Glutathione peroxidase 1 activity dictates the sensitivity of glioblastoma cells to oxidative stress
Abstract
The high intratumoral and intertumoral heterogeneity of glioblastoma (GBM) leads to resistance to different therapies, and hence, selecting an effective therapy is very challenging. We hypothesized that the antioxidant enzyme status is a significant feature of GBM heterogeneity. The most important reactive oxygen/nitrogen species (ROS/RNS) detoxification mechanisms include superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx). Expression and activity of these enzymes and the cellular response to induced oxidative stress were systematically analyzed and compared between GBM cells and nontransformed glial cells of both human and murine origin. Regardless of cell type or species, all tested cells expressed similar amount of catalase and MnSOD. All except one, GBM cell lines exhibited a deficiency in GPx1 expression and activity. Analysis of GBM tissue sections indicated a heterogeneous profile of weak to moderate expression of GPx1 in tumor cells. GPx1 deficiency led to an accumulation of ROS/RNS and subsequent death of GBM cells after induction of oxidative stress. Astrocytes, microglia/macrophages, and glioma stem cell lines expressed active GPx1 and resisted ROS/RNS-mediated cell death. Pharmacological inhibition or siRNA silencing of GPx1 partially reverted this resistance in astrocytes, indicating the contribution of various antioxidant molecules besides GPx1. The GPx1-expressing GBM cell line on the contrary, became extremely sensitive to oxidative stress after GPx1 inhibition. Altogether, these results highlight GPx1 as a crucial element over other antioxidant enzymes for oxidative stress regulation in GBM cells. Mapping the antioxidant enzyme status of GBM may prove to be a useful tool for personalized ROS/RNS inducing therapies. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Antioxidant enzymes provide cell homeostasis by regulating the redox state. These enzymes regulate the balance between production and/or accumulation of reactive oxygen/nitrogen species (ROS/RNS) and their neutralization. In absence of these enzymes, free radicals can cause damage to DNA, proteins, and lipids (Halliwell and Gutteridge,1985; Sies,1991). Under physiological conditions, this redox balance is maintained by various enzymes including superoxide dismutases (SODs), catalase, and glutathione peroxidases (GPx). The main function of SOD is to catalyze the conversion of superoxide to hydrogen peroxide and oxygen (Abreu and Cabelli,2010; Oberley and Buettner,1979). Hydrogen peroxide is a highly reactive oxidative species and must be degraded in order to prevent oxidative damage (Halliwell,1992). This is primarily accomplished by the enzymes catalase and GPx.
With a high oxygen consumption and hence a high oxidative metabolism, the brain is an organ that needs efficient redox-maintaining mechanisms. Studies with cultured cells or knockout mice have demonstrated the important role played by GPx in protecting cells and organisms against oxidative stress (Brigelius-Flohe and Kipp,2009; Dringen et al.,2000). Both microglia, the resident macrophages of the brain, and astrocytes are well-equipped for ROS detoxification, and can remove organic hydroperoxides such as tert-butyl hydroperoxide (TBHP) and cumene hydroperoxide that removal requires GPx activity (Dringen et al.,2000; Kussmaul et al.,1999). GPx enzymes exist in different isoforms and can be tissue-specific. GPx1 is the most abundant enzyme of the GPx family, and this cytosolic enzyme is present in all cells (Brigelius-Flohe,1999). The relative contribution of GPx1 and catalase to peroxide removal is not yet fully elucidated. In the heart, under in vivo mimicking conditions, detoxification of H2O2 seemed to occur mainly via GPx1 (Antunes et al.,2002; Simmons and Jamall,1989). However, cooperativity between these two enzymes has also been reported to be necessary for hydrogen peroxide removal in oligodendrocytes (Baud et al.,2004). Besides protection from peroxides, a role for GPx in peroxynitrite removal has also been observed (Sies et al.,1997). Peroxynitrite is a strong oxidant, and even though it is short-lived, it can result in high oxidative damage (Szabo et al.,2007).
Oxidative damage induction is frequently tested as a potential strategy for tumor treatment. One of the most challenging tumors to treat is glioblastoma (GBM). This highly invasive and aggressive type of glioma exhibits a high degree of cellular heterogeneity (Bonavia et al.,2011). Various molecules used in in vitro or in clinical studies have been reported to induce or increase oxidative stress in glioma cells through the inhibition of redox-maintaining mechanisms or through the increase of ROS/RNS production (El Sayed et al.,2012; Sharma et al.,2007; Smith et al.,2007). In contrast, overexpression of redox enzymes is often observed in different tumors and may support tumor cells' resistance to treatments involving an oxidative stress increase. Some of the examples are overexpression of GPx1 in MCF-7 cell line, peroxiredoxin and thioredoxin in breast cancer cells, and MnSOD in cervical cancer cells (Doroshow,1995; Nakano et al.,1996; Woolston et al.,2011b). Similarly, overexpression of antioxidant enzymes in glioma cells can contribute to their resistance to some chemotherapeutic agents and radiotherapy (Zhong et al.,1999). For instance, overexpression of SOD, catalase, GPx, and glutathione reductase (GR) in radioresistant clone of U251 glioma cell line leads to their resistance to cisplatin and radiotherapy (Lee et al.,2004).
We previously observed in the murine model that normal astrocytes and spontaneous murine astrocytoma (SMA-560) cells kept in culture differed in their response to oxidative stress induced by a combination of proinflammatory molecules composed of tumor necrosis factor alpha (TNF-α), lipopolysaccharide (LPS), and interferon-gamma (IFN-γ) [TLI]. Whereas treatment with TLI induced ROS/RNS production and death of astrocytoma cells within 2–3 days, astrocytes were preserved from death and displayed very low or undetectable level of ROS/RNS (Mora et al.,2010). These discrepancies between the capacity of astrocytes and tumor cells to respond to this oxidative stress suggested differences in the detoxification potential of these cells, which may have a therapeutic relevance. A better characterization of these differences would help in designing therapies with increased cellular specificity or in predicting patients' response to certain therapies. We hypothesized that GBM cell lines' specific sensitivity to induced oxidative stress is due to a lack or dysfunction of at least one of the main antioxidant enzymes. We therefore undertook the analysis of the antioxidant defense mechanisms in murine and human normal and transformed glial cells.
