Dicer1 and MiR-9 are required for proper Notch1 signaling and the Bergmann glial phenotype in the developing mouse cerebellum
Abstract
MicroRNAs (miRNAs) have important roles in the development of the central nervous system (CNS). Several reports indicate that tissue development and cellular differentiation in the developing forebrain are disrupted in the absence of miRNAs. However, the functions of miRNAs during cerebellar development have not been systematically characterized. Here, we conditionally knocked out the Dicer1 gene under the control of the human glial fibrillary acidic protein (hGFAP) promoter to examine the effect of miRNAs in the developing cerebellum. We particularly focused on the phenotype of Bergmann glia (BG). The hGFAP-Cre activity was detected as early as embryonic day 13.5 (E13.5) at the rhombic lip (RL) in the cerebellar plate, and later in several postnatal cerebellar cell types, including BG. Dicer1 ablation induces a smaller and less developed cerebellum, accompanied by aberrant BG morphology. Notch1 signaling appears to be blocked in Dicer1-ablated BG, with reduced expression of the Notch1 target gene, brain lipid binding protein (BLBP). Using neuronal co-culture assays, we showed an intrinsic effect of Dicer1 on BG morphology and Notch1 target gene expression. We further identified miR-9 as being differentially expressed in BG and showed that miR-9 is a critical, but not the only, miRNA component of the Notch1 signaling pathway in cultured BG cells. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
MicroRNAs (miRNAs), a class of small noncoding RNAs, are increasingly recognized as major players in regulating gene expression post-transcriptionally. MiRNA genes are transcribed and cleaved sequentially by RNase enzymes Drosha/DGCR8 and Dicer to release the mature miRNAs (Lund et al., 2004; Zeng et al., 2005). In the RNA-induced silencing complex (RISC), the guide strand of the mature miRNA can bind to the 3′-untranslated region (3′-UTR) of a target mRNA through imperfect base pairing, which results in either target degradation or translation inhibition (Bartel, 2004; Cao et al., 2006; Stefani and Slack, 2008). Dicer is essential for producing mature miRNAs; therefore, Dicer knockout mice have been widely used as models to study miRNA functions (Bernstein et al., 2003).
Studies using conditional knockouts of Dicer1 through Cre-mediated recombination have demonstrated that miRNAs play crucial roles in different regions and cell types during CNS development. For example, Dicer1 inactivation in forebrain excitatory neurons causes microcephaly, reduced dendritic branch width, and large increases in dendritic spine length (Davis et al., 2008). A study using Emx-1-Cre mediated Dicer1 knockout revealed that late, but not early, neural progenitors in the mouse embryonic neocortex are sensitive to miRNA ablation, exhibiting increased apoptosis and a dramatic impairment of neuronal differentiation (De Pietri Tonelli et al., 2008). In the developing spinal cord, miRNAs are not only necessary and sufficient for the timely differentiation of myelinating oligodendrocytes (Dugas et al., 2010; Zhao et al., 2010), but also have an essential role in astrogliogenesis in the ventral-most white matter area (Zheng et al., 2010). Dicer1 function in the cerebellum has been examined in several reports. Conditional knockout of Dicer1 in the early embryonic mid- and hindbrain region affects its proper patterning and results in ablation of the entire cerebellum (Huang et al., 2010; Soukup et al., 2009). On the other hand, Dicer1 ablation in postnatal astroglia (Tao et al., 2011) and mature Purkinje neurons (Schaefer et al., 2007) leads to ataxic walking behavior and degeneration of cerebellar cell types. However, miRNA function in the developing cerebellum, especially its early postnatal development, has not been systemically explored.
Bergmann glia (BG), with unique radial morphology, are essential glial cells for a precisely laminated neuronal structure of postnatal cerebella. BG cell bodies are located in the Purkinje cell layer (PCL), and their radial cellular processes in the molecular layer (ML) can be marked by antibodies against brain lipid binding protein (BLBP), nestin, and glial fibrillary acidic protein (GFAP). One critical function of BG is to serve as scaffolds during early postnatal cerebellar development, facilitating inward migration of postmitotic granule neurons from the external granule layer (EGL) to the inner granule layer (IGL) (Sotelo, 2004; Wang and Zoghbi, 2001). Genetic studies have demonstrated important functions of certain genes (Albanito et al., 2011; Baubet et al., 2012; Frick et al., 2012; Lopez-Juarez et al., 2011; Piper et al., 2011; Wang et al., 2011; Yue et al., 2005) and signaling pathways, including the Notch1 pathway (Eiraku et al., 2005; Komine et al., 2007; Tohgo et al., 2006; Weller et al., 2006), in the development and maintenance of the phenotype of BG; however, the involvement of miRNAs in this process remains elusive.
In the present study, we take advantage of the hGFAP-Cre-mediated Dicer1 conditional knockout to examine the effects of miRNAs in the developing cerebellum, with a focus on BG. We found that hGFAP-Cre-mediated Cre recombination occurs in multiple cerebellar cell types, including BG in the early postnatal cerebellum. Furthermore, we provide the first evidence that miRNAs contribute to the maintenance of BG morphology, at least in part, through intrinsically interacting with Notch1 signaling pathway. MiR-9 is differentially expressed in BG in the developing cerebellum and may be an important effecter of the Notch1 signaling pathway in maintaining the phenotype of BG.
MATERIALS AND METHODS
Mouse Lines and Genotyping
The hGFAP-Cre heterozygous mice (Zhuo et al., 2001) were mated with a loxP flanked Dicer1 line (Murchison et al., 2005) to generate hGFAP-Cre;Dicer1f/+ mice, which were then mated with Dicer1f/f mice to generate hGFAP-Cre;Dicer1f/f. The hGFAP-Cre mice were crossed with Gt(ROSA)26Sor;LacZ reporter mice (Soriano, 1999) to examine hGFAP-Cre activity. Genotyping the mice carrying the various hGFAP-Cre, Dicer1f/f and LacZ alleles was performed by PCR, using primers as follows. Cre F: 5′-CACCCTGTTACGTATAGCCG-3′; Cre R: 5′-GAGTCATCCTTAGCGCCGTA-3′; β-actin F: 5′-CCTAGGCACCAGGGTGTGAT-3′; β-actin R: 5′-TCACGGTTGGCCTTAGGGTT-3′; hGFAP-Cre F: 5′-ACTCCTTCATAAAGCCCTCG-3′, hGFAP-Cre R: 5′-ATCACTCGTTGCATCGACCG-3′ (Zhuo et al., 2001); Dicer1 F: 5′-CCTGACAGTGACGGTCCAAAG-3′, Dicer1 R: 5′-CATGACTCTTCAACTCAAACT-3′ (Harfe etal., 2005); and LacZ 1: 5′-GTTATCAGTAAGGGAGCTGCAGTGG-3′, LacZ 2: 5′-AAGACCGCGAAGAGTTTGTCCTC-3′, LacZ 3: 5′-GGCGGATCACAAGCAATAATAACC-3′ (Soriano, 1999). The Institutional Animal Care and Use Committee of West China 2nd University Hospital, Developmental & Stem cell Institute, Sichuan University at Chengdu, People's Republic of China approved the use of the animals and the experiments.
