased proportion of classical monocytes versus non-classical monocytes in peripheral blood mononuclear cells of MS versus healthy individuals (Fischer et al., 2019; Malhotra et al., 2020), other studies observed the opposite (Chuluundorj et al., 2014; Gjelstrup et al., 2018; Nakajima et al., 2004). Discrepancies between studies may have resulted from (i) variable inclusion of patients treated with disease-modifying therapies (DMTs) or length of time since treatment, (ii) differences in average disability (EDSS) scores or disease duration between cohorts, or (iii) use of a mixed population without distinction between MS types or disease activity. Indeed, Haschka et al. recently revealed differences in the proportions of monocyte subsets between inactive and active forms of MS, and that this is influenced by MS type and DMTs (Haschka et al., 2020). For instance, non-classical monocytes were found to be increased in active relapse-remitting MS (RRMS) and inactive progressive MS (PMS), in RRMS versus PMS, and in RRMS following DMT treatment. Conversely, classical monocytes were seen to be increased in inactive RRMS (Haschka et al., 2020).
Further investigations of monocyte subsets in human disease may be improved by use of mass cytometry, where the purity of subsets is increased to ~99% by combining CD16 and CD14 assessment with that of CCR2, CD36, HLA-DR, and CD11c (Thomas et al., 2017). In addition, recent work has identified additional subsets which may need to be considered in future. Eight different monocyte subsets have now been identified in a mouse model of autoimmune-mediated demyelination (experimental autoimmune encephalomyelitis; EAE). Two of these subsets (Cxcl10+ and Saa3+) were found to be pathogenic, as their specific depletion using a CCR2 antibody resulted in an improved clinical outcome (Giladi et al., 2020). Additionally, Merah-Mourah et al. identified a novel monocyte subset in humans by analyzing CD14+ peripheral blood mononuclear cells (Merah-Mourah et al., 2020). Thought to comprise ~8% of circulating monocytes, this subset had a markedly increased cell size confirmed using imaging flow cytometry, could be subdivided into CD14+CD16− and CD14+CD16+ subgroups, and had different expression levels of cell surface molecules and adhesion proteins than their smaller counterparts. Responses of large versus small monocytes to inflammatory stimuli were found to be highly heterogeneous between individuals (Merah-Mourah et al., 2020). Whether these new monocyte subsets are present or relevant in disease, however, is not yet known. Altogether, these studies suggest that monocyte subsets may play a role in aging and disease, yet their differential contribution to remyelination is unknown. Understanding this contribution could potentially lead to measures of monocyte subsets in the blood as biomarkers for remyelination, and development of novel regenerative therapeutics.
3 MONOCYTE ROLES IN REMYELINATION
3.1 Tools to distinguish between macrophage populations
Macrophages play a critical role in remyelination by creating a favorable environment for OPC recruitment and maturation, via phagocytosis of myelin debris that normally inhibits remyelination, modulation of the extracellular matrix, and secretion of regenerative factors (Davies & Miron, 2018; Kotter et al., 2006; Lampron et al., 2015; Lloyd & Miron, 2019; Robinson & Miller, 1999). With aging, these macrophage functions are impaired, leading to poor remyelination (Cantuti-Castelvetri et al., 2018; Rawji et al., 2018; Ruckh et al., 2012). In a lesion environment, both microglia and MDMs are indistinguishable from one another when analyzing their morphology or expression of macrophage markers, for example, IBA1 and CD68. Consequently, microglia and MDMs have often been analyzed as one macrophage population (referred to as microglia/macrophages) and their distinct contributions to remyelination are therefore unclear. Of note, differential roles of these populations have been documented during demyelination, in the experimental autoimmune encephalomyelitis (EAE) model (Davies & Miron, 2018; Yamasaki et al., 2014).