Abbreviations
-
- AO
-
acridine orange
-
- CM- H2DCFDA
-
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
-
- FeTPPs
-
5,10,15,20-tetrakis(4-sulfonatophenyl) prophyrinato iron (III)
-
- FFPE
-
formalin-fixed paraffin-embedded
-
- GBM
-
glioblastoma
-
- GPx
-
glutathione peroxidase
-
- GR
-
glutathione reductase
-
- GSC
-
glioma stem cells
-
- GSH
-
reduced glutathione
-
- GSSG
-
oxidized glutathione
-
- GST
-
glutathione S-transferase
-
- HPRT
-
hypoxanthine phosphoribosyltransferase
-
- IDH
-
isocitrate dehydrogenase
-
- IFN-γ
-
interferon gamma
-
- LPS
-
lipopolysaccharide
-
- MSA
-
mercaptosuccinic acid
-
- NHA
-
normal human astrocytes
-
- NO
-
nitric oxide
-
- PMSF
-
phenylmethylsulfonyl fluoride
-
- SOD
-
superoxide dismutase
-
- TBHP
-
tert-butylhydroperoxide
-
- TLI
-
TNF-alpha/lipopolysaccharide/interferon gamma
-
- TNF-α
-
tumor necrosis factor alpha
-
- TRAIL
-
TNF-related apoptosis-inducing ligand
MATERIALS AND METHODS
Cell Lines and Culture Media
Maintenance in culture
Normal human astrocytes (NHA) of fetal cortical origin (ScienCell) were grown in 2% fetal calf serum (FCS)-containing astrocyte growth medium (ScienCell). Human primary (NCH82, 149, 210) and secondary (NCH199) GBM cell lines were used at low passages (between 20 and 50) and grown in complete growth medium (cDMEM), consisting of Dulbeccos's Modified Eagle Medium (DMEM; Sigma) supplemented with 10% heat-inactivated FCS (PAA), 2 mM glutamine (Invitrogen), and 50 μg mL−1 Gentamycin (Invitrogen; Karcher et al.,2006). NCH441, NCH601, NCH620, NCH627, NCH644 glioma stem cell lines (Campos et al.,2010; Herold-Mende, unpublished results) were generated at the Department of Neurosurgery (Heidelberg Medical University, Germany). Stem cells were grown in stem cell medium made of DMEM complemented with BIT [bovine serum albumin, insulin, transferrin (ProVitro)], serum-free supplement (ProVitro) containing basic fibroblast and epidermal growth factors (20 ng mL−1 each; ProVitro), 100 U mL−1 penicillin G (ProVitro), and 100 μg mL−1 streptomycin (Gibco). Human tumor-infiltrating microglia/macrophages (TIM) were isolated from the brain tumor tissues as described (Kees et al.,2012). Informed consent was obtained from patients according to the research proposals approved by the Institutional Review Board at Heidelberg Medical University. After isolation, TIM were cultured in cDMEM supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF; 50 U mL−1; ProVitro) for 1 week before subsequent culture in cDMEM for 1–2 weeks. Murine astrocytes (mAst), prepared from VM/Dk new-born mice brains (Burudi et al.,1999), and the murine astrocytoma cell line SMA-560 were grown in cDMEM. All cells were incubated at 37°C in an atmosphere of 5% CO2.
Experimental setups
To avoid variations in the selenium content of media, all cells except GBM stem cell lines, were cultured and treated in 5% FCS/DMEM (DMEM, 5% FCS, 2 mM Glutamin, 50 μg mL−1 Gentamycin) for 24–48 h prior to experiments and during experiments. Glioma stem cell neurospheres did not survive in the 5% FCS/DMEM and therefore had to be cultured and treated in DMEM complemented with BIT, growth factors, and antibiotics, as described above. Seeding densities of the cells for different time points are presented in Table 1.
| Cell type | Seeding density (cells/mL) for analysis after: | |||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| NCH82 | 105 | 105 | 5 × 104 | 3 × 104 |
| NCH149 | 2 × 105 | 2 × 105 | 105 | 105 |
| NCH199 | 105 | 105 | 5 × 104 | 3 × 104 |
| NCH210 | 2 × 105 | 2 × 105 | 105 | 105 |
| NCH441 | 2 × 105 | 105 | N/D | N/D |
| NCH601 | 2 × 105 | 105 | N/D | N/D |
| NCH620 | 2 × 105 | 105 | N/D | N/D |
| NCH627 | 2 × 105 | 105 | N/D | N/D |
| NCH644 | 2 × 105 | 105 | N/D | N/D |
| NHA | 2 × 105 | 2 × 105 | 105 | 105 |
| TIM | 2 × 105 | 2 × 105 | 105 | 105 |
| mAst | 2 × 105 | 2 × 105 | 105 | 105 |
| SMA-560 | 2 × 105 | 105 | 5 × 104 | 2.5 × 104 |
- Seeding in 96-well plate, 24-well plate, 12-well plate, and 10-cm dish was done with 100 μL, 600 μL, 1 mL, and 6 mL of cell suspension, respectively.
- N/D: not done.
Reactive Oxygen/Nitrogen Species Measurement
The fluorescent probe CM-H2DCFDA (Invitrogen) was used to detect ROS/RNS. CM-H2DCFDA is reported to primarily detect hydrogen peroxide, but it also reacts with other reactive species like superoxide, nitric oxide, hydroxyl radical, and peroxynitrite (Hempel et al.,1999; Matsumoto et al.,2006; Shanker et al.,2004). NCH82, NCH149, NCH199, NCH210 GBM cell lines and human astrocytes were seeded in 12-well plates and loaded with 5 μM CM-H2DCFDA in PBS for 30 min at 37°C. After the incubation time, cells were washed three times with PBS and cultured in 5% FCS/DMEM or treated with TLI (recombinant human TNF-α 20 ng mL−1, Sigma; LPS 0.1 μg mL−1, Sigma; recombinant human IFN-γ 33 ng mL−1, Peprotech; in 5% FCS/DMEM). Accumulation of intracellular ROS/RNS was measured after 24 and 48 h of the treatment. Adherent and floating cells were harvested and labeled with TO-PRO-3 iodide (1 μM; Invitrogen) and analyzed at the FACS Calibur flow cytometer (BD Biosciences). Intensity of CM-H2DCFDA in living, TO-PRO-3 negative cells, was measured on fluorescence-1 channel. Analyses were performed using the CellQuest software.
Cytotoxicity Assays
Assays were performed as previously described (Mora et al.,2010). Briefly, cells were seeded in 5% FCS DMEM in 96-well plates and treated 24 h later with 5% FCS/DMEM (control) or TLI for 72 and 96 h, in the presence or absence of FeTPPs (100 μM; Calbiochem). At the end of the treatment, cells were incubated for 1 h with 2.5 μg mL−1 of acridine orange (AO; Sigma). Supernatants and cells were harvested, centrifuged in V-shaped 96-well plates, and resuspended in FACS Sheath Solution with Surfactant (BD Biosciences). Analysis was performed at the FACSArray flow cytometer (BD Biosciences) at the yellow parameter (532 nm excitation, 564–606 nm emission) in order to detect positive AO staining. Living cells contain intact acidic vesicles that are stained by AO. Dead cells were therefore distinguished by their lack of AO labeling. They were quantified as AO-negative cells using the FACSArray internal analysis software and expressed as percentage of total cells in each sample.
Alternatively, cells were seeded in 12-well plates. They were treated with 25-μM TBHP (Sigma) for 24 h, or with TLI for 96 h. Adherent and floating cells were harvested and labeled with TO-PRO-3 iodide (1 μM) and analyzed at the FACS Calibur flow cytometer. TO-PRO-3 iodide positive and negative cells (dead and living cells, respectively) were quantified and analyzed using the CellQuest software.
Protein Expression Analysis
Cells were seeded on 10-cm dishes in adequate growth medium for 24 h and afterward treated with 5% FCS/DMEM (control) and TLI for 24 h. TLI was formulated with recombinant human cytokines when used for treating human cells, and with recombinant murine TNF-α (Peprotech) and IFN-γ (Peprotech) when used for treating murine cells. Cells were harvested with a cell scraper and lysed in lysis buffer [10 mM Tris-HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 10 mM NaF, 1 mM, Vanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF; Roche), 1× protease inhibitor cocktail complete™ (Roche)]. Protein concentration in the lysates was determined using the BCA (bicinchoninic acid) protein assay reagent (Pierce). Proteins (60 μg per sample) were separated by electrophoresis in 12% SDS-PAGE gels (murine proteins) and in 4–12% Bis-Tris NuPage gels (Invitrogen; human proteins) under reducing conditions and transferred to nitrocellulose membrane (Schleicher and Schuell) by semidry transfer. After blocking for 1 h at room temperature in PBS containing 5% milk and 0.1% Tween-20 (PMT), membranes were incubated with primary antibodies against human and murine MnSOD (1:250; Santa Cruz), catalase (1:2,000; Abcam), and 14-3-3 (1:300; Santa Cruz) for 1 h at room temperature in PMT or with the rabbit polyclonal antibody against human and murine glutathione peroxidase 1 (GPx1; 1 μg μL−1; Abcam) over night at 4°C in PBS containing 0.1% Tween-20. Antibody against human and mouse thioredoxinreductase 2 (1:500; Abcam) was incubated overnight at 4°C in PBS containing 5% milk and 0.1% Tween-20. Following incubation with appropriate secondary peroxidase-conjugated antibodies (Santa Cruz), labeled proteins were detected using the enhanced chemiluminescence immunodetection kit (GE Healthcare). Membranes were exposed to Amersham Hyperfilm-MP for various periods of time.