RNA Extraction and Quantitative Reverse Transcriptase PCR (qRT-PCR)
Total RNA was isolated from tissue or cell culture samples using TRIzol® reagent, according to the manufacturer's instructions (Invitrogen). Total RNA (0.5 μg) was reverse-transcribed into cDNA using a PrimeScript® RT reagent kit (TaKaRa Biotechnology, Dalian, P.R. China). qRT-PCR analysis of gene expression was analyzed using a SYBR® Premix DimerEraser™ (TaKaRa Biotechnology, Dalian, P.R. China) on a BIO-RAD CFX96™ Real-time System (BIO-RAD). The relative gene expression was normalized to internal controls such as β-actin (Li et al., 2008b). The primer sequences of the target genes are shown in Table 1. MiRNA-specific primers were purchased from Guangzhou Ribobio Co., Ltd (Guangzhou, P.R. China), and the relative expression level of individual miRNAs was analyzed according to the manufacturer's instructions and normalized to that of U6 snRNA.
| Gene name | Forward | Reverse |
|---|---|---|
| β-Actin | CTTTGCAGCTCCTTCGTT | TTCTGACCCATTCCCACC |
| Dicer1 | ACCAGCGCTTAGAATTCCTGGGAG | GCTCAGAGTCCATTCCTTGC |
| GFAP | AACAACCTGGCTGCGTAT | CGAACTTCCTCCTCATAGA |
| BLBP | ACCCGAGTTCCTCCAGTT | TGCCACCTTCCTGACTGA |
| Notch1 | GGTGAACAATGTGGATGCTG | ACTTTGGCAGTCTCATAGCTG |
| Jag1 | CTGTCCCACTGGTTTCTCTG | GTTCTTGCCCTCATAGTCCTC |
| Jag2 | TCGTCATTCCCTTTCAGTTCG | GTCATTGTCCCAGTCCCAG |
| Dll1 | ATTCCCCTTCGGCTTCAC | CTTTCTGGGTTTTCTGTTGCG |
| Dll3 | ACTGGATGCCTTTTACCTGG | ACATCGAAGCCCGTAGAATC |
| Dll4 | CTTTACCGGCTCTAACTGTGAG | GGCTTGGACCTCTGTTCAG |
| Hes1 | GGTGGGCTAGGGACTTTAC | TACACCAGCAACAGTGGG |
| Onecut2 | GGAGGAGAAAGCCCAGTC | CAGGAGGAATCACCTACCAA |
In Vivo BrdU Pulse-Labeling
Pulse-labeling of dividing BG was carried out by intraperitoneal injection of bromodeoxyuridine (5-bromo-2′-deoxyuridine, BrdU) (Sigma) into postnatal day 6 (P6) mice at 100 mg/kg body weight. Mice were sacrificed 20 h after injection. BrdU staining was performed by incubating vibratome sections at 37°C in 2 M HCl for 30 min before standard immunohistochemistry.
β-Galactosidase (β-gal) Staining
Cerebella of different ages were fixed with phosphate buffered saline (PBS) containing 1% formaldehyde, 0.2% glutaraldehyde, 2 mM MgCl2, 5 mM EGTA, and 0.02% NP-40 for 24 h at 4°C. The tissues were then sagittally sectioned into 40-μm thick sections on a vibratome (Leica VT1000s). After washing the sections three times with PBS containing 0.02% NP-40, staining was performed at 37°C for 1 to 12 h in staining solution (1 mg/mL X-Gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 0.01% nadeoxycholate, 0.02% NP-40, and 2 mM MgCl2 in PBS) until the appropriate blue color developed.
Immunohistochemistry, Immunocytochemistry and Fluorescence Microscopy
Cerebella were collected after intracardiac perfusion with 4% paraformaldehyde (PFA). The tissues were then sagittally sectioned into 40-μm thick sections on a vibratome and postfixed in 4% PFA overnight at 4°C. Tissue sections or fixed cell cultures were incubated with monoclonal antibodies against β-III tubulin (TuJ1, mouse IgG, 1:500, Covance), nidogen (rat IgG, 1:1,000, Novas Biological), BrdU (rat IgG, 1:200, Accurate), reelin (mouse IgG, 1:200, a gift from Dr. Gabriella D'Arcangelo) and NeuN (mouse IgG, 1:400, Chemicon); polyclonal antibodies against β-gal (chicken IgY, 1:500, Abcam), BLBP (rabbit IgG, 1:400, Abcam), Zic1 (rabbit IgG, 1:2,000, Rockland Immunochemicals), parvalbumin (rabbit IgG, 1:800, ImmunoStar), GFAP (chicken IgY, 1:1,000, Aves or rabbit IgG, 1:200, DAKO), calbindin D-28K (rabbit IgG, 1:500, ImmunoStar), cleaved caspase-3 (rabbit IgG, 1:1,000, Cell Signaling), nestin (chicken IgY, 1:1,000, Aves), Notch1 (rabbit IgG, 1:200, Santa Cruz), and Ki67 (rabbit IgG, 1:500, Millipore), followed by appropriate species-specific secondary antibodies (Molecular Probes). DAPI (10 μg/mL, Sigma) was included in the secondary antibody incubations to label nuclei. The tissue sections or cultured cells were then mounted in mounting medium (Zhong Shan-Golden Bridge Biotech, P.R. China) and analyzed by conventional (Nikon Eclipse Ti) or confocal (Zeiss LSM 5 duo) fluorescence microscopy.