Techniques have been developed to distinguish the two cell types from one another. This includes differential CD45 expression using flow cytometry, where MDMs have a higher expression (CD45hi) compared to CD45lo microglia (Ford et al., 1995). Bone marrow chimeric mice also provide useful information on the recruitment of MDMs to injured tissue, whereby body irradiation removes the host monocyte supply which is then replenished with transplanted monocytes harboring a distinct allele of CD45 (i.e., bone marrow from CD45.1 donor mice transplanted into a CD45.2 host) (Larochelle et al., 2016). Another approach is to take advantage of the expression of the chemokine receptor CCR2 by classical monocytes, and the absence of expression by microglia, to track or manipulate classical monocytes. This includes Ccr2RFP/+ knock-in reporter mice allowing for the tracking of RFP+ classical monocytes in vivo to distinguish them from RFP− brain resident microglia (Saederup et al., 2010). As classical monocytes require CCR2 to exit the bone marrow, use of Ccr2 −/− mice allows for analysis of injury or repair responses in tissue in the absence of this subset (Boring et al., 1997; Kurihara et al., 1997; Kuziel et al., 1997). More recently, single-cell RNA sequencing identified Msa43 (membrane-spanning 4-domains, subfamily A, member 3) as a marker of monocyte-committed progenitors which led to the development of new Cre-driver (Ms4a3Cre, Ms4a3CreERT2) and reporter (Ms4a3TdT) transgenic lines for monocyte manipulation or tracking in vivo (Liu et al., 2019), although neutrophils and basophils in the blood may also be also targeted or traced. Another approach is to use parabiosis, whereby a shared vasculature between a wildtype mouse and a reporter (e.g., GFP) expressing mouse allows tracking of GFP+ blood cells (including monocytes) in the wildtype parabiote; this was particularly useful in identifying that young monocytes are able to enter demyelinated lesions in aged mice, and enhance remyelination in part via phagocytosis of myelin debris (Ruckh et al., 2012). When the young parabiote was Ccr2−/−, this regenerative influence was significantly reduced, indicating the rejuvenating potential of classical monocytes in the aged CNS (Ruckh et al., 2012). However, how endogenous monocytes are dysregulated with aging and how this links with remyelination failure are not known.
3.2 Monocyte and microglia crosstalk following demyelination
Distinguishing monocytes from microglia is further complicated by changing cell signatures during health and disease (Hammond et al., 2019; Masuda et al., 2019). During injury or disease, microglia can downregulate microglia-specific markers including P2ry12 and Tmem119 (Keren-Shaul et al., 2017; Plemel et al., 2020) and upregulate CD45 (Plemel et al., 2020), further obscuring their distinction from monocytes. Furthermore, single-cell sequencing of macrophages in disease and injury have identified microglia heterogeneity in mice (Hammond et al., 2019; Keren-Shaul et al., 2017; Masuda et al., 2019; Plemel et al., 2020) and humans (Masuda et al., 2019; Mathys et al., 2019), including during remyelination (Hammond et al., 2019; Masuda et al., 2019). Encouragingly, a unique monocyte signature in MS has been detected, including CCR2, PLAC8, CLEC12A, and FCN1 (Masuda et al., 2019). In addition, MRP14/ S100A9 is commonly used to detect infiltrating monocytes in vivo and does not detect microglia (Brück et al., 1995; Masuda et al., 2019; Zhao et al., 2012), although it is known to also be expressed by neutrophils.
Despite these challenges, recent studies have uncovered the crosstalk between monocytes and microglia. Following myelin toxin-induced demyelination in the mouse spinal cord, activated microglia were found to restrict the influx of infiltrating monocytes, as microglia ablation induced peripheral macrophage repopulation of the demyelinated lesion (Plemel et al., 2020). This suggests that microglia modulate monocyte infiltration into the damaged CNS. In addition, preventing monocyte infiltration into injured spinal cord using Ccr2−/− mice increased microglial expression of genes associated with inflammation and apoptosis, increased expression of activation markers CD11b and CD86, worsened demyelination, and perturbed functional recovery (Greenhalgh et al., 2018), suggesting a regenerative role for monocytes in regulating microglia. Given that microglia show regional heterogeneity in transcriptome and function, it will be interesting to determine whether these microglial functions and responses are retained in the demyelinated brain. In vitro co-culture between MDMs and microglia revealed a direct interaction between the cell types. In both mouse and human cells, co-culture reduced lipopolysaccharide (LPS)-induced pro-inflammatory cytokine expression in microglia (interleukin (IL)-1β, tumor necrosis factor (TNF), IL-6) indicating an immuno-modulatory influence of MDMs (Greenhalgh et al., 2018). Furthermore, myelin phagocytosis was suppressed in microglia and increased in MDMs, suggesting an impact of MDM on microglia function (Greenhalgh et al., 2018). These studies suggest that MDM and microglia communication can influence their function, yet how this influences remyelination efficiency is unclear.