Films were scanned after development using a Scan Maker X12 USL device.
Semiquantitative densitometry for Western blot was performed using the gel analyzer tool in ImageJ software developed by the National Institute of Health (http://rsb.info.nih.gov/ij/). The area under curve (AUC) of the specific signal was corrected for the AUC of the loading control. The value for the “NHA + TLI” condition served as initial value and was set to 1. Fold change was calculated for each other condition to allow ratio comparisons.
Immunohistochemistry
Sections from formalin-fixed paraffin-embedded (FFPE) tissue of the NCH82 and NCH149 primary GBM were obtained from the Tissue Bank of the National Centre for Tumor-Related Illnesses (NCT) in Heidelberg. CD68 immunohistochemistry was performed under standard conditions using a Ventana BenchMark XT automatic immunostainer (Ventana Medical Systems). Staining for GPx1 was performed as follows. Tumor sections were first deparaffinized and subjected to thermal antigen retrieval (7 min, 400 W in microwave) in 10 mM citric acid pH 6 (Antigen retrieval solution; Dako). The sections were treated with 5% hydrogen peroxide for 10 min, washed in PBS, and blocked 20 min in normal 2.5% horse serum (ImmPRESS™ Detection Systems, Vectorlabs). The sections were incubated for 30 min at 37°C with the rabbit polyclonal antibody against human GPx1 (1:2,000; AbCam) in antibody diluent (Dako), or with antibody diluent without primary antibody (control). Primary antibody was visualized by ImmPRESS Anti-Rabbit Ig (peroxidase) secondary antibody (ImmPRESS Detection Systems, Vectorlabs) and ImmPACT diaminobenzidin (DAB) peroxidase substrate (ImmPACT DAB substrate kit, Vectorlabs). Sections were stained in Mayer's Hematoxylin Solution (Sigma) for 1 min and washed with running tap water for 15 min. Sections were then labeled in Eosin Y solution (Sigma) for 2 min and extensively washed with water. Sections were examined and photographed at a 20× magnification using Zeiss Axioplan microscope and AxioCamICc3 Rev.3 (O) camera. Images were analyzed by AxioVisionLE software. GBM tumor sections were semiquantified by analyzing DAB staining with the ImmunoRatio plugin for ImageJ software (Tuominen et al.,2010). The ImageJ ImmunoRatio plugin measured the DAB (brown) and nuclei hematoxylin (blue) stains with an image resolution of 4.4 pixels μm−1. The imaged areas (n = 4) within tumor sections were randomly chosen and analyzed with the same light filter settings for each section. Areas with large blood vessels and necrotic regions were excluded from the quantification analysis.
Enzymatic Activities
Catalase, GPx, and GR specific enzymatic activity was determined using the Catalase Fluorometric Detection Kit (Enzolifesciences), GPx Assay Kit (Cayman), and GR Activity Assay Kit (BioVision) respectively, according to the manufacturers' instructions.
Briefly, cell lysates were prepared as described above (see section “Protein expression analysis”) and 100 μg of proteins per sample were subjected to enzymatic activity assays.
The Catalase Fluorometric Detection Kit is an assay that utilizes a nonfluorescent detection reagent to measure hydrogen peroxide substrate left over from the catalase reaction. Left over of hydrogen peroxide reacts with the nonfluorescent detection reagent and with peroxidase leading to the production of fluorescent resofurin, which fluorescence can be measured at Ex/Em = 570/590 nm using Ascent Fluoroskan FL (Thermo Scientific). Catalase supplied by the manufacturer was used as a standard.
GPx assay measures GPx activity indirectly by a coupled reaction with GR. The assay involves two enzymatic steps. First, GPx reduces hydroperoxide whereby oxidized glutathione (GSSG) is being produced. In the second step, GSSG is recycled to its reduced state by GR and NADPH. NADPH oxidizes to NADP+, as observed by a decreased absorbance at 340 nm that is directly proportional to the GPx activity in the sample. Cellular GPx supplied by the manufacturer was used as a positive control. The rate of reduction in absorption of NADPH at 340 nm was measured at FluoStar Omega multimode microplate reader (BMG-Labtech), and the amount of NADPH consumed was calculated using the extinction coefficient for NADPH (0.00622 in μM−1 cm−1).
GR Activity Assay Kit (BioVision): in this assay, GR reduces GSSG with the help of NADPH to GSH (reduced glutathione). GSH reacts with 2-nitrobenzoic acid to generate 5-thio-2-nitrobenzoic acid (yellow color product) that is measured at 405 nm on Ascent Multiskan (Thermo Scientific).
Reduced and Oxidized Glutathione Measurement
Total and oxidized glutathione were measured in 20,000 cells per sample by the GSH/GSSG-Glo Assay (Promega). Briefly, cells were seeded in 96-well plates (white, opaque; Nunc) and left in culture for 24 h. At the time of the assay, cells were either counted or lysed directly in the wells with the lysis buffer provided with the kit. The assay is based on the conversion of a luciferin derivative to luciferin through a reaction catalyzed by glutathione S-transferase (GST). The signal generated in a coupled reaction with firefly luciferase is proportional to the amount of total glutathione present in the sample. Oxidized glutathione is measured in the reaction, where a reagent is added that blocks all the GSH while leaving the GSSG intact. This blocking step is followed by a reducing step that converts the GSSG to GSH for quantification in the luminescent reaction scheme. The amount of reduced GSH was obtained by subtracting oxidized GSSG from total glutathione. Luminescence was measured using the microplate reader Ascent Fluoroskan FL (Thermo Scientific).
RNA Isolation and RT-PCR
RNA was extracted from the cells cultured in 75-cm2 flask at 80% confluency using the RNAeasy kit (Qiagen). RNA concentrations were measured by NanoDrop Spectrophotometer ND-100 (PeQLAB Biotechnologie GmbH). RNA (150 ng) per sample was used for performing the RT-PCR using QuantiTectVirusKit 2nd (Qiagen). Forward and backward primers for full length GPx1 were: AAGGTACTACTTATCGAGAATGTG and GTCAGGCTCGATGTCAATGGTCTG, respectively (Li et al.,2000). Primers for ubiquitine and HPRT were used as controls. Ubiquitine forward and backward primers (GGGTGTG-GCACAGCTAGTTCCG and CATTGTCAAGTGACGATC- ACAG, respectively), were kindly provided by G. Halec, DKFZ. Primers for HPRT and GPx1 were purchased from Sigma. Eighteen microliters of the cDNA product were loaded in 2.5% agarose gels. cDNA bands were visualized by SybrSafe (Invitrogen) and pictures were taken using ImageQuant100 (GE Healthcare) and Olympus photo camera. Semiquantitative densitometry of cDNA bands was performed using the gel analyzer tool in ImageJ software similarly as described for Western blot semiquantitative analyses. The AUC of the specific signal was corrected for the AUC of the RT-PCR control. The value for the “TIM” GPx1 cDNA expression band served as an initial value and was set to 1. Fold change was calculated for each other condition to allow ratio comparisons.
Inhibition of Proteasomal and Lysosomal Degradation
MG-132 (Sigma) is a reversible inhibitor of proteasome (Lee and Goldberg,1998). NCH82 cells were treated with different concentrations of MG-132 in nM: 0, 50, 70, 100, 150. After 24 h of treatment, cell lysates were prepared and analyzed for GPx1 expression by Western blot.
As an inhibitor of lysosomal degradation, we used NH4Cl dissolved in cell culture medium (DMEM with 5% FCS; Koh et al.,2005; Tanaka et al.,1986). NCH82 cells were treated for 24 h with different concentrations of NH4Cl in culture medium. After the treatment, cell lysates were prepared and analyzed for GPx activity, using the GPx Assay Kit (Cayman).
Knockdown and Inhibition of GPx1 Activity
Inhibition by siRNAs
Human astrocytes were transfected for 48 h with 25 nM siRNA against GPx1 (siRNA-Premix; Qiagen) and scrambled siRNA (siRNA-Premix; Qiagen) as a negative control. After the first 24 h of transfection, 25 μM TBHP was added on the cells and incubation continued with siRNA and TBHP for the next 24 h. Adherent and floating cells were harvested and labeled with TO-PRO-3 iodide (1 μM) and analyzed at the FACS Calibur flow cytometer. TO-PRO-3 iodide positive (dead) and negative (living) cells were quantified and analyzed using the CellQuest software. For determination of specificity and activity of siRNAs, cell lysates were prepared from nontransfected cells and cells transfected with 25 nM siRNA against GPx1 and scrambled siRNA for 48 h. These lysates were tested for GPx activity, using the GPx Assay Kit (Cayman).
Pharmacological inhibition
The human GBM NCH149 cells and murine astrocytes were resistant to the transfection with siRNA. Therefore, these cells were treated with mercaptosuccinic acid (MSA; Sigma), a pharmacological inhibitor of GPx1 activity (Chaudiere et al.,1984), at efficient, nontoxic concentrations that were determined in preliminary experiments. NCH149 cells were cultured in medium containing 2.5 mM MSA with or without 25 μM TBHP for 24 h. Adherent and floating cells were harvested, labeled with TO-PRO-3-iodide (1 μM), and the number of dead cells analyzed at the FACS Calibur flow cytometer as described above. Murine astrocytes were treated with 10 mM mercaptosuccinic acid in medium with or without TLI for 96 h. After AO labeling for 1 h, cell death was assessed by FACSarray as described above. GPx activities of NCH149 cells and murine astrocytes were measured in untreated and MSA-treated samples in order to assess the efficiency of MSA treatment on GPx1 inhibition.
Statistical Analysis
Data are presented as means and standard deviations. Statistical significance was determined using the Student's t-test or ANOVA where appropriate, using GraphPad Prism software. The asterisks represent significantly different values: * P < 0.05; ** P < 0.01; *** P < 0.005.
RESULTS
Response of Human GBM Cell Lines to Induced Oxidative Stress
We have recently demonstrated that murine astrocytoma cell lines, but not murine astrocytes, responded to a combination of TNF-α, LPS, and IFN-γ (TLI) by an increased ROS/RNS production that led to tumor cell death (Mora et al.,2010).
Here, we analyzed whether TLI treatment can similarly induce oxidative stress in human GBM cell lines and normal human astrocytes (NHA). Moreover, we tested their sensitivity to induced oxidative stress. Both human GBM cell lines and human astrocytes were treated with TLI for up to 96 h. Treatment for 24 and 48 h led to accumulation of ROS/RNS in human GBM cell lines and, to a lower extent, in human astrocytes (Fig. 1A). ROS/RNS could not be measured at later time points, since TLI-treated glioma cells were dying after 72 and 96 h (Fig. 1B,C). Remarkably, human astrocytes, similar to their murine counterparts, were not affected by this treatment (Fig. 1C).
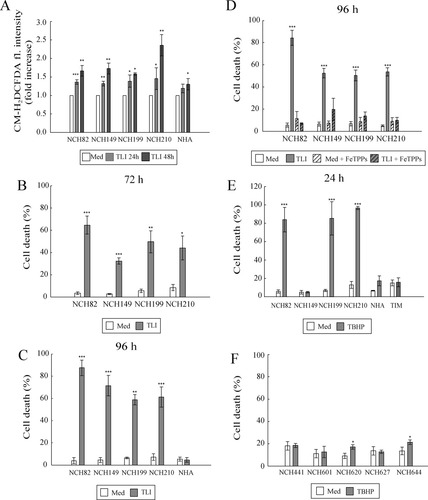
Cell response to induced oxidative stress. (A) Accumulation of ROS in human GBM (NCH) cell lines and normal human astrocytes (NHA). Data are expressed as fold increase in mean fluorescence intensity over medium condition. For each cell line, treatment condition (TLI for 24 or 48 h) was compared with the corresponding medium condition that was normalized to 1 (Med). Significance was calculated using paired Student's t-test. (B–D) FACSArray-based cytotoxicity assay. Cell death is expressed as percentage of the dead cells number in untreated (Med) and treated conditions. (B, C) significance (Med vs. TLI condition) for each cell line was calculated using unpaired Student's t-test. (D) ANOVA was performed and P-values for pairwise comparisons were adjusted by Bonferroni. (E, F) Cell cytotoxicity assay based on measurement of TO-PRO-3 iodide positive cells was performed to determine the percentage of dead cells in untreated (Med) and treated (TBHP) conditions. Significance (Med vs. TBHP condition) for each cell line was calculated using unpaired Student's t-test. Data presented in A–F are means of at least three independent experiments and their standard deviations.
To determine whether the increased ROS/RNS production/accumulation was responsible for GBM cell death, we tested the effects of the peroxynitrite scavenger, FeTPPs, on TLI-induced cell death. Cytotoxicity assays performed after 96 h of treatment showed that FeTPPs protected human GBM cells from death (Fig. 1D). Thus, in both murine (Mora et al.,2010) and human (this study) models, TLI induced ROS/RNS-mediated cell death specifically in glioma cells.
The high level of protection achieved by FeTPPs (between 60 and 90%) strongly suggests that TLI-induced oxidative stress increase was the main cause of death. However, we could not exclude that perturbations in other biological processes, such as the sphingolipid metabolism as observed in the murine model (Mora et al.,2010), would also be induced by TLI. We therefore further tested the sensitivity of human GBM cell lines and astrocytes to the organic peroxide, TBHP, as a genuine inducer of oxidative stress. As shown in Fig. 1E, the cell lines NCH82, NCH199, and NCH210 were strongly sensitive to TBHP treatment that induced up to 90% cell death after 24 h of treatment. On the contrary, astrocytes and the NCH149 cell line were resistant to TBHP treatment and did not undergo cell death. Human TIM were resistant to TBHP treatment. This latter was expected since microglia/macrophages are known for their strong antioxidative defense mechanisms (Dringen,2005).
Given the likely presence of cancer stem cells in GBM, we also tested glioma stem cell lines (GSC; NCH441, NCH601, NCH620, NCH627, NCH644) for their sensitivity to TBHP treatment. Contrary to the GBM cell lines, none of the tested glioma stem cell lines underwent cell death upon treatment with TBHP for 24 h (Fig. 1F) or upon TLI treatment (data not shown).
Redox Enzymes Expression in Human and Murine Cells
The differences in the sensitivity to oxidative stress observed within the panel of cells we analyzed, led us to postulate differences in their redox-maintaining mechanisms. We therefore tested the expression of catalase, MnSOD, and GPx1, which are the most important enzymes that regulate superoxide and hydrogen peroxide levels in cells.
As shown in Fig. 2A, catalase was constitutively expressed in all tested human GBM cell lines and in the human astrocytes. CuZnSOD was hardly detectable in any tested cells (data not shown), whereas constitutive levels of MnSOD varied from very low to detectable levels within the GBM cells and the astrocytes. Three of the four human GBM cell lines (NCH82, NCH199, NCH210) showed a significantly lower GPx1 expression than human astrocytes (Fig. 2A, see quantification in Fig. 2B). On the contrary, the GBM NCH149 cell line and the glioma stem cell lines we tested, displayed levels of GPx1 expression comparable to that of human astrocytes (Fig. 2C). TIM expressed high amounts of catalase, MnSOD, and especially GPx1 (Fig. 2A). We observed no discrepancy between murine astrocytoma cell line SMA-560 and murine astrocytes in their expression of catalase and MnSOD. We could not detect GPx1 expression in SMA-560 cells, whereas murine astrocytes prominently expressed it (Fig. 2D). Treatment with TLI for 24 h (Fig. 2A,D) or for 48 h (data not shown) did not affect the basal levels of catalase, GPx1 nor did it induce that of CuZnSOD (data not shown), whereas it upregulated that of MnSOD in both human and murine cells, as expected from the reported abilities of TNF-α, LPS, and IFN-γ to regulate MnSOD expression in different cell types (Harris et al.,1991; Tsan et al.,2001; Wong and Goeddel,1988).
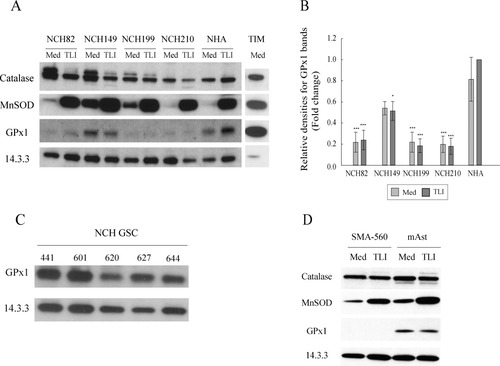
Redox enzymes expression in human and mouse cells. (A) Protein extracts prepared from human cells left untreated (Med) or treated with TLI (TLI) for 24 h were analyzed by Western blot. Levels of 14.3.3 expression were used as a protein loading control. Data from one representative experiment are shown for glioma and astrocytes (n = 4) and for TIM (n = 3). (B) Quantification of GPx1 protein expression by semiquantitative densitometry. Protein level is expressed as relative density fold increase compared with the condition NHA + TLI (set to 1). GPx1 expression levels for each cell line and condition (Med or TLI) were compared with NHA (Med) condition using ANOVA post hoc comparison with Dunnett adjustment. (C, D) GPx1 expression in human glioma stem cell lines (NCH GSC), murine astrocytoma cells (SMA-560), and murine astrocytes (mAst) was determined by Western blot as described in (A). Data are from one representative experiment (C: n ≥ 2; D: n = 4).
Evaluation of GPx1 Expression in Tumor Tissues
To evaluate the profile of GPx1 expression in vivo, we performed immunohistochemical analysis on FFPE sections of the primary GBM tissues from which the NCH82 and NCH149 cell lines are derived. In Fig. 3, we present two representative regions of NCH82 (A) and NCH149 (B) tissue sections. Adjacent sections were labeled against GPx1 (upper panel) or CD68 (lower panel) under the same conditions. Control sections are shown in Supp. Info. Fig. 1. Numerous CD68-positive cells, that is, TIM, were present in these sections. They were identified on the basis of their morphology in the GPx1-labeled sections (arrow heads) and showed the strongest GPx1 staining. This level of expression was in agreement with the level of GPx1 expression detected by Western blot and expressed in vitro by TIM isolated from primary GBM (see Fig. 2A). Reactive astrocytes, identified as strongly GFAP-positive cells with a typical stellate morphology (data not shown), were difficult to detect in the GPx1-stained sections. Contrary to astrocytes, tumor cells were easily identified by their morphology (arrows in Fig. 3) and presented a heterogeneous intensity of labeling for GPx1. In panel A (NCH82 tumor), most of the GBM cells displayed an undetectable signal for GPx1. In panel B (NCH149 tumor), tumor cells were clearly GPx1 positive.
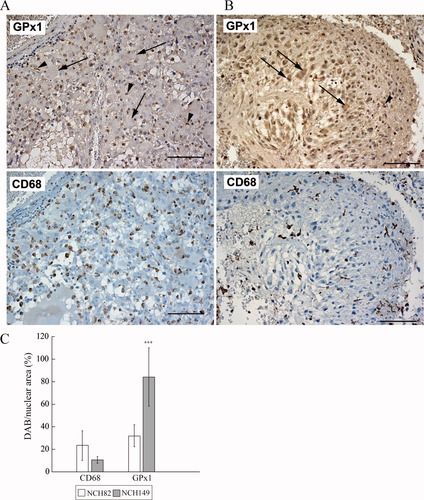
Evaluation of GPx1 expression in tumor tissues. Panels A (NCH82 tumor) and B (NCH149 tumor) display two adjacent different tumor sections stained for GPx1 (upper panel) or CD68 (lower panel). Cells were identified by their morphology in the upper panel; arrow = GBM cells; arrow head = TIM. Size of the scale bars = 100 μm. (C) Quantification of the number of CD68 and GPx1 positive cells present in the sections using ImmunoRatio application. Data are presented as the mean of the percentage of values and standard deviation of DAB-positive cells. GPx1 expression between NCH82 and NCH149 cells was compared using unpaired Student's t-test; n = 4.
A quantitative analysis of these images (see description in Supp. Info. Fig. 2) was performed according to Tuominen et al. (2010). Using the ImmunoRatio application software, the percentage (number of DAB-positive cells/nucleus area) of GPx1-positive cells and of CD68-positive cells was determined. Whereas the amount of CD68-positive cells did not grossly differ between the two tissues, the amount of GPx1-positive cells was significantly higher in the NCH149 tumor section (Fig. 3C). We could also observe, but not quantitate variations in the level of GPx1 signal intensity/cell.
In summary, the heterogeneous levels of GPx1 expression observed both in vitro and in vivo strongly suggest that cellular variations in levels of GPx1 expression were genuine, and not related to culture conditions.
Redox Enzymes Activity in Human and Murine Cells
We next investigated the enzymatic activities of catalase and GPx in human and murine cells. All the tested tumor cell lines and astrocytes had very similar levels of basal catalase activity in both human and murine models (Fig. 4A, medium). Assays for GPx activity (Fig. 4B) showed that the human GBM cell lines NCH82, NCH199, and NCH210 displayed lower basal GPx activity levels than human astrocytes and the NCH149 cell line. The GSC lines and astrocytes displayed similar levels of GPx1 that were 2- to 3-fold lower than that of human TIM. Murine astrocytes displayed a high level of GPx activity, whereas SMA-560 cells did not show any significant GPx activity. This latter was tested with an assay that measures the activity of any GPx and hence would detect activity of GPx1 and GPx4, the two GPx expressed in brain. We could not detect GPx4 expression by Western blot analysis in either murine or human cells (not shown). We therefore are confident that the activity we measured is that of GPx1. Moreover, levels of GPx activity correlated with GPx1 protein expression patterns. Treatment with TLI for 24 h (Fig. 4A,B, TLI) or for 48 h (data not shown) did not affect the patterns of the basal enzymatic activity of GPx1 and catalase observed in tumor cell lines and astrocytes, in the human or the murine model.
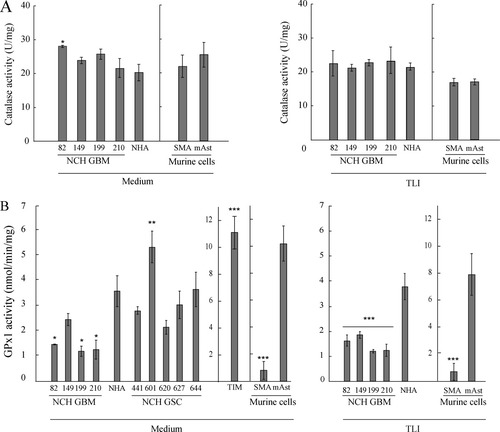
Catalase and glutathione peroxidase activities in human and murine cells. (A) Catalase activity in untreated (Medium) and 24 h TLI-treated (TLI) cells. Data are presented as mean of specific activity (U mg−1) and standard deviation (n = 3). Human cells: ANOVA with post hoc comparison to NHA with Dunnett adjustment was performed and showed no significant differences for all GBM cell lines, except NCH82. Murine cells: unpaired Student's t-test was used for comparison of catalase activities in SMA-560 (SMA) and astrocytes and showed no significant differences. (B) GPx activity in untreated (Medium) and 24 h TLI-treated (TLI) cells. Data are presented as mean of specific activities (nmol min−1 mg−1) and standard deviations. Astrocytes and cell lines: n = 3 independent experiments; TIM: one representative experiment is shown (n = 2 independent experiments, each performed in triplicates). Human cells: ANOVA with post hoc comparison to NHA with Dunnett adjustment was performed. Murine cells: unpaired Student's t-test was used for comparison of GPx activities in SMA-560 and astrocytes.
Regulation Mechanisms of GPx1 Expression and Activity
To gain insights in the possible mechanisms involved in the regulation of GPx1 expression, we first analyzed GPx1 mRNA expression in the different cell types. As shown in Fig. 5A,B, RT-PCR analysis indicates that human astrocytes and GBM cell lines, as well as TIM, expressed similar levels of GPx1 mRNA. GSC cell lines displayed various levels of mRNA expression that corresponded to that of protein expression (Fig. 2C) and enzymatic activity (Fig. 4B).
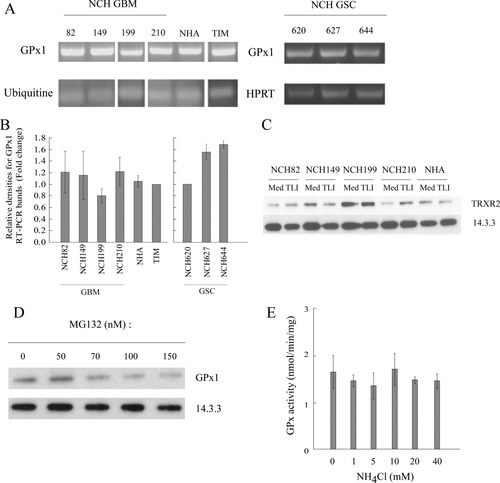
Regulation mechanisms of GPx1 expression. (A) Determination of GPx1 mRNA expression by RT-PCR in GBM cells and in glioma stem cells (GSC). Primers for ubiquitine and HPRT were used as mRNA expression control. Data from one representative experiment are shown. (B) GPx1 cDNA bands were quantified by semiquantitative densitometry. Data for GBM and NHA cells (left; n = 3) and GSC cells (right; n = 2) are expressed as relative density fold change compared with GPx1 cDNA expression by TIM and by NCH620, respectively. Means and standard deviations are shown. Nonstem cells: ANOVA with post hoc comparison to TIM (control sample, set to 1) with Dunnett adjustment was performed and showed no significant differences. (C) Expression of TRXR2 was assessed by Western blot in untreated (Med) or 24 h TLI-treated (TLI). Levels of 14.3.3 expression were used as a protein loading control (n = 1). (D) NCH82 cells were treated for 24 h with various concentrations of the inhibitor of proteasome degradation (MG132) and GPx1 expression was assayed. One representative experiment is shown (n = 2). (E) NCH82 cells were treated for 24 h with various concentrations of the inhibitor of lysosomal degradation (NH4Cl) and GPx1 activity was measured. Data are presented as mean and standard deviations (n = 3). ANOVA with post hoc comparison to untreated cells with Dunnett adjustment was performed and showed no significant differences.
GPx1 is a selenoprotein that contains a selenocystein (Sec) in its sequence and requires the presence of selenium for its normal expression and functioning. Variation in GPx1 expression hence may result from variation in the amount of selenium present in the culture medium and available to the cell. The cell lines and primary cells we used were kept in medium containing various percentage (2% for NHA; 10% for GBM, TIM, mAst, SMA-560) of FCS, the source of selenium in vitro. To ensure the same conditions in selenium content in the experimental setup, all cells were kept for 24–48 h before experiment in 5% FCS DMEM (see Materials and Methods), except glioma stem cells that could not survive in this medium. Selenium supplementation through cultivation of GBM and NHA cells in DMEM containing 30 nM sodium selenite for 10 days prior and during the TBHP treatment did not affect cells' sensitivity to oxidative stress (data not shown). Since a deficiency in selenium uptake would affect the synthesis of other selenoproteins, the expression of another selenoprotein, thioredoxin reductase 2 (TRXR2), was tested. As shown in Fig. 5C, human GBM cell lines and NHA all expressed TRXR2, suggesting that selenium uptake in GBM cell lines was not impaired.
We next tested the possibility that GPx1 is faster degraded in GBM cells than in astrocytes. For that purpose, we treated NCH82 cells with proteasomal and lysosomal degradation inhibitors (MG132 and NH4Cl, respectively) at different concentrations for 24 h. The use of inhibitors of degradation pathways would allow the accumulation of GPx1, and would lead to an increase of GPx1 activity in NCH82 GBM cell line. As shown in Fig. 5D,E, the inhibition of degradation pathways did not increase the GPx1 expression and activity.
Glutathione Reductase Activity and GSH Status in Human GBM Cell Lines and Human Astrocytes
The positive correlation observed between the high sensitivity of GBM cells to organic peroxide (Fig. 1E) and the deficiency in GPx1 expression (Fig. 2A) and activity (Fig. 4B) strongly suggested that GPx1 was the main player for an efficient peroxide reduction in GBM cells. However, (Liddell et al.,2006) have reported that murine GPx1−/− astrocytes were resistant to the organic peroxide (cumene hydroperoxide), but died after depletion of GSH. This indicates a strong dependency on a fully intact GSH system in astrocytes. We therefore investigated the status of the GSH system in the GBM cell lines and in astrocytes. A functional GR is essential as it allows the swift reduction of the oxidized GSSG, formed upon reduction of peroxides by GPx, into the reduced GSH that serves as an electron donor to GPx1 (Lubos et al.,2011). Measurement of GR enzymatic activity showed that all GBM cell lines possessed an active GR (Fig. 6A). GR activity was similar in GBM and astrocytes, with the exception of the NCH149 and NCH210 cell lines that showed respectively a higher and a lower activity when compared with that of astrocytes. As shown in Fig. 6B, GSH was present in astrocytes and each of the GBM cell line. Measurement of GSSG indicated its accumulation in the GBM cell lines, but not in astrocytes (Fig. 6C). As a result, the GSH/GSSG ratio, an indicator of the cellular health, was significantly higher in astrocytes (Fig. 6D). This was expected, because tumor cells are known to exist in a pro-oxidant state when compared with normal, nontransformed cells (Halliwell,2007). There were no significant differences in the GSH/GSSG ratios among the four GBM cell lines, suggesting that the deficiency in GPx1 expression did not impair the net redox status of the cells.
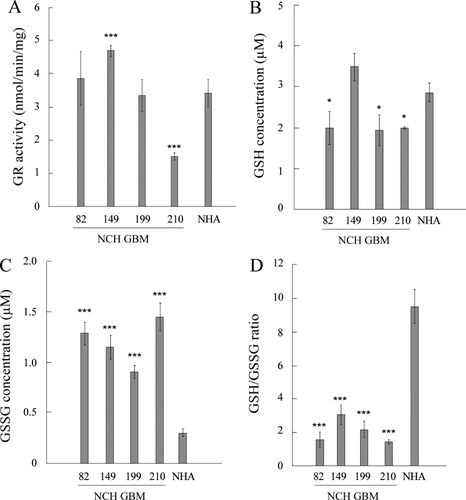
GR activity and GSH status in GBM cells and NHA. (A) Glutathione reductase (GR) activity in human GBM cell lines and NHA (n = 3). ANOVA with post hoc comparison to NHA with Dunnett adjustment was performed. (B–D) Reduced glutathione (GSH), oxidized glutathione (GSSG), and their ratio in human GBM cell lines and NHA. Glutathione content (GSH or GSSG) represents the amount in 20,000 cells per sample. Data are shown as means and standard deviations (n = 3). ANOVA with post hoc comparison to NHA with Dunnett adjustment was performed.
Response of Astrocytes and NCH149 Cells to Induced Oxidative Stress After GPx1 Inhibition
To further assess the relation between the level of GPx1 expression and activity (i.e., its detoxification efficacy) and the degree of sensitivity to the induced oxidative stress, we inhibited GPx1 expression in astrocytes and in NCH149 GBM cells, and analyzed cell death rate upon treatment with the organic hydroperoxide TBHP or with TLI. For that purpose, knockdown experiments were conducted in human astrocytes. NCH149 cells and murine astrocytes were resistant to siRNA transfection and were treated with mercaptosuccinic acid, a specific inhibitor of GPx (Chaudiere et al.,1984).
Human astrocytes were transfected for 48 h with specific (GPx1 siRNA) and control (scrambled) siRNA (Ctrl siRNA) and treated with TBHP during the last 24 h. Transfection with GPx1-specific siRNA, but not with Ctrl siRNA, led to a significant decrease in GPx1 activity (Fig. 7A). This sensitized human astrocytes to TBHP treatment (Fig. 7B). Treatment of NCH149 cells with mercaptosuccinic acid significantly decreased GPx1 activity in these cells (Fig. 7C), which led to their sensitization to TBHP treatment and cell death (Fig. 7D). Similarly, treatment of murine astrocytes with MSA resulted in a loss of GPx1 activity (Fig. 7E) and, upon treatment with TLI, led to a concentration-dependent increase in cell death (Fig. 7F).
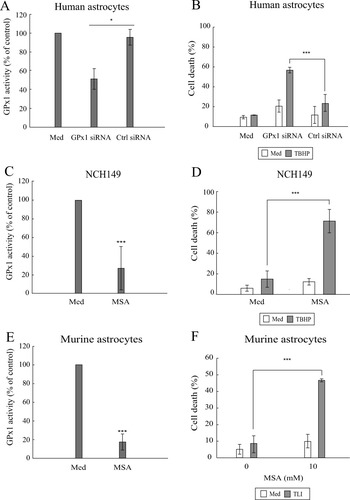
Sensitization of human and mouse cells to induced oxidative stress. (A) Specificity and efficacy of siRNA against GPx1 were assessed through GPx activity measurement in human astrocytes that were: nontransfected (Med); transfected with 25 nM siRNA against GPx1 (GPx1 siRNA); transfected with 25 nM control siRNA (Ctrl siRNA). GPx activities for transfected cells are expressed as percentage of activity of control (untreated) cells. Data are presented as mean and standard deviation (n = 3). Unpaired Student's t-test was performed for comparison between GPx1 siRNA and Ctrl siRNA conditions. (B) Human astrocytes transfected as described in (A) were left untreated (Med) or treated with 25 μM TBHP for 24 h. Cell cytotoxicity assay (TO-PRO-3 iodide labeling) was performed to determine the percentage of dead cells. (n = 3). Unpaired Student's t-test was performed for comparison between GPx1 siRNA and Ctrl siRNA conditions in TBHP-treated cells. (C, E) Efficacy of mercaptosuccinic acid (MSA) inhibition was assessed through GPx activity assay. Cells were left untreated or treated with 2.5 mM MSA for 24 h (NCH149) or with 10 mM MSA for 48 h (murine astrocytes). GPx activity is presented as percentage of activity of untreated (Med) cells. Data are presented as mean and standard deviation (n = 3). Unpaired Student's t-test was performed for comparison between untreated (Med, normalized to 100%) and MSA-treated conditions. (D) NCH149 cells were treated with MSA in absence or presence of TBHP for 24 h. Cell cytotoxicity assay (TO-PRO-3 iodide labeling) was performed to determine the percentage of dead cells (n = 3). Unpaired Student's t-test was performed for comparison between (Med + TBHP) and (MSA + TBHP) conditions. (F) Murine astrocytes were treated with MSA in absence or presence of TLI for 96 h. FACSArray-based cytotoxicity assay was performed. Cell death is expressed as mean of the percentage of the dead cells number in untreated and treated conditions and standard deviations (n ≥ 3). Unpaired Student's t-test was performed for comparison between TLI and (MSA + TLI) conditions.
DISCUSSION
GPx1, a Determinant Enzyme for Redox Homeostasis in Brain-Transformed Cells
Given the complexity of the redox regulation, therapeutic strategies that aim at altering the redox status of transformed cells may lead to adverse and unwanted effects. Moreover, alterations of the redox status in transformed cells may differ from cell to cell as a consequence of the frequent cellular heterogeneity of tumors. In this study, most of the glial cells present in a brain tumor or in its surrounding, that is, tumor and tumor stem cells, normal astrocytes, and microglia/macrophages, were analyzed for their redox status, with a focus on their antioxidant enzymes. Among these enzymes, GPxs, and particularly GPx1, play an important role in the catabolism of hydrogen peroxide (Lubos et al.,2011) and detoxification of peroxynitrite (Sies et al.,1997). The effective detoxification of peroxides by GPx1 in astrocytes requires an intact GSH/GSSG system that maintains GSH, the electron donor in the reaction catalyzed by GPx, in excess of GSSG (Hirrlinger and Dringen,2010). This GSH/GSSG ratio is furthermore determined by the NADPH-consuming enzyme, GR. A correlation between low level of total GPx and GR activities and high level of protein oxidation suggestive of oxidative damage has been recently reported in samples of human glioma tissues (Tanriverdi et al.,2007).
Remarkably, we observed that the presence of an active GPx1 is an essential element that determines the sensitivity to oxidative stress of a panel of human and murine brain cells. A decreased or lack of detectable expression of GPx1 correlated with a high rate of cell death induced by ROS/RNS despite the presence of catalase activity in the cells, indicating their dependency on GPx1 for ROS/RNS detoxification. The essential role of GPx1 in the antioxidant response of the GBM cell lines was supported by the following observations. Irrespective of their level of GPx1 expression/activity, the GBM cells we tested appear to possess a functional GSH system (as measured by GR activity, GSH, and GSSG levels) and a functional catalase. The presence of intracellular GSH in these GBM cells did not protect the GPx1-deficient cells from organic peroxide-induced cell death, contrary to what has been reported for GPx1-deficient murine astrocytes (Liddell et al.,2006). Finally, GPx1 inhibition in the GPx1-positive NCH149 cells and in astrocytes promoted almost complete reversion (more than 80% dead cells) of their resistance to TBHP-mediated cell death. It is worth to note that this prominent loss in resistance to TBHP occurred in cells in which inhibition of GPx1 activity was not complete (∼ 50% of activity). Moreover, catalase and other antioxidant molecules might have contributed to a protective effect and compensate a loss of GPx1 activity (Haapasalo et al.,2003). Our results suggest that it was not the case. This observation hence reinforces the determinant role of GPx1 in the GBM cells for ROS/RNS detoxification. On the contrary, nontransformed cells, such as astrocytes and microglia/macrophages, as well as glioma stem cell lines, comparatively displayed a higher GPx1 expression and activity than GBM cells. They accordingly survived an increase in oxidative stress. Considering astrocytes and microglia, such a survival was expected, given their strong potential for an anti-oxidant defense (Dringen,2005; Wilson,1997). Inhibition of GPx1 activity in murine or human astrocytes, similar to GPx1-positive GBM cells, reversed their resistance to TBHP-mediated cell death. The rate of cell death was however lower (∼ 50% dead cells) than that observed for GBM cells. This suggests that in these cells, contrary to GBM cells, antioxidant molecules such as GSH might have contributed to the removal of organic peroxides. Considering glioma stem cells, their redox mechanisms still are largely unknown and this precludes any hasty conclusion about the role of GPx1 over other antioxidant molecules in these cells. The cell lines used in our study were resistant to siRNA transfection, and treatment with MSA was toxic per se. Their resistance to oxidative stress however indicates that their antioxidant potential differs from that of the GBM cell lines. These differences may rely not only on their capacity to express antioxidant enzymes but also on metabolic differences, namely glycolysis and oxidative phosphorylation, as recently reported (Vlashi et al.,2011).
Altogether, this comparative analysis of the redox enzymes in GBM cell lines and primary glial cells, brings evidence that further strengthens a crucial role for GPx1 in the maintenance of the redox status, both in murine and human brain cells. More importantly, it reveals a strong and striking dependency of GBM cells on GPx1 to resist oxidative stress.
Regulation of GPx1 Expression in the GBM Cells
Analysis and comparison of the levels of GPx1 expression/activity in nontransformed (astrocytes), glioma stem, and GBM cells indicates alteration in the regulation of this enzyme. The observed loss of GPx1 expression might accompany differentiation from glioma stem cells to GBM cells or occur in GBM cells at given stages of carcinogenesis (Brigelius-Flohe and Kipp,2009). To understand the mechanisms underlying this loss, we first checked the levels of GPx1 mRNA present in these cells. All the cells we tested expressed GPx1 mRNA at a similar level. This indicates that loss of GPx1 expression in the GBM cells we tested did not result from loss of the gene, as observed in some types of cancer (Hu et al.,2005) or from alterations in the transcription machinery. It is worth noting here that approaches conducted at gene expression level for the search of specific alterations of therapeutic interest would have missed GPx1 and its differential expression in tumor versus nontransformed cells in the brain. GPx1 is a selenoprotein that contains a selenocystein (Sec) in its sequence. Sec incorporation during translational process is a unique, complex mechanism (Driscoll and Copeland,2003; Lubos et al.,2011). The presence of available selenium in culture medium has been reported to impact on the stability of selenoproteins' mRNA (Driscoll and Copeland,2003). Our preliminary evidence however indicates that differences in GPx1 expression cannot be attributed (i) to variations in selenium content, (ii) to an impaired selenium uptake by GBM cells nor can it be attributed to an increased degradation of GPx1 within these cells.
With regards to protein degradation, we made the intriguing observation that treatment of the GPx1-deficient NCH82 cells with the proteasome inhibitor MG132 led a drastic decreased level of expression of GPx-1 after 24 h of treatment with increased doses of MG132. Since none of these doses were cytotoxic, we hypothesize that treatment with the inhibitor might have affected the rate of GPx1 synthesis. In support of this hypothesis, data reported in two recent articles indicate that treatment of liver cells with a protease inhibitor led to a reduction of SREBP1c mRNA expression (Oliva et al.,2012), a transcription factor that was shown by (Kallin et al.,2007) to induce the expression of GPx3 mRNA in liver cells. Whether the family of SREBP transcription factors is indeed involved in controlling GPx1 mRNA expression has still to be proven.
Based on our observations, we favor the hypothesis that regulation of GPx1 expression in the GBM cells we tested takes place at a specific translational or post-transcriptional step. The mechanisms that control Sec incorporation in the nascent protein might be impaired in GBM cells. Post-translational modifications of GPx1 might occur and affect its structure and/or its enzymatic activity. A deeper analysis of these mechanisms shall provide us with a better understanding of the role of GPx1 not only in normal but also in tumor stem and transformed cells.
GPx1, Tumor Heterogeneity, and GBM Therapy Perspectives
Among the four human GBM cell lines tested, one cell line (NCH149) showed GPx1 expression and activity that was similar to that of the nontransformed astrocytes and of glioma stem cell lines. This level of GPx1 activity protected NCH149 cells as well as astrocytes and glioma stem cells from an oxidative stress induced by organic peroxide. It did not protect NCH149 cells however from cell death induced by TLI, contrary to astrocytes or to glioma stem cells. The TLI combination of proinflammatory compounds must have targeted pathways that are altered in GBM cells, but not in glioma stem cells and not in astrocytes. Identification of these pathways may be of therapeutic relevance.
This singular profile of GPx1 expression among the tested GBM cell lines might have indicated that variations in GPx1 expression either reflected heterogeneity among the four different tumor tissues from which the NCH cell lines were derived, or resulted from culture artifacts. Analysis of GPx1 expression in tumor cells from GBM tissues showed intercellular variation that reflected variation in GPx1 expression in cultured glioma cells. We observed a clear regional heterogeneity in the level of signal intensity, with tumor areas lacking GPx1 staining and other areas showing a weak to moderate staining. Expression of the GPx1 protein therefore appears to be a marker of the intertissue and intratissue heterogeneity of GBM. As such, it could be a very valuable marker for defining the success of a therapy. Variability in GPx1 expression could not be correlated with other known genetic alterations or drug resistance profiles of the GBM cells we studied. For instance, the GPx1-positive NCH149 cells and the GPx1-deficient NCH82 cells derive from primary GBM tumors (Karcher et al.,2006) that express the wild type isocitrate dehydrogenase (IDH) 1 and IDH 2, whereas secondary GBM tumors frequently express mutated forms of IDH 1 and 2 (Hartmann et al.,2009). These enzymes play an important role in the redox status of a cell, since they reduce NADPH that is mandatory for the regeneration of GSH through the action of the GR. The tumor suppressor genes PTEN and INK4 are deleted or altered in both GPx1-positive NCH149 cells and GPx1-deficient NCH82 cells (Di Piazza et al.,2007). These two cell lines display a similar profile of resistance to the death ligand TRAIL or sensitivity to cisplatin (Di Piazza et al.,2007). This similar sensitivity to cisplatin, a drug known to be detoxified by GST, suggests that the differences observed in GPx1 expression and activity do not impact on GST activity. In addition, it has been shown for glioma cell lines and tumors that their sensitivity/resistance to chemotherapy does not depend on GST levels (Winter et al.,2000).
As already pointed out, the heterogeneous nature of GBM impedes therapy efficiency (Bonavia et al.,2011). With regards to oxidative stress enzymes, heterogeneous expression of redox proteins has been correlated to the clinical outcome in breast cancer patients undergoing radiotherapy (Woolston et al.,2011a). Here, we show that the tumor cell's potential for oxidative stress defense, specifically GPx1 expression and activity, is one of GBM biological features responsible for their heterogeneity in response to an increase in oxidative stress. According to our observations, the average level of GPx1 expression/activity within the tumor mass would be contributed not only by tumor cells but also tumor stem cells and microglia/macrophages. Tumor stem cells are characterized by high proliferation capacity (Singh et al.,2004). This, together with a high GPx1 expression and activity may highly contribute to the tumor resistance to oxidative stress induction and leads to therapy failure. Our data therefore suggest that the overall level of GPx1 expression in the tumor should be taken into account and included as a relevant parameter to estimate the success of a therapy. For instance, conventional radiotherapy that induces free radical formation (Ross,1999) may be more efficient for patients presenting GBM with low GPx1 expression. This might also hold true for new therapies based on the induction of oxidative stress in GBM, such as that reported by El Sayed et al. (2012), which is based on H2O2 generation by the D-amino acid oxidase enzyme.
In conclusion, we have shown in this study that GPx1 is a decisive antioxidant enzyme which its decreased expression in brain tumor cells leads to high sensitivity to oxidative stress. The intratissue and intertissue heterogeneity of expression confers a therapeutic value to GPx1 in treatment planning strategies for personalized therapies, which deserves to be further investigated.
Acknowledgements
The authors are greatly indebted to Prof. Rommelaere for his continuous support, to Prof. Dr. Annette Kopp-Schneider (DKFZ, Heidelberg, Germany) for her expertise and help in statistical analyses, and to Dr. Tobias Dick (DKFZ, Heidelberg, Germany) for helpful discussion. Support of the DKFZ Light Microscopy Facility is gratefully acknowledged.