Western Blot Analysis
Cerebellar tissues or cultured cells were harvested in TRIzol® reagent, and protein portions were isolated according to the manufacturer's instructions (Invitrogen). Proteins were separated on Any kD™ Mini-PROTEAN® TGX™ precast polyacrylamide gels and transferred onto PVDF membranes. The membranes were then incubated with primary antibodies against BLBP (rabbit IgG, 1:100, Abcam), GFAP (chicken IgY, 1:5,000, Aves), nestin (chicken IgY, 1:5,000, Aves), and Notch1 (rabbit IgG, 1:1,000, Santa Cruz). The primary antibodies were detected by appropriate species-specific DyLight 700 or 800-conjugated secondary antibodies (1:10,000, Thermo Scientific) on a LI-COR Odyssey® Infrared Imaging System. Anti-GAPDH (mouse IgG, 1:1,000, Sigma) was used to normalize sample loadings.
BG Co-Cultures, DAPT Treatment, and miRNA Transfection
Immortalized neural progenitor clone GE6 was propagated in a serum-free medium containing FGF2, as previously described (Li et al., 2012). BG cells were purified from P3-5 cerebella by percoll-gradient centrifugation (Li et al., 2003) and cultured in DMEM medium containing 10% fetal bovine serum (FBS) for several passages. For N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) treatment, control BG cultures were preincubated with either dimethylsulfoxide (DMSO) or 50 μm DAPT (Sigma) for 2 h before GE6 cells were added and cultured for 2 days. For miRNA transfection, BG were transfected with miR-9 mimics or inhibitor (Guangzhou Ribobio Co., Ltd, P.R. China) using GenoFect™ transfection reagent (Geno Biotech, Beijing, P.R. China; available at: http://www.geno-bio.cn) according to the manufacturer's instructions and/or co-cultured with equal numbers of GE6 cells on poly-D-lysine (50 μg/mL, Sigma) coated substrates for 2 days at 37°C with 5% CO2. The cells were then either harvested for RNA extraction or fixed with 4% PFA and subjected to immunostaining.
Measurements and Statistical Analysis
For quantification of bipolar BG in culture, nestin+ cells with cell processes at least twice the length of their cell body diameters were counted, and the percentage was calculated among total nestin+ cells. All quantifications on sections were done with tissues from at least three animals, unless indicated otherwise. All data were presented as means ± standard deviation. Statistical analysis was performed in Microsoft Excel using Student's t-test.
RESULTS
hGFAP-Cre;Dicer1 f/f Mice Show Dicer1 Inactivation in the Cerebellum and Develop Ataxic Behavior
To determine the function of miRNAs in the developing cerebellum, we crossed the hGFAP-Cre mouse line with Dicer1f/f line to conditionally knock out the Dicer1 gene in the developing CNS. The fidelity of hGFAP-Cre has been questioned because it exhibits variable recombination activity (Requardt et al., 2009). Therefore, we were cautious in confirming the mutant mice both pre- and post-experimentally. The genotype of hGFAP-Cre;Dicer1f/f mice was confirmed by PCR with two pairs of Cre primers and these mice were referred to as mutants, whereas mice with other genotypes showed phenotypes similar to wild-type mice and were referred to as controls (Fig. 1A). Mutant mice survived and appeared normal at birth. They gained weight at a similar rate compared with their littermates until approximately 2 weeks after birth, when a weight loss was observed. At 3 weeks of age (weaning time), mutant mice had significantly smaller body sizes than controls (controls, 10.47 ± 0.82 g, n = 12; mutants, 6.32 ± 2.00 g, n = 6; P < 0.005) and exhibited smaller ears (Fig. 1B), ungroomed hair, clasping behavior in a tail-suspension test (Fig. 1C), and ataxic walking behavior (Supp. Info. Movie). In more severe cases, mutant mice could not even maintain their standing gesture. Mutants displayed whole body tremor behavior as early as P12. Approximately 80% of mutant mice died around weaning time, and those who survived started to gain weight and some of them lived up to 2 months of age. At P22, mutant mice had a smaller cerebellum as well as a smaller cerebral cortex (Fig. 1D). Coronal section staining showed a thinner cortical wall, larger lateral ventricles, and a diminished hippocampal structure in the forebrain of mutant mice compared with controls (Fig. 1E). These phenotypic defects in the cerebral cortex of the mutant are similar to what others have reported in Dicer1 conditional knockout mice using different cortex-specific promoters (Davis et al., 2008; De Pietri Tonelli et al., 2008; Kawase-Koga et al., 2009). To further confirm the inactivation of Dicer1 in mutant cerebella, we performed qRT-PCR analysis on postnatal cerebellar tissues and showed that Dicer1 expression was reduced in mutant tissues at the mRNA level (Fig. 1F). MiRNAs miR-9 and miR-124 are highly expressed in normal developing CNS, including the cerebellum (Hohjoh and Fukushima, 2007; Lagos-Quintana et al., 2002), and we found that their expression levels were also dramatically decreased, which further confirmed the functional inactivation of Dicer1 in the mutant mice (Fig. 1F).
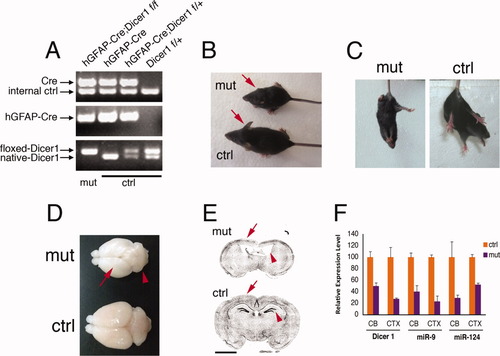
Phenotypic analysis of the hGFAP-Cre;Dicer1f/f mice. (A) To ensure the accuracy of mouse genotyping, two different Cre PCR primer pairs were used. Mice with the hGFAP-Cre;Dicer1f/f genotype were termed mutants (mut), while mice with other genotypes were termed controls (ctrl). Mutant mice have smaller body sizes and smaller ears (arrows) than controls at P21 (B), and exhibit the classic clasping behavior in a tail-suspension test (C), indicative of abnormal motor function. (D) The mutant cerebral cortex (arrow) and cerebellum (arrowhead) are also smaller in the mutants than in the controls. (E) Coronal sections of the mutant cerebral cortex show a thinner cortical wall (arrows), larger lateral ventricle, and diminished hippocampal structure (arrowheads) compared with the control, as revealed by inverted DAPI staining images. (F) qRT-PCR analysis showing a reduction of Dicer1 mRNA in the mutant cerebellum (CB) and the cerebral cortex (CTX) at P13 compared with the controls. The expression of two representative miRNAs, miR-9 and miR-124, was also reduced in mutant tissues, confirming the functional inactivation of Dicer1. Scale bars: 250 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
hGFAP-Cre-Mediated Recombination Starts at the Rhombic Lip (RL) and Occurs in Multiple Cell Types, Including BG, in the Developing Cerebellum
Although the activity of the hGFAP promoter is restricted to astrocytes in the adult brain, hGFAP-Cre expression starts as early as E13.5 in the dorsal forebrain and labels both astrocytes and neurons at later developmental stages in hGFAP-Cre;lacZ reporter mice (Zhuo et al., 2001). In the cerebella of these reporter mice, major cell types, such as BG and granule neurons, are positive for the lacZ signal, with the exception of Purkinje neurons, which are negative for lacZ, as has been previously reported (Zhuo et al., 2001). To identify the origin of mutant cerebellar defects, we analyzed hGFAP-Cre-mediated recombination in the hGFAP-Cre;LacZ reporter mice during cerebellar development. At E13.5, β-gal staining was first detected at the RL of the cerebellar plate (Fig. 2A). Consistent with the previous report (Zhuo et al., 2001), the dorsal forebrain of this reporter mouse also showed β-gal staining at this age (data not shown). At E14.5, the RL continued to be β-gal positive, as was the EGL, which is derived from the RL and is reelin-immunoreactive (Fig. 2B′, arrows). The nestin-positive ventricular zone (VZ) of the cerebellar plate showed little β-gal staining (Fig. 2B,B′, arrowheads). In the E17.5 cerebellum, the VZ acquired a β-gal signal, suggesting that Cre-mediated recombination has occurred in this region (Fig. 2C, arrowheads). β-gal was also observed in the cells that were scattered in the cerebellar parenchyma, which had presumably migrated from the VZ (Fig. 2C, arrows). The most intense β-gal staining in P3 cerebella was in the EGL (Fig. 2D), indicating that granule neuron precursors, which were Zic1 positive (Fig. 2F), were widely affected by hGFAP-Cre activity and might be a major cell type responsible for the mutant cerebellar defects. BG in the parenchyma were also β-gal positive, as demonstrated by anti-BLBP co-staining (Fig. 2H, arrows). At P22, consistent with the observation of β-gal positive EGL at P3, granule neuron-enriched IGL were packed with strong β-gal positive cells (Fig. 2E) that were also neuN positive (Fig. 2I). Therefore, our analysis confirmed that hGFAP-Cre-mediated recombination occurred in granule neurons (and their precursors), in BG and in small interneurons (Fig. 2K, arrows) in the ML, but not in calbindin-positive Purkinje neurons (Fig. 2G and arrows in Fig. 2J). Furthermore, the RL is the first region affected by Dicer1-ablation in the mutant cerebellum and could be the origin of the mutant phenotype.
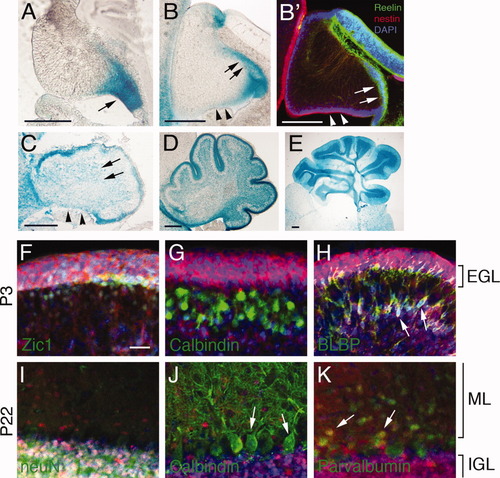
Cre-mediated recombination starts in the RL of embryonic cerebella and occurs in multiple cerebellar cell types, including BG in the hGFAP-Cre;LacZ reporter line. Cre-mediated recombination was examined by β-gal staining of cerebellar sections of E13.5 (A), E14.5 (B and B′), E17.5 (C), P3 (D), and P22 (E). Cerebellar structures are indicated: RL (arrow in A), VZ (arrowheads in B, B′, and C), early EGL (arrows in B and B′) and parenchyma (arrows in C). Anti-β-gal immunostaining (in red) was performed on cerebellar sections of P3 and P22 hGFAP-Cre;LacZ reporter mice (F–K) and co-labeled with anti-Zic1 (F), anti-calbindin (G and J), anti-BLBP (H), anti-neuN (I), or anti-parvalbumin (K). Purkinje neurons are β-gal negative (arrows in J) while granule neurons (I) and precursors in the EGL (F), BG (arrows in H), and small interneurons (arrows in K) are β-gal positive. EGL, external granule layer; ML, molecular layer; IGL, internal granule layer. Scale bars, 200 μm in A–E and 50 μm in F–K. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Dicer1 Ablation Disrupts Tissue Morphogenesis in the Developing Cerebellum
The smaller cerebellum of the mutant mice led us to examine their tissue morphogenesis at the cellular level during development. DAPI staining of cerebellar sagittal sections showed that cerebellar morphology was grossly normal in the mutant mice until P1 (Fig. 3). At P5, however, while the control cerebellum developed elongated fissures and lobules, folia were barely recognized in the mutant cerebellum (Fig. 3). At P16, the central lobe of the mutant cerebellum was less developed, with one fissure missing, which resulted in the formation of three, instead of four, lobules (Fig. 3, arrows). The nonprincipal fissure that separates lobules IXb and IXc was also missing. On the other hand, lobes IV and V of the mutant cerebellum were separated earlier than those of the controls (Fig. 3). In general, the length of each cerebellar lobe was shorter in the mutants than in the controls, which resulted in a smaller cerebellum in the mutant mice. Further examination revealed defects in neuronal cell types, especially granule neurons and their precursors, in Dicer1-ablated cerebella (being reported elsewhere).
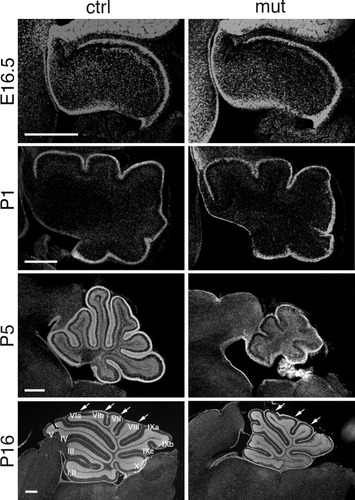
Defective cerebellar foliation in the mutant cerebellum. DAPI staining of cerebellar sections of mutants (mut) were compared with those of controls (ctrl), from E16.5 to P16. Starting from P5, mutant mice showed a smaller cerebellum, defects in EGL formation and impaired foliation. In the mutant cerebellum at P16, major folia were formed, but their lengths were generally shorter than in the controls. The central lobes were often seen to contain fewer lobules than controls (arrows). I-X, Number of cerebellar folia. Scale bars: 200 μm.
Dicer1 Ablation Disrupts the Phenotype of BG in the Developing Cerebellum
BG provide scaffolding for the inward migration of postmitotic granule neurons and play an essential role in organizing the laminated structure of the developing cerebellum. Given our observation of disrupted tissue morphogenesis in mutant cerebella, we examined whether Dicer1 ablation affects the phenotype of BG. BLBP, nestin, and GFAP are markers that label BG in the postnatal cerebellum (Feng et al., 1994; Soriano et al., 1997). Mutant BG had a decreased intensity of anti-BLBP staining at P5 compared with control BG, especially in their cell bodies (Fig. 4A, arrowheads). Mutant BG fibers were disorganized, as revealed by anti-nestin and anti-GFAP staining (Fig. 4A). At P16, the morphological defects of mutant BG fibers was extensive, as revealed by anti-GFAP immunostaining (Fig. 4Ba,b), with some BG having detached their endfeet off the basement membrane (Fig. 4B,b′, arrows). This detachment was not caused by the interruption of the basement membrane, as we observed continuous anti-nidogen staining around the region where the “falling-off” of BG as noted (Fig. 4B,c, arrows). In regions where BG endfeet were still intact, their cellular processes were often less organized compared with controls, and displayed uneven process length and poor alignment (Fig. 4B,d,e). Correlated with the morphological defect in mutant BG, NeuN+ granule neurons were loosely packed in the IGL and showed an absence of clear borders, as seen in the control cerebellum (Fig. 4B,d,e). The morphology of mutant BG was also examined in acutely isolated cerebellar cultures. The percentage of bipolar nestin+ BG among total nestin+ cells in mutant cultures was consistently and significantly reduced compared with controls at P5 (Fig. 5). We then asked if the altered phenotype of BG in the mutants was caused by aberrant proliferation of these cells. To test this, the proliferative capacity of BG was assessed in a BrdU labeling experiment. The percentage of BLBP+/BrdU+ (proliferative) among total BLBP+ BG cells at P7 (27.9 ± 0.8%) was not significantly different from the controls (21.1 ± 2.3%). We also found that proliferative BG (nestin+/Ki67+) showed a similar percentage between mutants (73.7 ± 1.9 %) and controls (68.2 ± 8.7%) in acute cultures isolated from P5 cerebella. In parallel, we examined the survival of mutant BG and did not detect a significant number of apoptotic BG in either mutant or control mice at P5, as determined by anti-cleaved caspase-3 staining (data not shown). Therefore, the disturbed phenotype of BG in the mutants could not be explained by defects in cell proliferation or survival.
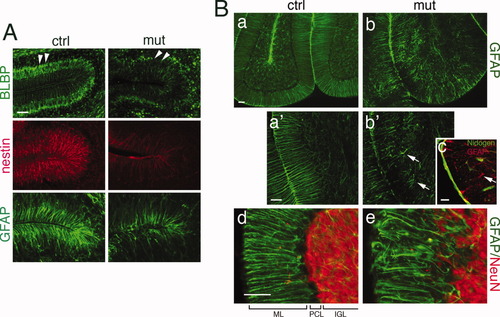
Defective phenotype of BG in the developing mutant cerebellum. (A) BG labeled by antibodies against BLBP (arrowheads), nestin and GFAP, showing decreased expression of these markers in P5 mutant cerebella. Additionally, mutant BG fibers labeled by GFAP and nestin display a less organized pattern in comparison with controls. (B) At P16, the disorganized phenotype of BG in the mutants became more severe (B, a and b), with some BG having retracted their processes from the basement membrane (arrows in B, b′ and c). The local basement membrane was still intact, as revealed by anti-nidogen staining (B, c). In less affected regions of the mutant cerebellum, BG fibers often exhibited uneven process length and poorly aligned patterns, which correlated with obscured boundary between the ML (labeled by GFAP) and IGL (labeled by NeuN) (B, d and e). ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granule layer. Scale bars: 50 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
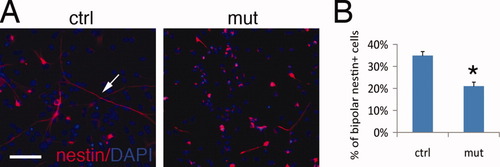
Dicer1-ablated BG exhibit a morphological defect in vitro. (A) BG were identified by their bipolar morphology and nestin immunoreactivity (arrows) in P5 cerebellar primary cultures incubated overnight on a laminin substrate. DAPI was used to label nuclei. (B) Percentage of bipolar BG among total nestin+ cells was quantified and compared between controls and mutants (*P < 0.05). Scale bar: 50 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Altered Phenotype of BG is not Caused by Insufficient Expression of Notch1 Receptor or Its Ligands in the Mutant Cerebellum
Active Notch1 signaling is critical in maintaining the proper phenotype of BG during postnatal cerebellar development, and suboptimal Notch1 signaling in these cells could be responsible for the altered phenotype of BG in Dicer1-ablated cerebella. Therefore, we examined whether the Notch1 receptor and its ligands have reduced expression in mutant cerebella. qRT-PCR analysis showed a significant decrease of BG markers (e.g. GFAP and BLBP) in P7 mutant cerebella (Fig. 6A), which was also confirmed by Western blot analysis (Fig. 6B). This was consistent with our immunostaining results on cerebellar sections (Fig. 4). We then examined the expression of Notch1 and its ligands in these tissues. Surprisingly, no significant difference was observed in Notch1 expression at the mRNA level between mutants and controls, although Notch1 showed a slightly higher level in the mutants (Fig. 6A). This was also true at the protein level (Fig. 6B). Among the examined Notch1 ligands, only Jagged2 exhibited a significant decrease in controls, but not in the mutants (Fig. 6A). These data suggest that the defective phenotype of BG and decreased Notch1 target gene expression (BLBP) in mutant cerebella is unlikely to be a result of insufficient levels of Notch1 receptor and its ligands in the tissue.
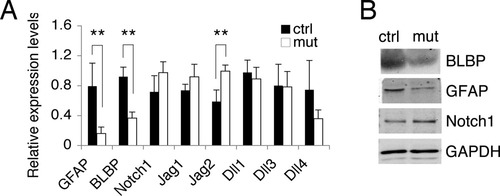
Altered phenotype of BG in mutant cerebella is not caused by decreased expression of Notch1 receptor or its ligands. (A) Gene expression levels of BG markers, Notch1 receptor and ligands in P7 cerebellar tissues (n = 5 for both mutants and controls) were analyzed by qRT-PCR (**P < 0.01). Jag1, Jagged1; Jag2, Jagged2; Dll1, Delta-like1; Dll3, Delta-like3; Dll4, Delta-like4. (B) Western blot analysis of P5 cerebellar tissues comparing protein levels of BLBP, GFAP, and Notch1 between mutants and controls. GAPDH was used as a loading control.
Dicer1-Ablated BG Display Intrinsic Blockage of Notch1 Signaling
We then determined if Dicer1-deletion in BG could lead to intrinsic defects of these cells. We isolated BG cells from early postnatal cerebella and performed a neuron-glial co-culture assay (Li et al., 2008b). Percoll-gradient-purified BG were cultured for several passages, and mutant cultures were confirmed by their reduced expression of Dicer1 and miR-9 (Fig. 7A). More than 90% of GFAP+ BG were presumably affected by Dicer1 ablation as revealed by their β-gal immunopositivity (Fig. 7B). No obvious differences were observed between the mutant and control cultures in terms of their expression levels of BG markers (Fig. 7C,D). BG cells were then co-cultured with a GFP+ rat neurogenic neural progenitor clone, GE6 (Li et al., 2012) for 2 days. As expected, control BG cells displayed a bipolar radial morphology, as revealed by anti-GFAP staining and elevated level of BLBP, a direct downstream target of Notch1 activation (Anthony et al., 2005), upon co-culturing (Fig. 7E). This GE6-induced upregulation of BLBP, as well as another target gene Hes1, was confirmed at the mRNA level by qRT-PCR using mouse-specific primers (Fig. 7F). In addition, this upregulation could be blocked by pretreatment with the γ-secretase inhibitor DAPT, further confirming the involvement of Notch1 signaling in this process (Fig. 7F). By contrast, mutant BG did not change their morphology nor increase their BLBP expression, indicating a blockage of Notch1 signaling in the mutant cells (Fig. 7E). However, this blockage was probably not caused by a lack of Notch1 receptor on the mutant BG membranes, because similar amounts of Notch1 protein were detected on the control and mutant cells by immunostaining and Western blot analysis (Fig. 7C,D). Thus, the data indicated that Dicer1 ablation disrupted BG morphology and differentiation during postnatal cerebellar development, at least in part, by blocking the intrinsic Notch1 signaling pathway. Many GFP+ GE6 cells differentiated into TuJ1+ neurons, with no obvious difference in percentage between mutant (24.9 ± 7.4%) and control (23.4 ± 4.7%) co-cultures (Fig. 7E).
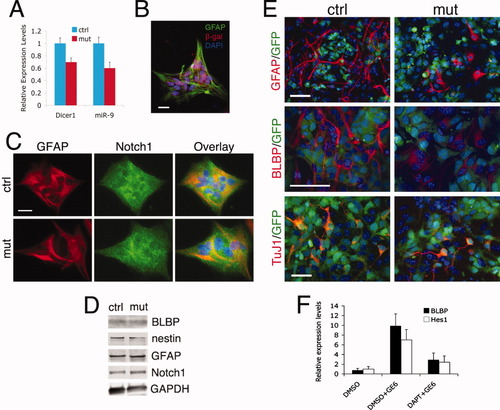
Dicer1-ablated BG display a Notch1 signaling defect in vitro. BG cell cultures, derived from P5 cerebella of control or mutant mice, were cultured for two to three passages in DMEM + 10% FBS. (A) Decreased expression of Dicer1 and miR-9 in mutant BG cultures confirmed by qRT-PCR analysis. (B) Immunostaining showing co-localization of GFAP and β-gal in these cultures. (C) The GFAP+ BG from mutant and control cultures showing similar morphologies and comparable Notch1 protein expression on their cell surfaces. (D) Western blot analysis of these cultures showing similar protein levels of markers between mutants and controls. (E) BG purified from either control or mutant P5 cerebella were then co-cultured with a GFP+ rat neurogenic progenitor clone GE6 for 2 days. The morphology of BG was analyzed by anti-GFAP staining, while Notch1 signaling pathway activation was examined by anti-BLBP staining. The neuronal differentiation of GE6 cells was also compared by TuJ1 staining. DAPI was used to label nuclei. (F)The mRNAs of Notch1 downstream targets BLBP and Hes1 were upregulated as measured by qRT-PCR in control BG cultures when co-cultured with GE6 cells for 2 days. This upregulation was blocked by pretreatment with DAPT (50 μM), a γ-secretase inhibitor. DMSO was used as the solvent. This is a representative result from at least two independent experiments. Scale bars: 20 μm in B and C; 50 μm in E. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
MiR-9 is Differentially Expressed in BG and is Required for Proper Notch1 Signaling
To gain an insight into the molecular mechanism of Dicer1-mediated effects on the phenotype of BG, the expressions of two well-known miRNAs (i.e. miR-124 and miR-9) were examined in Percoll-gradient-purified cerebellar granule neurons and in BG by qRT-PCR analysis. MiR-124, a neuronal miRNA, was expressed at a higher level in granule neurons, whereas miR-9 expression was higher in BG (Fig. 8A). MiR-9 is strongly expressed in the developing CNS, including the cerebellum (Krichevsky et al., 2003). Therefore, the differential expression of miR-9 in BG suggests a functional role in maintaining the phenotype of these cells. To assay miR-9′s functional contribution to the phenotype of BG, miR-9 was knocked down or overexpressed in our cultures. Antisense miR-9 inhibitor was successfully transfected into control BG cultures and decreased the level of miR-9 by 40% (Fig. 8B). Synthesized miR-9 mimics downregulated the level of Onecut2 mRNA, a validated target of miR-9 (Plaisance et al., 2006), by 50% in mutant BG cultures (Fig. 8C), as measured by qRT-PCR. We then altered miR-9 levels by transfection in the neuron-glial co-culture assays mentioned above. In control BG cultures, the miR-9 inhibitor was introduced to decrease cellular miR-9 level before GE6 cells were added and incubated for 2 days. Two Notch1 target genes, BLBP and Hes1, were chosen as indicators of Notch1 signaling activation. To measure gene expression changes in BG only, we designed mouse-specific primers that did not recognize rat genes in GE6 cells and validated their specificity by qRT-PCR (data not shown). Similar to the anti-BLBP immunostaining results (Fig. 7E), the BLBP mRNA level in the control BG culture increased by approximately five fold after co-culturing with GE6; knocking down miR-9 expression blocked this increase by more than 40% (Fig. 8D), indicating that miR-9 is an essential component for normal Notch1 signaling. The Hes1 mRNA level exhibited a similar trend in these experiments, but to a lesser degree than BLBP (Fig. 8D). By contrast, miR-9 mimics were transfected to boost the miR-9 level in mutant BG before GE6 co-culturing. Consistent with the previous observation (Fig. 7E), the expression levels of BLBP and Hes1, which were lower than that of control cultures, showed only a marginal increase upon GE6 co-culturing and, more importantly, exogenous miR-9 was unable to increase the expression of these genes to a comparable level to the controls (Fig. 8D). Therefore, miR-9 overexpression failed to rescue disrupted Notch1 signaling in mutant BG. These results indicated that miR-9 has a critical function in mediating proper Notch1 signaling pathway, but is not the only miRNA required.
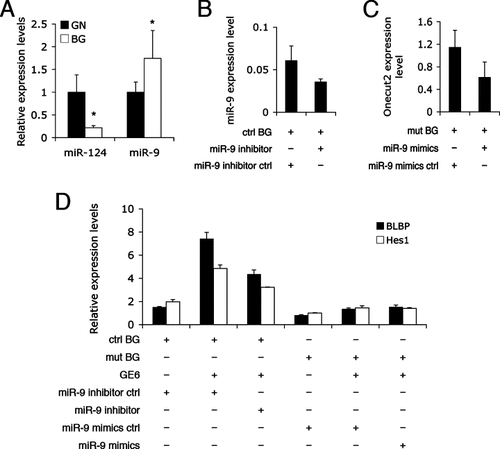
MiR-9 is differentially expressed in BG and is required for proper Notch1 signaling. (A) qRT-PCR analysis of miR-9 and miR-124 expression levels in granule neurons (GN) and BG isolated from P5 control mouse cerebella (n = 3; *P < 0.05). (B) MiR-9 inhibitor transfection efficiency as measured by the miR-9 level using qRT-PCR. (C) MiR-9 overexpression was evaluated by the knockdown of a known miR-9 target gene, Onecut2. MiR-9 mimics ctrl and miR-9 inhibitor ctrl are scrambled sequences used as transfection controls for miR-9 mimics and miR-9 inhibitor, respectively. (D) Notch1 pathway activation in BG cultures was measured by the expression levels of BLBP and Hes1 using qRT-PCR with mouse-specific primers, before and after GE6 co-culturing in the presence of miR-9 mimics or inhibitor. Shown are representative results from at least three independent experiments.
DISCUSSION
In this study, we took advantage of the hGFAP promoter to knock out Dicer1 and examine the effect of miRNAs in early postnatal cerebellar development, with a focus on BG. Our data showed that Dicer1 ablation resulted in a smaller cerebellum, disrupted tissue morphogenesis, and caused aberrant morphology of BG. Dicer1-ablated BG also displayed decreased expression of BG markers and an intrinsic blockage of Notch1 signaling. In addition, miR-9 is required for proper Notch1 signaling in early postnatal BG. A decreased miR-9 level contributes, at least in part, to the altered phenotype of BG in the mutants. These results indicate that miRNAs are required for normal BG development and cerebellar morphogenesis.
The morphology of radial glia (RG) is important in providing physical scaffolds for neuronal migration and maintaining the integrity of laminated tissue structures in various regions of the developing CNS. In the early postnatal cerebellum, BG function as RG to facilitate inward migration of postmitotic granule neurons. In the reported experimental settings, including those in the present study, where misbehavior of RG was observed in the absence of miRNAs, their neighboring neurons were also depleted of miRNAs. Therefore, it is hard to distinguish whether RG defects are mediated by cell-autonomous mechanisms or by aberrant signals presented by neighboring neurons. However, our neuron-glial co-culture results strongly argue for the former possibility, by showing that the intrinsic Notch1 signaling pathway was inhibited in the Dicer1-deleted BG; however, we cannot exclude the possibility that neurons may also play a role in vivo. In support of the hypothesis that miRNAs intrinsically contribute to the properties of BG, Tao et al. performed an astroglia-specific Dicer1 deletion by mGFAP-Cre recombination (Tao et al., 2011). The study focused on the physiological functions of mature astrocytes and demonstrated a massive neuronal cell death in cerebella and neurological decline in this Dicer1-deleted mutant 5 weeks postnatally. Gene expression analysis allowed the authors to conclude that astroglia failed to mature and stalled at a more primitive stage in the absence of miRNAs, which in turn caused non-cell-autonomous neuronal dysfunction and degeneration (Tao et al., 2011). By contrast, we focused our study on the effects of Dicer1 in developmental aspects of early postnatal cerebella, and found that Dicer1 ablation severely disrupted the phenotype of BG and that miRNAs, including miR-9, are essential to intrinsic Notch1 signaling in developing BG. Surprisingly, Tao et al. detected few morphological defects in Dicer1-deleted astroglia, including BG, in P15 cerebellum of their mutants, which argues that extrinsic mechanisms may also play a role in the defective phenotype of BG that we observed in hGFAP-Cre;Dicer1f/f mice. Alternatively, the two mGFAP-Cre mouse lines that Tao et al. used showed Cre activity no earlier than P4 in cerebella, in contrast to the mid-embryonic stage in hGFAP-Cre mouse. This timing difference of Cre expression may also have contributed to the ambiguous phenotypes of BG observed in these two different types of mutant mice.
Genes that regulate RG morphology have been described. For example, the Notch1 signaling pathway is crucial for maintaining RG phenotypes in the developing mouse forebrain (Gaiano et al., 2000; Li et al., 2008a). Corfas' group provided evidence that both Notch1 and erbB2/neuregulin signaling are essential for reverting differentiated astroglia back into an RG-like state bearing a bipolar morphology and RG marker expression (Gierdalski et al., 2005; Patten et al., 2003; Rio et al., 1997). The miRNA system may well be interacting with these signaling pathways in normal animals and thus participates in the regulation of RG identity. In the present study, we demonstrated that Dicer1-ablated BG are deficient in mediating Notch1 signaling when activated by neuronal contact from GE6 cells in co-cultures (Fig. 7). Consistent with our results, two recent reports showed that Dicer1 ablation results in downregulation of Notch1 target genes during mouse retinal development confirming the sensitivity of this signaling pathway to miRNA alterations (Davis et al., 2011; Georgi and Reh 2011).
MiR-9, an evolutionarily conserved miRNA, has been implicated in numerous biological processes during CNS development (Delaloy and Gao, 2008; Leucht et al., 2008; Shibata et al., 2008; Zhao et al., 2009). In this study, we showed that miR-9 is differentially expressed in BG in the developing cerebellum. Furthermore, using an in vitro neuronal co-culturing assay, we provided evidence that miR-9 might be an important effecter of the Notch1 signaling pathway in maintaining the phenotype of BG. The direct functional analysis of miR-9 during forebrain development was recently reported in an miR-9 knockout mouse (Shibata et al., 2011). By deleting two of the three miR-9 genomic loci in mice, the authors showed that the mutant exhibited a larger lateral ventricle, thinner cortical wall, and reduced early neurogenesis, all of which were very similar to the phenotype of the Dicer1-deleted mouse forebrain (De Pietri Tonelli et al., 2008). These data suggest that miR-9 may be the most important, if not the only, player in the miRNA-mediated effect during forebrain development. Unfortunately, the RG were not thoroughly examined in this miR-9 knockout mouse, being described as having an “almost normal” morphology at E12.5 by nestin immunostaining (Shibata et al., 2011). It will be intriguing to look at the phenotype of BG during cerebellar development in this mouse.
Dissecting out miRNA targets that are responsible for defective phenotypes of Dicer1-deleted BG will be critical in understanding the mechanisms of miRNA-mediated effects and will be of great interest in our future studies. The arrangement of the cytoskeleton may be important for maintaining the unique morphology of BG, forming potential targets for miRNA-mediated actions. Our previous study showed that microtubules, but not actins, are important for BG bipolar morphology, and that several microtubule-associated proteins (MAPs) and microtubule-affinity regulating kinases (MARKs) are differentially expressed in the RG-like clone C6-R and in postnatal BG (Li et al., 2003). Among the predicted cytoskeleton-related gene targets of miR-9 are Map1a, Map1b, and Map7 (Table 2). Moreover, microtubule-dependent motor protein kinesin family members, Kif1c and Kif21a, are also targets of miR-9 (Table 2). These target genes will be candidates for future validation of miRNA function in the regulation of the morphology of BG. Interestingly, a recent report indicated that the microtubule-regulatory protein, stathmin, is a novel target of miR-9 to coordinate proliferation and migration of human embryonic stem cell-derived neural progenitors (Delaloy et al., 2010).
| Gene symbol | Gene title | Gene ID | Total context score |
|---|---|---|---|
| Myo1d | Myosin ID | NM_012983.2 | −0.47 |
| Myh9 | myosin, heavy chain 9, nonmuscle | NM_013194.1 | −0.36 |
| Myo1c | Myosin IC | NM_023092.1 | −0.36 |
| Esr1 | Estrogen receptor 1 | NM_012689.1 | −0.34 |
| Ank2 | Ankyrin2 | NM_001034168.1 | −0.33 |
| Lmna | Lamin A | NM_001002016.1 | −0.32 |
| Capza1 | F-actin-capping protein subunit alpha-1 | NM_001109625.2 | −0.29 |
| Hunk | Hormonally upregulated Neu-associated kinase | NM_001191662.1 | −0.26 |
| Jup | Junction plakoglobin | NM_031047.2 | −0.25 |
| Arpc1a | Actin-related protein 2/3 complex subunit 1A | NM_031146.2 | −0.24 |
| Map7 | Microtubule-associated protein 7 | NM_001106270.2 | −0.24 |
| Vcl | Vinculin | NM_001107248.1 | −0.21 |
| Drd2 | Dopamine receptor D2 | NM_012547.1 | −0.2 |
| Sgcd | Sarcoglycan, delta (dystrophin-associated glycoprotein) | NM_001109029.1 | −0.2 |
| Tes | Testis derived transcript | NM_001039344.2 | −0.2 |
| Actr1a | ARP1 actin-related protein 1 homolog A | NM_001106364.1 | −0.19 |
| Tlk1 | Tousled-like kinase 1 | NM_001107734.1 | −0.19 |
| Kif1c | Kinesin family member 1C | NM_145877.1 | −0.17 |
| Map1b | Microtubule-associated protein 1B | NM_019217.1 | −0.17 |
| Arhgef2 | rho/rac Guanine nucleotide exchange factor (GEF) 2 | NM_001012079.1 | −0.16 |
| Kif13b | Kinesin family member 13B | NM_213626.1 | −0.16 |
| Kif21a | Kinesin family member 21A | NM_001109040.1 | −0.16 |
| Utrn | Utrophin | NM_013070.1 | −0.14 |
| Map1a | Microtubule-associated protein 1A | NM_030995.1 | −0.13 |
| Src | v-src Sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian) | NM_031977.1 | −0.13 |
| Itga6 | Integrin, alpha 6 | NM_008397.3 | −0.11 |
| Sh3bp4 | SH3-domain binding protein 4 | NM_022693.3 | −0.11 |
| Dlgap2 | Disks large-associated protein 2 | NM_053901.1 | −0.1 |
| Sh3bgrl2 | SH3 domain binding glutamic acid-rich protein like 2 | NM_001137647.1 | −0.1 |
| Ssx2ip | Synovial sarcoma, X breakpoint 2 interacting protein | NM_175597.3 | −0.09 |
| Myo10 | Myosin X | NM_001107657.1 | −0.05 |
- The functional annotation clustering of the predicted targets (TargetScan5.1) was performed by DAVID-EASE software; available at: http://david.abcc.ncifcrf.gov/ease/ease.jsp. The list is sorted by total context score from TargetScan5.1 software.
Acknowledgements
The authors thank Dr. Richard Q. Lu and members of his laboratory for their technical support.