3.3 Monocyte contribution to remyelination
Monocytes may contribute to remyelination by phagocytosis of myelin debris, to prevent impedance of the OPC responses which are required for remyelination. Depletion of monocytes in the circulation using clodronate liposomes impairs early phase remyelination (Kotter et al., 2001) and reduces OPC differentiation, likely due to an accumulation of inhibitory myelin debris (Kotter et al., 2005, 2006; Syed et al., 2008); of note, blood–brain barrier damage may have allowed liposome leakage into the CNS to also deplete microglia, whose influence on remyelination cannot be discounted. Interestingly, monocytes isolated from people with MS show reduced capacity for phagocytosis of myelin debris in vitro (Healy et al., 2017; Natrajan et al., 2015b). Likewise, aged mice show deficits in myelin debris clearance following demyelination (Ruckh et al., 2012). Ex vivo live imaging of microglia/MDMs in lesioned spinal cord indicated that this reflects both reduced macrophage numbers and phagocytosis of myelin debris (Rawji et al., 2018). Interestingly, human monocytes isolated from aged individuals show similar deficits in phagocytosis in vitro (Natrajan de la Fuente et al., 2015a), suggesting that monocytes in MS may have a prematurely aged phenotype. Although aged mice can remyelinate following demyelination, the process occurs at a much slower pace (Shields et al., 1999) and is nonetheless incomplete compared to young mice (Gingele et al., 2020). Due to these similarities between MS and aging, aged mice provide a useful model for understanding the obstacles to efficient repair in MS (Gingele et al., 2020).
4 THERAPEUTIC TARGETING OF MONOCYTES
4.1 Targeting dysregulated pathways in monocytes
Monocytes are an attractive therapeutic target for neurological disease, as exposure to drugs in the peripheral blood eliminates the necessity for blood–brain barrier permeability, and monitoring of monocyte responses to drugs is facilitated by easy access. Although no drug currently exists which is aimed to specifically target monocytes for remyelination, recent studies have identified pathways and drugs of interest.
One strategy to target monocytes in disease is to influence their inflammatory profile. Recent RNA sequencing of peripheral blood mononuclear cells (PBMCs) in primary progressive MS, which comprises of not only monocytes but also lymphocytes and dendritic cells, indicated a pro-inflammatory signature. This included higher expression levels of genes whose protein products are components of the pro-inflammatory complex the inflammasome (IL-1 β and NLRP3; Malhotra et al., 2020). The inflammasome is part of the innate immune system that reacts in response to foreign molecules, initiating release of pro-inflammatory cytokines such as IL-1 β, and can promote pyroptotic cell death. Dysregulation of the NLRP3 inflammasome has been implicated in a number of neurological conditions including perinatal brain injury (Holloway et al., 2021), AD (Heneka et al., 2013), stroke (Ren et al., 2018) and MS (Guo et al., 2017). Malhotra and colleagues went on to confirm NLRP3 expression in IBA-1+ macrophages in MS lesions using immunohistochemistry (Malhotra et al., 2020), however whether both microglia and MDMs have inflammasome activation remains to be determined. This study also found that inhibiting the inflammasome pathway in the EAE model using the NLRP3 inhibitor MCC950 reduced disease severity (Malhotra et al., 2020), suggesting a potential therapeutic avenue for MS. Although increased expression of IL-1β by PBMCs isolate from people with primary progressive MS (PPMS) PBMCs, treatment with the IL-1R inhibitor Anakinra did not reduce EAE disease severity (Malhotra et al., 2020). One possibility which may account for this result is that CCR2+ monocytes require IL-1β signaling to migrate into the CNS (Paré et al., 2018), and IL-1R inhibition may have therefore prevented the presence of regenerative infiltrating monocytes in lesions. In addition, another drug previously explored for treatment of MS, Laquinimod, acts to reduce secretion of IL-1β by monocytes via NF-kB signaling (Engel et al., 2021), and has shown limited efficacy in clinical trials (Giovannoni et al., 2020; Thöne & Linker, 2016). It is therefore conceivable that inflammasome-targeting drugs may compromise adequate influx of classical monocytes to aid in remyelination – a postulate which warrants further investigation.
4.2 Stimulating phagocytosis in monocytes
A second strategy to target monocytes in disease is to stimulate their phagocytosis, as monocytes isolated from individuals with MS have impaired myelin uptake in vitro (Healy et al., 2017; Natrajan Komori et al., 2015b). Both mRNA and protein analysis showed a distinct downregulation of the tyrosine kinase receptor, MerTK, in MDMs derived from MS monocytes, and that the deficit could be reversed with exposure to transforming growth factor β (TGFβ) in vitro (Healy et al., 2017). Another study performed microarray analysis of total human monocytes (CD14+) and showed that poor myelin phagocytosis is associated with downregulation of the retinoid X receptor (RXR) pathway in both aging and in MS (Natrajan de la Fuente et al., 2015a). This included a number of genes associated with the RXR pathway including RXRα and RXRβ receptor subunits and a receptor binding partner, peroxisome proliferator-activated receptor (PPAR) (Natrajan de la Fuente et al., 2015a). Myelin phagocytosis was equally poor in young human monocytes following inhibition of RXR signaling with an antagonist, whereas this was rescued in aged monocytes by stimulation of the RXR pathway with the agonist 9-cis retinoic acid (9cRA). Furthermore, knockout of RXRα in myeloid cells in young mice impaired myelin debris phagocytosis and remyelination (Natrajan de la Fuente et al., 2015a). These studies highlight the potential of therapeutically targeting the RXR pathway for remyelination. Accordingly, the RXR agonist Bexarotene, normally used to treat skin cancer, improved myelin debris phagocytosis by MS monocytes in vitro (Natrajan de la Fuente et al., 2015a). Although a phase 2 clinical trial in MS suggested that Bexarotene improved remyelination, adverse side effects halted further development (Brown et al., 2021). An alternative strategy to stimulate this pathway is via PPARγ targeting. Monocytes in RRMS have reduced PPARγ expression (Wouters et al., 2020), and exposing MS monocytes to the PPARγ activator Pioglitazone, currently used to treat diabetes, increased monocyte phagocytic function (Natrajan Komori et al., 2015b). Interestingly, the reduced PPARγ levels in MS monocytes are associated with higher levels of intracellular cholesterol, typical of inflammation-associated foamy macrophages (Wouters et al., 2020), suggesting monocytes in MS have altered lipid and inflammatory profiles. Pioglitazone was also shown to switch MS monocyte gene expression from pro-inflammatory (related to CD14 pathway activation) to immune-regulatory (related to IL-10, IL-4, and transforming growth factor beta (TGFβ) signaling) (Natrajan et al., 2015b). These findings suggest that targeting the RXR pathway in monocytes may improve remyelination via promoting phagocytosis and regulating inflammation.
4.3 Impact of DMTs on monocytes
In addition to these prospective therapies, approved disease modifying therapies (DMTs) can also impact monocytes (Carlström et al., 2019; Chuluundorj et al., 2017; Dalla Costa et al., 2018; Dallari et al., 2015; Fingerle-Rowson et al., 1998; Haschka et al., 2020; Waschbisch et al., 2016). For example, dimethyl fumarate stimulates monocytes to produce reactive oxygen species (ROS) (Carlström et al., 2019), whereas both glatiramer acetate and the glucocorticoid methylprednisolone promote monocyte expression of the anti-inflammatory cytokine IL-10 (Fischer et al., 2019; Spadaro et al., 2017). One study found that DMTs can differentially affect the production of monocyte-derived cytokines, with increased production of IL-6 by classical monocytes in RRMS patients treated with dimethyl fumarate treatment versus those treated with fingolimod, glatiramer acetate, natalizumab, or IFN-β-1a (Fiedler et al., 2017); IL-6 has roles in both demyelination and remyelination (Makinodan et al., 2016; Petković & Castellano, 2016), yet the impact of these drugs on the regenerative function of monocytes remains to be resolved. In addition, DMTs can also affect the number of microvesicles (MVs) secreted by monocytes (Blonda et al., 2017), which enable cell–cell interaction (Sáenz-Cuesta et al., 2014; Verderio et al., 2012). Furthermore, an impact of DMTs on monocyte chemotaxis has been documented. Whereas methylprednisolone stimulates monocyte chemotaxis, glucocorticoid treatment does not alter CCR2 expression by monocytes (Fischer et al., 2019). The sphingosine receptor modulator fingolimod (FTY720, Gilenya) causes downregulation of genes associated with monocyte trafficking and activation (Sferruzza et al., 2021).
These studies suggest the potential of monocytes as therapeutic targets, yet point to the need to investigate the impact of prospective and current therapies on the regenerative roles of monocytes in remyelination.
5 PERSPECTIVES AND FUTURE DIRECTIONS
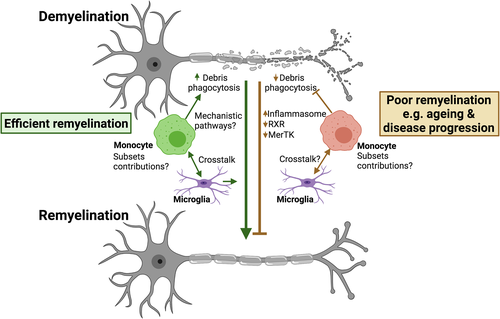
Monocytes are increasingly being implicated in CNS health and neurodegenerative disease, in part due to recent developments in tools and technologies to profile these cells and distinguish them from tissue resident macrophages. With monocytes clearly showing changes in aging and disease, we now need to fully understand how monocytes contribute to remyelination and the monocyte subsets involved (summarized in Figure 1).
Although therapeutically targeting monocytes is a promising regenerative strategy, an important issue to consider would be potential off-target effects on other immune cells in the circulation. One approach to overcome this could be isolation of monocytes from peripheral blood for ex vivo treatment before transplantation back into the circulation. Indeed, isolated monocytes loaded with biodegradable drug-containing microparticles have been administered back into the blood stream and found to migrate to injury sites where they adopted an anti-inflammatory macrophage profile (Wofford et al., 2020). An alternative strategy is the delivery of non-toxic nanoparticles of a specific molecular weight and charge. To enhance monocyte-specific delivery of drugs, Saito and colleagues discovered monocytes can show specificity to internalize nanoparticles made of high molecular weight poly(lactide-co-glycolide) (PLG-H) in vitro. Following intravenous injection into EAE mice, PLG-H-nanoparticles were internalized by classical monocytes and improved clinical outcome (Saito et al., 2019). However, the exact mechanism of action by which internalized PLG-H nanoparticles ameliorate the clinical symptoms of EAE is not yet fully understood. Although these approaches are promising, due to the nature of monocyte plasticity and their short survival within the blood stream, alternative methods may need to be developed for sustained therapeutic effect.
Targeting monocytes in aging and disease may also be achieved through diet. A high fat diet promotes pro-inflammatory transcriptomic changes in monocytes (Christ et al., 2018), whereas fasting reduces the proportion of pro-inflammatory monocytes in the blood (Jordan et al., 2019) and enhances experimental remyelination (Choi et al., 2016; Neumann et al., 2019). Recently, Vitamin B3 (Niacin) has been shown to reverse phagocytic deficits in demyelinated aged mice, stimulating increased myelin uptake by microglia and MDMs and promoting remyelination (Rawji et al., 2020); whether monocytes are directly targeted remains to be determined. In addition, as neurodegenerative diseases like MS and AD are more prevalent in females than in males, it is important to understand how sex differences in monocytes may impact disease progression, response to therapies, and remyelination. Indeed, sex hormones modulate monocyte numbers and function (Bouman et al., 2005), and monocytes display sex differences in autoimmune and neurodegenerative conditions (Carlisle et al., 2021; Jiang & Gilkeson, 2014). Therefore, in any future development of monocyte-specific therapies, it may be important to account for the potential impact of diet and sex on monocytes.
In summary, monocytes present an attractive therapeutic target to promote remyelination in neurological disease (summarized in Figure 2). Efficient monocyte targeting for remyelination requires further understanding of the roles of monocyte subsets in remyelination and the specific pathways involved, and how disruption of these functions could be altered with aging and disease thereby contributing to remyelination failure.

ACKNOWLEDGMENTS
This works was supported by a project grant from the United Kingdom Multiple Sclerosis Society and a Senior Non-Clinical Fellowship from the Medical Research Council to Veronique E. Miron.
CONFLICT OF INTEREST
Veronique E. Miron has received consultancy or research funds from Novartis, Biogen, GlaxoSmithKline, and ReWind Therapeutics.
AUTHOR CONTRIBUTIONS
L.H.F. wrote the article and made the figures, and V.E.M. consulted on article content and edited the article.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed.