Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury
Abstract
Chronic neuropathic pain is a frequent consequence of spinal cord injury (SCI). Yet despite recent advances, upstream releasing mechanisms and effective therapeutic options remain elusive. Previous studies have demonstrated that SCI results in excessive ATP release to the peritraumatic regions and that purinergic signaling, among glial cells, likely plays an essential role in facilitating inflammatory responses and nociceptive sensitization. We sought to assess the role of connexin 43 (Cx43) as a mediator of CNS inflammation and chronic pain. To determine the extent of Cx43 involvement in chronic pain, a weight-drop SCI was performed on transgenic mice with Cx43/Cx30 deletions. SCI induced robust and persistent neuropathic pain including heat hyperalgesia and mechanical allodynia in wild-type control mice, which developed after 4 weeks and was maintained after 8 weeks. Notably, SCI-induced heat hyperalgesia and mechanical allodynia were prevented in transgenic mice with Cx43/Cx30 deletions, but fully developed in transgenic mice with only Cx30 deletion. SCI-induced gliosis, detected as upregulation of glial fibrillary acidic protein in the spinal cord astrocytes at different stages of the injury, was also reduced in the knockout mice with Cx43/Cx30 deletions, when compared with littermate controls. In comparison, a standard regimen of post-SCI treatment of minocycline attenuated neuropathic pain to a significantly lesser degree than Cx43 deletion. These findings suggest Cx43 is critically linked to the development of central neuropathic pain following acute SCI. Since Cx43/Cx30 is expressed by astrocytes, these findings also support an important role of astrocytes in the development of chronic pain. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
As many as 80% of patients who suffer spinal cord injury (SCI) experience chronic pain that develops shortly following the primary injury and often persists indefinitely. Chronic pain of this nature is distinct from acute pain in that it provides no neuroprotective benefits with its negative effects lasting long after the injury site has healed (Hulsebosch et al.,2009; Norenberg et al.,2004; Turner and Cardenas,1999). Recent progress in the field of pain research has demonstrated important roles of spinal cord glial cells (e.g., microglia and astrocytes), in the genesis of chronic pain (Gao and Ji,2010; Milligan and Watkins,2009; Tsuda et al.,2005). It is generally believed that activation of glial cells induces central sensitization and enhances chronic pain via producing glial mediators, such as proinflammatory cytokines (IL-1β, IL-6, TNF-α; DeLeo and Yezierski,2001; Kawasaki et al.,2008; Watkins et al.,2001). Activation of ATP receptors such as P2X7 receptors in microglia is essential for the release of proinflammatory cytokines (Clark et al.,2010). However, the cellular source of ATP and the molecular mechanisms controlling ATP release are still elusive.
Initial astrocyte and microglial activation is thought to represent a generalized neuro-protective response aimed at ameliorating the damage done by the initial SCI (Bethea,2000; Farahani et al.,2005; Faulkner et al.,2004). However, if activation goes unopposed, a secondary phase of reactive gliosis occurs that triggers cell death among neurons and glia, and is associated with peritraumatic expansion of neural damage (Bethea,2000; Springer et al.,1999). This secondary phase has been shown to correlate with high levels of sustained ATP release, in the peritraumatic regions (Wang et al.,2004). Although the source of ATP remains controversial, high extracellular levels have been shown to activate purinergic P2X receptors on microglia and leukocytes, and P2X7 activation in particular has been shown to contribute to the secretion of a host of cytokines and proinflammatory molecules (e.g., ROS, IL-1β, IL-6, TNF-α, etc.; Collo et al.,1997; Di Virgilio et al.,1999, 2009; Peng et al.,2009). Exposure to cytokines is known to contribute to neuronal sensitization in the spinal cord and is also thought to contribute to chronic pain (Cotrina and Nedergaard,2009; Gwak and Hulsebosch,2011; Kawasaki et al.,2008). Furthermore, pharmacological blockade and genetic deletion of P2X receptors have been shown to attenuate rodent pain-like-behaviors, which suggest that activation of cell P2 receptors is an essential step in the development of chronic pain. (Chessell et al.,2005; Dell'Antonio et al.,2002a,b; Labasi et al.,2002; Tsuda et al.,2009). A more in depth understanding of the causes and consequences of ATP release in the setting of SCI is, therefore, of great clinical importance, as it would allow specific targeting for novel therapies.
We have previously shown that ATP is released after SCI (Peng et al.,2009) and that genetic deletion of connexin 43 (Cx43), a principle connexin expressed in spinal cord astrocytes, dramatically decreases the extracellular concentration of ATP following acute SCI (Huang et al.,2012). Unopposed hemichannels that directly link the cytosol and the interstitium (Bennett et al.,2003; Kang et al.,2008) constitute ideal candidates for potential ATP release pathways, since the biophysical profile of these channels shows them to be capable of high levels of ATP efflux (Bennett et al.,2003; Cotrina et al.,1998; Parpura et al.,2004). Studies have also shown that astrocytes upregulate the expression of Cx43 following traumatic SCI (Theriault et al.,1997), and that exposure to several types of cytokines reduces astrocyte–astrocyte gap junctional communication (Contreras et al.,2002; Meme et al.,2006; Retamal et al.,2007). Additionally, blocking the expression of Cx43 has been shown to attenuate inflammation and improve functional recovery following SCI (Cronin et al.,2008), which further implicates Cx43 as a mediator of reactive changes following injury. Here, we sought to evaluate the role of Cx43 in chronic neuropathic pain and inflammation, as it is well established that purinergic signaling plays a role in acute pain transmission and proinflammatory cytokine release.
The aim of this study was to evaluate the role Cx43 plays in the development and maintenance of chronic pain following SCI. We show that transgenic mice with deletions of both Cx43 and Cx30 exhibited improved pain scores, and reduced upregulation of glial fibrillary acidic protein (GFAP) following SCI, as compared with controls.
Abbreviations
-
- ATP
-
adenosine triphosphate
-
- BBB
-
blood brain barrier
-
- BMS
-
Basso mouse scale
-
- CNS
-
central nervous system
-
- Cx30
-
connexin 30
-
- Cx43
-
connexin 43
-
- DAPI
-
4′,6-diamidino-2-phenylindole
-
- ERK
-
extracellular receptor kinase
-
- GFAP
-
glial fibrillary acidic protein
-
- IL-1β
-
interleukin-1 beta
-
- IL-6
-
interleukin-6
-
- ROS
-
reactive oxygen species
-
- SCI
-
spinal cord injury
-
- TNF-α
-
tumor necrosis factor-alpha
MATERIALS AND METHODS
Animals
In addition to wild-type C57BL/6 (Charles River) mice, Cx30−/−Cx43fl/fl:hGFAP–Cre (Cx43−/−Cx30−/−, double knockout) and their littermate controls, Cx30−/−Cx43fl/fl (Cx30−/− single KO), which were originally generated in 2006 (Wallraff et al.,2006), were used in evaluating the role of Cx43 on chronic pain development. All mice were 8- to 10-week females with a weight of 20–25 g. In addition, C57BL/6 (Charles River) age-matched female mice were used for the minocycline studies. In accordance with UCAR protocol, animals were housed under a 12 h light–dark cycle and had free access to water and food. All experiments were approved by the Animal Care and Use Committee of the University of Rochester.
Surgery
Adult female mice were anesthetized with a mixture of ketamine (60 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). A laminectomy over the dorsal portion of T11 was performed and the vertebral column was held with fine clamps at the T10 and T12 level. The exposed dorsal surface of the spinal cord was subjected to a 3-g weight-drop with tip diameter of 0.5-mm flat surface, modified NYU impactor (Peng et al.,2009), from a height of 6.75 mm using a modified NYU impactor. Accordingly, animals were monitored post-SCI via Basso Mouse Scale (BMS) scoring and deurinated twice a day until leg movement returned to animals.
Behavioral Studies
Mechanical allodynia and heat hyperalgesia were examined over the course of 2 months after SCI to determine the development of SCI-induced neuropathic pain. Mechanical allodynia was tested using Von Frey Filaments. Each session involved the exertion of a filament with 0.02g of force onto the plantar surface of the foot. The percentages of negative responses were calculated for each foot. A total of 10 trials were done per day (Goldman et al.,2010).
Heat hyperalgesia was evaluated using Plantar Test (Ugo Basile). The Plantar test apparatus was calibrated before and after each set of data to ensure accuracy. A mobile heat source was placed under the hind paw and calibrated to an IR intensity of 20. Paw withdrawal latency was defined as the time taken by a mouse to withdraw its foot from radiant heat. Each foot was measured three times, giving an average. To avoid conditioning to stimulation, there was a 5-min rest period between each measurement. Furthermore, to account for stress to the environment, each animal was allowed to habituate for 1 h before any measurements were taken (Goldman et al.,2010).
Tissue Preparation
The mice were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) and perfused transcardially with 4% paraformaldehyde in PBS at 8 weeks after SCI. The spinal cords were dissected, postfixed overnight at 4°C, and then transferred to 30% sucrose for processing into 20-μm longitudinal cryosections. One set of sections was stained with cresyl violet, and the remaining sections were used for immunohistochemistry. These sections were blocked for 30 min at room temperature in a solution containing 10% normal donkey serum and 0.5% Triton X-100, and incubated overnight at 4°C with primary antibodies against GFAP (1:500, Sigma), and Cx43 (1:500, Sigma). All fluorescent-conjugated secondary antibodies (Jackson Immunoresearch) were used at 1:250. After immunolabeling, the sections were counterstained with DAPI (1:5,000, Invitrogen) for 10 min at room temperature and a coverslip mounted. Images were collected with a confocal microscope (FV500, Olympus) with FluoView (Olympus) software by using a 20× oil objective lens (NA 1.3). Nonbiased image collection was used to evaluate the injury volume and fluorescence intensity (see below). Images were subsequently analyzed with custom-made MatLab software (Peng et al.,2009). Since mild injury was studied, no obvious tissue lesions were identified in the spinal cords harvested 2 months after the traumatic injury. Instead, the spinal cords exhibited clear atrophy close to the site of impact. The spinal cord atrophy volume was quantified as the tissue missing in serial longitudinal sections of the spinal cord stained with cresyl violet as shown in Fig. 2. For immunohistochemical analysis, 4–6 fields (640–640 μm2; 2–3 from rostral left and right and 2–3 from caudal left and right) of gray and white matter were chosen as the near injury fields; 4–6 fields at least 10-mm distance from the site of injury were likewise chosen as distant fields. The parameters for confocal image capture (laser, power, photomultiplier tube voltage, gain, and offset) were set from a wild-type spinal cord and remained constant for all remaining image capturing. The average intensity of each field (intensity per μm2) was quantified, and the data were expressed as % intensity increase in near-injury area with respect to the far-injury area.
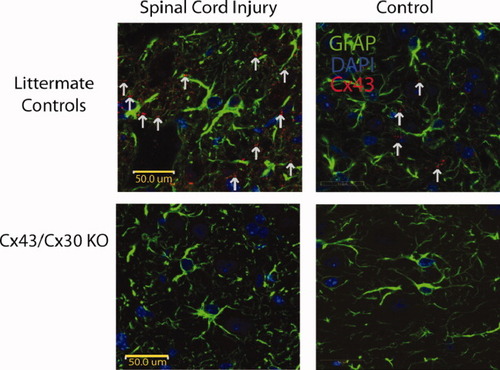
Immunohistochemical staining of injured and noninjured spinal cord. (A) Immunohistochemical staining of GFAP (green), DAPI (blue), and Cx43 (red) in longitudinal spinal cord slices of Cx43/Cx30 KO mice and littermate controls. As expected, in Cx43/Cx30 KO, GFAP-expressing cells express no Cx43. Meanwhile, littermate controls express Cx43 in GFAP-expressing cells and exhibit an upregulation of CX43 at injury sites. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
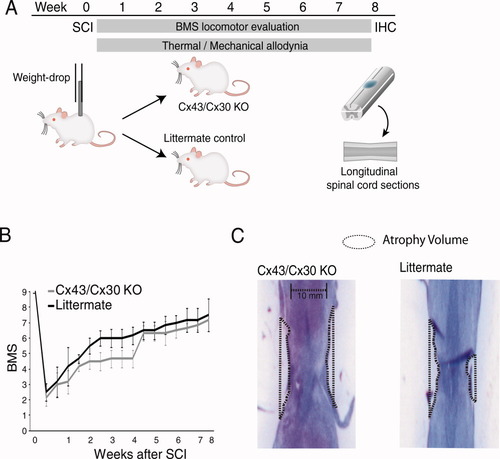
Deletion of Cx43 does not significantly change lesion volume or locomotor recovery following mild spinal cord injury. (A) Schematic diagram of experimental design. Mild spinal cord injury was inflicted by a weight-drop impact (3-g weight-drop with tip diameter of 0.5-mm flat surface at T10 and T12) in adult female mice. Locomotor function was scored according to the Basso Mouse Scale (BMS) on a bi-weekly basis, whereas thermal hyperalgesia and mechanical allodynia were quantified daily. Animals were perfusion-fixed after 2 months and spinal cords were harvested for histology. (B) The graph depicts BMS scores for locomotor functions as a function of time after spinal cord injury. Deletion of Cx43/Cx30 has no significant effects on recovery of motor function (n = 6, P > 0.05, ANOVA, mean ± SEM). (C) The severity of spinal cord injury was quantified as the atrophy volume, since it is difficult to delineate the borders of the tissue lesion following mild spinal cord injury. Cx43/Cx30 KO and their littermate controls exhibit a comparable degree of atrophy close to the injury site. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RESULTS
Littermate Controls Express CX43 on GFAP-Expressing Cells While Cx43/Cx30 KO Do Not
It has been well established that Cx43 is well expressed in spinal cord astrocytes (Huang et al.,2012). However to confirm that our model was appropriate, double immunohistochemistry staining of Cx43 and GFAP demonstrated co-localization of Cx43 expression with astrocytic cells (GFAP-expressing cells) in littermate controls (Fig. 1). Consistent with previous findings, we found that Cx43 levels and plaque formation were elevated in injured animals when compared with noninjury site in littermate controls (Theriault et al.,1997). Meanwhile, double immunohistochemistry staining of Cx43 and GFAP in Cx43/Cx30 KO confirmed that Cx43 was not present at injury sites or noninjured sites, confirming our transgenic animal (Fig. 1).
Exposure to Mild SCI Results in Impaired Locomotor Function and Loss of Spinal Cord Tissue in Both Wild-Type and Knockout Mice
Cx43 is recognized as the primary gap junctional protein expressed by astrocytes (Teubner et al.,2003; Theis et al.,2003). To delete Cx43 in astrocytes, we used a mouse line developed by Klaus Willecke in which conditional knockout in astrocytes is accomplished using mice-expressing Cre under the human glial fibrillary acidic protein (hGFAP) promoter (Theis et al.,2001). However, astrocytes also express connexin 30 (Cx30), and Cx30 has been reported to exhibit a compensatory upregulation in response to deletion of Cx43 (Nagy et al.,1999; Teubner et al.,2003; Theis et al.,2003). To prevent compensatory increases in Cx30 expression, the Cx43 mice were therefore crossed with Cx30 knockout mice. We compared double-deficient Cx30−/−, Cx43fl/fl: GFAP-Cre (double knockouts) with their littermate, Cx30−/−, Cx43fl/fl (Cx30 KO) and wild-type (WT) controls to evaluate the roles of astrocytic Cx43 hemichannels/gap junctions in the development of SCI-induced neuropathic pain.
All mice were exposed to mild SCI (3 g weight with tip diameter of 0.5 mm dropped from a height of 6.75 mm; Fig. 2A). Motor behavior was assessed on a biweekly schedule in open-field testing with the aid of the 9-point BMS for Locomotion rating scale after traumatic SCI (Fig. 2B). All measurements were taken at the same time of day during the wake cycle of the animal (Basso et al.,2006; Beare et al.,2009). No significant difference was found between Cx43/Cx30 mice and the littermate controls in the BMS scoring during the 2 months observation period (P-value > 0.05; Fig. 2B). Immediately after SCI, all animals displayed scores of 0–2, signifying complete paraplegia with movement limited to slight ankle movement. Both Cx43/Cx30 KO and their littermates exhibited steady recovery with partial recovery of motor function over a period of 4–5 weeks after injury (BMS score > 5, which correlates with the first signs of plantar placement defined, as thumb and last toe of paw placed on surface with each step; Fig. 2A).
A milder injury induced via weight-drop was selected to ensure that the injury was accompanied with extensive functional recovery (BMS score) and to ensure proper recovery of somatosensory function (Rosenzweig et al.,2010). As the focus of this study was on chronic pain development, extensive functional recovery is crucial to prevent permanent paraplegia. As a consequence of this mild injury, no clear lesions could be identified in longitudinal sections of the spinal cord after weight-drop injury. Instead, we quantified atrophy volume in Cx43/Cx30 KO and their littermate controls exposed to the same injury (Fig. 2C). Spinal cord atrophy was quantified in cresyl violet-stained serial longitudinal sections of the spinal cord, as shown in Fig. 2 and previously described (Peng et al.,2009).
Littermate Controls and WT Controls Exhibit Indistinguishable Neuropathic Pain Following SCI
As the study was focused on Cx43 in astrocytes, the first step is to confirm that Cx30 KO and wild-type controls are indistinguishable in regards to chronic pain development. We first observed baseline pain sensitivity between Cx30 KO mice and wild-type controls. We found that mechanical allodynia (Fig. 3A) and heat hyperalgesia (Fig. 3B) are not significantly different at baseline (P > 0.05). We next observed chronic pain development following SCI. We found that mechanical allodynia (Fig. 3A) and heat hyperalgesia (Fig. 3B) following SCI injury are also not significantly different (P > 0.05). Following this confirmation, we compared Cx43/Cx30 double KO with their littermate controls, exclusively, as no significant difference was found between Cx30 and wild-type controls or littermate controls and wild-type controls.
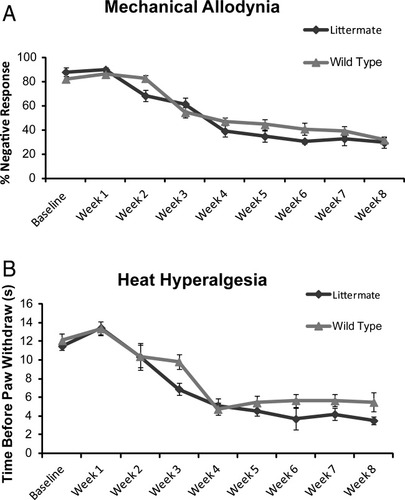
Cx30 KO plays no role in the development in chronic pain after spinal cord injury. Cx30KO and wild-type animals do not differ in development of mechanical allodynia (A) or heat hyperalgesia (B) after spinal cord injury. At all time points tested, no significant difference was noted (P > 0.1, one-way ANOVA, n = 10, mean ± SEM).
Cx43/Cx30 KO Mice Do Not Develop Neuropathic Pain Following SCI
We first compared baseline pain sensitivity in Cx43/Cx30 KO mice and littermate controls before SCI. We found that mechanical paw withdrawal frequency to mechanical Von Frey filament (0.02g) stimulus (Fig. 4A) and paw withdrawal latency to radiant heat stimulus (Fig. 4B) were almost identical in Cx43/Cx40 KO and littermate control, indicating normal pain perception in both strains.
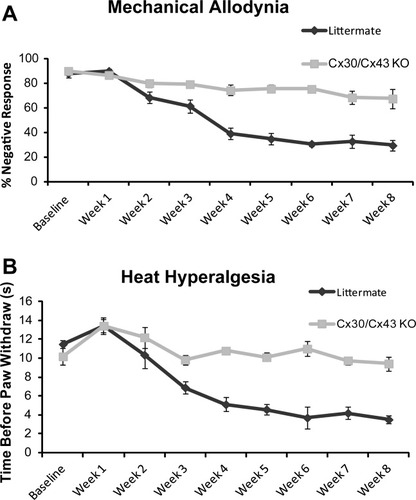
Deletion of Cx43 reduced mechanical allodynia and heat hyperalgesia after mild spinal cord injury. (A) Graph comparing the development of mechanical allodynia over the course of 2 months after mild spinal cord injury. Four weeks after spinal cord injury, mechanical allodynia was significantly less pronounced in Cx43/Cx30 KO than in littermate controls (*P < 0.01, one-way ANOVA, n = 6, mean ± SEM). (B) Development of heat hyperalgesia over the course of 2 months after mild spinal cord injury. Starting from 4 weeks after spinal cord injury and lasting for the remaining observation period, thermal hyperalgesia was significantly reduced in Cx43/Cx30 KO compared with littermate controls (*P < 0.01, ANOVA, n = 6, mean ± SEM).
Neuropathic pain after SCI is characterized by mechanical allodynia, nociceptive response to previously innocuous low-threshold mechanical stimulus (Tan et al.,2009). We assessed the development of mechanical allodynia weekly for 8 weeks. As a result of the paraplegia, during the first 3 weeks after injury, no allodynia was observed (Fig. 4A). Four weeks following SCI or at the time point where the mice started to regain significant motor functions (BMS score 4 or higher), littermate mice began to develop mechanical allodynia, which was still maintained after 8 weeks. However, SCI-induced mechanical allodynia was prevented in Cx43/Cx30 KO mice. From week 4 to 8, Cx43/Cx30 KO mice consistently exhibited significantly higher negative mechanical nociceptive response rate than their littermate controls (P < 0.05; Fig. 4A). At the last time point evaluated (2 months after SCI), negative response rate decreased from 90% ± 1.9% to 40% ± 4.29% in littermate controls, but only from 86.67% ± 2.9% to 72.2% ± 6.9% in Cx43/Cx30 KO mice.
In addition to mechanical allodynia, SCI-induced neuropathic pain is also characterized by heat hyperalgesia, which is defined as an increased response to a noxious heat stimulus. In the first 3 weeks after SCI, prior to motor recovery, no significant difference in heat sensitivity was noted between Cx43/Cx30 KO mice and their littermate controls (P > 0.1). However after partial motor recovery at 4 weeks, heat nociceptive threshold levels (withdrawal latencies) were significantly higher in CX43/Cx30 KO mice when compared with littermate controls (Fig. 4B). At the last time point evaluated (2 months after SCI), paw withdrawal latency decreased from 13.03 ± 0.60 to 3.22 ± 0.54 s in littermate controls (P < 0.05), but only decreased from 12.93 ± 0.85 to 9.88 ± 0.66 s in Cx43/Cx30 KO mice (P > 0.1; Fig. 4B).
Minocycline Moderately Reduces Neuropathic Pain Following SCI Less Efficiently than Deletion of Cx43/Cx30
The effect of minocycline, an anti-inflammatory agent and also a microglia inhibitor, on the development of neuropathic pain following SCI was next evaluated (Hains and Waxman,2006; Marchand et al.,2005). A standard regimen consisting of daily injections of 0.3-mL minocycline (50 mg/kg, i.p) or saline (control littermates) were given to C57/Bl wild-type animals for 5 consecutive days following SCI (Tan et al.,2009). Weekly analysis of heat hyperalgesia and mechanical allodynia in the first 3 weeks after mild SCI showed no effects of minocycline on these neuropathic pain behaviors (Fig. 5A,B). However, after 4 weeks, or upon the recovery of motor function (defined as BMS score of >4), the animals treated with minocycline exhibited significantly less mechanical allodynia (P < 0.05; Fig. 5A) and heat hyperalgesia (P <0.01; Fig. 5B).
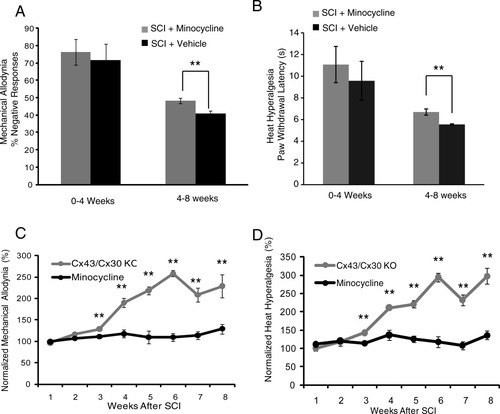
Minocycline also reduces the development of neuropathic pain symptoms after mild spinal cord injury, but less efficiently than deletion of Cx43. (A) Bar histogram shows the effects of minocycline on mechanical allodynia. Significant attenuation of mechanical allodynia was observed 4–8 weeks after spinal cord injury in mice receiving minocycline compared with vehicle controls exposed to the same injury (**P < 0.05, ANOVA, n = 12) (B) Bar histogram shows the effects of minocycline on the development of heat hyperalgesia. Heat hyperalgesia was significantly reduced in mice receiving minocycline 4–8 weeks after spinal cord injury compared with vehicle controls (**P < 0.05, ANOVA, n = 12, mean ± SEM). (C and D) Graph comparing the analgesic effects of minocycline versus deletion of Cx43/Cx30. Both set of data was normalized to littermate controls exposed to the same injury. Deletion of Cx43/Cx30 leads to a significantly greater reduction of mechanical allodynia and heat thermal hyperalgesia after spinal cord injury (P < 0.01, ANOVA, n = 6).
Deletion of Cx43/Cx30 more robustly reduced both mechanical allodynia and heat hyperalgesia than minocycline (P < 0.01; Fig. 5C,D). A comparison of the efficacy by which the two manipulations reduced allodynia showed that deletion of Cx43/Cx30 consistently reduced allodynia more than minocycline treatment. The comparison was performed after normalization of the hyperalgesia and allodynia scores to corresponding controls. Although minocycline reduced mechanical allodynia (% of response) by 1–30%, after being normalized to corresponding vehicle control, Cx43/Cx30 deletion reduced mechanical allodynia (% of response) by 1–200%, after being normalized to littermate control (Fig. 5C). Moreover, minocycline only changed heat hyperalgesia (paw withdrawal latency) by 1–40%, while Cx43/Cx30 deletion changed heat hyperalgesia by 1–300%, after being normalized to corresponding controls (Fig. 5D). This suggests that targeting Cx43/Cx30 hemichannel/gap junctions is more effective in reducing the development of neuropathic pain than administration of minocycline, a traditional anti-inflammatory agent (Fig. 5C,D).
Deletion of Cx43/Cx30 Reduces the Severity of Reactive Gliosis in the Injured Spinal Cord
To evaluate the role of Cx43/Cx30 KO in reactive gliosis, immunohistochemistry was next used to quantify GFAP expression in the peritraumatic areas at various time points after SCI. Immunolabeling revealed that 3-day post-SCI littermate control mice exhibited significantly higher levels of GFAP immunoreactivity in the peritraumatic regions in contrast to Cx43/Cx30 KO controls (P < 0.05, Student's t-test). GFAP expression peaked 7 days post-SCI with littermate controls mice exhibiting significantly higher GFAP expression than Cx43/Cx30 KO mice (P < 0.05, Student's t-test). GFAP expression began to decline from the peak by 1-month post-SCI. However, littermate controls continued to show increased GFAP expression than Cx43/Cx30 KO (P < 0.05, Student's t-test; Fig. 6A,B). Thus, GFAP expression in the peritraumatic areas was significantly reduced in Cx43/Cx30 KO mice, suggesting that Cx43 play a role in astrogliosis after SCI. In addition to GFAP, slices were stained for Iba1. Littermate controls exhibited significantly higher fluorescent intensity of Iba1 when compared with Cx43/Cx30 KO mice. Iba1 intensity peaked 3-day post-SCI and declined by 1-month post-SCI (data not shown). This is consistent with the literature as only GFAP expression remains elevated 6–8 weeks after SCI (Peng et al.,2009). Other markers of inflammation, including CD68 (microglia activation), MPO (neutrophils), and CD8 (cytotoxic T-lymphocytes) normalized to preinjury level within a few weeks after traumatic injury of the spinal cord (data not shown).
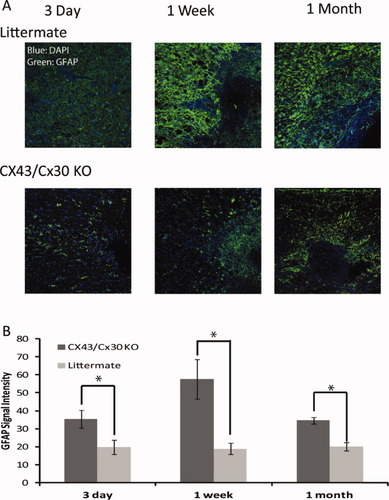
Deletion of Cx43 reduced astrogliosis after mild spinal cord injury. (A) Representative confocal images of longitudinal sections of spinal cord at site of injury 3 day, 1 week, and 1 month after the traumatic event. The sections were immunolabeled against GFAP (Top: littermate controls, Bottom: Cx43/Cx30 KO). Blue: DAPI; Green: GFAP. (B) Quantitative analysis of immunofluorescence intensity of GFAP in Cx43/Cx30 KO and littermate controls. Deletion of Cx43 reduced GFAP immunolabeling after traumatic injury in Cx43/Cx30 KO compared with littermate controls, reflecting reduced gliosis (P < 0.05, ANOVA, n = 6). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Statistical Analysis
All data are plotted as mean ± SEM. Student's t-test (two-tailed, unpaired) and one-way ANOVA were used in all data comparison. Data were graphed using Adobe Illustrator and Adobe Photoshop.
DISCUSSION
The present study demonstrates, for the first time, that astrocytic Cx43 plays an essential role in the development of chronic neuropathic pain following SCI. Prior studies have demonstrated that ATP activation of spinal glia via P2X receptors represents a critical step in the development and facilitation of chronic pain (Di Virgilio et al.,2009; Ferrari et al.,2006; Milligan and Watkins,2009). A major driving force in this process consists of the release of proinflammatory molecules such as cytokines, which can sensitize neurons and exacerbate pathological plasticity following injury (Di Virgilio et al.,1999; Gwak and Hulsebosch,2011; Hughes et al.,2007). We propose that Cx43-mediated ATP release constitutes a critical upstream pathway that facilitates the development of chronic pain. Using a weight-drop injury model, we show that transgenic mice with Cx43/Cx30 deletion, exhibited significantly reduced neuropathic pain and reduced levels of gliosis, when compared with littermate controls and wild-type controls exposed to the same injury (Figs. 4 and 5). These findings, to date, appear to identify the furthest upstream step involved in purinergic-mediated chronic pain and, therefore, suggest that effective therapeutic targets may be found immediately downstream from ATP release.
In addition, increases in extracellular ATP have been documented in a wide range of peripheral and central nervous system injuries, such as sciatic nerve entrapment (Matsuka et al.,2008), traumatic brain injury (Davalos et al.,2005; Franke et al.,2006), and various models of SCI (Peng et al.,2009; Wang et al.,2004). Spinal astrocytes, which are in close contact with neuronal synapses, have been shown to respond to SCI-induced changes by releasing gliotransmittors and neuromodulators, such as ATP and glutamate (Thompson et al.,2006; Ye et al.,2003). More interestingly, peritraumatic regions have also been shown to exhibit ATP release, which lasts more than 6 h (Wang et al.,2004), suggesting the presence of a continuous release pathway from viable ATP-producing cells; one possible source being gap junction-coupled networks of astrocytes with an increased number of open hemichannels. Bioluminescent imaging of cells in culture, and two-photon imaging in vivo (Newman,2005) have shown that ATP release through connexin hemichannels triggers astrocytic activation via calcium waves (Bennett et al.,2003). These calcium waves have been implicated as a feedback mechanism in response to traumatic injury (Neary et al.,2003), and have been shown to trigger additional ATP release (Gallagher and Salter,2003; Scemes and Giaume,2006; Suadicani et al.,2004).
Furthermore, for several decades, ATP has been known to elicit pain responses when applied to the skin or peripheral nerves and, more recently, ATP was found to play a critical role in pain transmission, through its role as a fast neurotransmitter among nociceptive neurons in the dorsal horn (Brederson and Jarvis,2008; Chen and Gu,2005; Kennedy,2005). Extracellular ATP is also an important mediator of CNS inflammation, through action on glial P2 receptors (Gwak and Hulsebosch,2011; Hughes et al.,2007; Milligan and Watkins,2009). Although little is known about the role of P2Y metabotropic receptors in chronic pain (Kobayashi et al.,2008; Tozaki-Saitoh et al.,2008), the P2X subfamily of ATP receptors are heavily expressed on microglia and leukocytes (Collo et al.,1997) and, P2X7 in particular has been shown to facilitate the maturation and secretion of proinflammatory cytokines and other signaling molecules (e.g., ROS, IL-1, IL-6B, TNF, etc.) that contribute to neuronal sensitization and chronic pain (Di Virgilio et al.,2009; Ferrari et al.,2006; Minami et al.,2006). Additionally, blockade or deletion of P2X7 has been shown to decrease cytokine production and secretion (Gourine et al.,2005; Solle et al.,2001), attenuate neuropathic and inflammatory pain (Dell'Antonio et al.,2002a,b; Labasi et al.,2002; Sorge et al.,2012), and promote functional recovery following SCI (Peng et al.,2009; Wang et al.,2004). P2X4 has also been implicated in chronic pain, and its expression has been shown to upregulate following peripheral nerve injury, while pharmacological blockade and genetic deletion attenuated tactile allodynia (Tsuda et al.,2003, 2009). Cx43/Cx30 deletions were expected to inhibit microglial activation and, thereby, cytokine release, via preventing astrocytic release of ATP, which may also exert direct action on neuronal P2X receptors. Our finding that these deletions improved pain scores is, therefore, consistent with a microglial and cytokine-mediated model of chronic pain that is downstream from astrocytic ATP release.
Thus, we theorize that astrocytic hemichannels are one of the key sources of ATP after injury. Although it is true that, in addition to efflux from unopposed connexin hemichannels, vesicular exocytosis (Garre et al.,2010), opening of pannexin hemichannels (Iglesias et al.,2009), and release from P2X7R (Duan et al.,2003) have been proposed as possible candidates for ATP release, in a previous study, our lab used in vivo bioluminescent imaging to demonstrate a significant reduction in ATP release, in mice with Cx43 deletions, but not in littermate controls, following SCI (Huang et al.,2012). This finding demonstrated the crucial role Cx43 plays in SCI-induced ATP efflux. Additionally, considerable further evidence points to Cx43 as a likely candidate for ATP release since, (1) the inner pore diameter of Cx43 hemichannels is consistent with the passage of ATP, and Cx43 is shown to open under conditions of reduced extracellular Ca2+, ischemia, and metabolic strain (John et al.,1999; Kang et al.,2008; Retamal et al.,2007); (2) astrocytic upregulation of GFAP, which is representative of gliosis, correlates with upregulation of Cx43, at the site of the injury and in peritraumatic tissues, which also coincides with increased concentrations of extracellular ATP (Contreras et al.,2002; Retamal et al.,2007; Theriault et al.,1997); and (3) several studies have shown that injury-induced upregulation of Cx43 corresponds to a diminishing of normal gap junctional communication (Contreras et al.,2002; Garre et al.,2010). These observations are consistent with the hypothesis that upregulation of Cx43 corresponds to the addition of hemichannels capable of ATP efflux.
In addition to Cx43 hemichannels, Cx43 gap junctions could also play a key role in modulating chronic pain. Gap junctions allow for communication between cells via exchange of ions and small molecules that act as secondary messengers, such as Ca2+, NAD+, cAMP, IP3, ATP, glutamate, and glucose (Bennett et al.,2003; Evans et al.,2006). As they are highly expressed by spinal cord astrocytes, they are in an ideal location to modulate chronic pain following SCI. Hightened astrocyte–astrocyte communication following SCI, through gap junction networks, may result in extensive Ca2+ waves, and long range signaling via the release of ATP. This could directly excite nociceptive neurons, by binding to neuronal P2 receptors, in addition to causing the glial-mediated release of proinflammatory cytokines or pronociceptive molecules, such as ATP, prostaglandin or glutamate, in local or peritraumatic tissue (Evans et al.,2006; Stout et al.,2002; Wu et al.,2012). Furthermore, decoupling of gap junctions has been previously shown greatly reduced the concentrations of IL-1 and IL-6 in tissues and in the CSF, which as a result inhibited the development of mechanical allodynia and heat hyperalgesia after nerve injury (Spataro et al.,2004). In other studies, decoupling of gap junctions has been linked to a reduction in astrocytic activation in the spinal cord, which has also been shown to result in inhibition of mechanical allodynia and heat hyperalgesia (Roh et al.,2010). Although our current mouse model cannot dissect the roles of Cx43 hemichannels and Cx43 gap junctions, both likely have mechanisms that contribute to chronic pain.
As a measure of comparison, administration of minocycline, following a standard protocol (Tan et al.,2009), attenuated mechanical allodynia and heat hyperalgesia (Fig. 5A,B) but to a much lesser degree than deletion of Cx43/Cx30 (Fig. 5C,D). In the past decade, minocycline, a tricyclic antibiotic, has been shown to attenuate inflammation and tissue loss following acute SCI, by preventing the activation of microglia (Ledeboer et al.,2005; Lee et al.,2003; Raghavendra et al.,2003). Although the mechanism for its action remains ill-defined, minocycline's anti-inflammatory effects have also been shown to attenuate chronic pain (Hua et al.,2005; Nie et al.,2010). Given that minocycline is thought to partially derive its anti-inflammatory and antinociceptive effects from a similar, albeit, downstream pathway from astrocytic ATP release, we sought to compare its antinociceptive effects with those from Cx43/Cx30 deletion. Our finding that treatment with a standard regimen of minocycline attenuated pain behaviors to a lesser degree than Cx43/Cx30 deletion, is consistent with a microglial-driven model of chronic pain downstream from astrocytic release of ATP. This finding also suggests that future therapeutic interventions, which target upstream events (i.e., ATP release or purinergic receptors), may be more efficient than those targeting more complicated downstream pathways, where redundancies in pain signaling are more likely to occur.
No significant difference was found in the BMS scoring, or in atrophy volume, between Cx43/Cx30 KO mice and littermate controls, and additionally atrophy volume did not correlate with reductions in chronic pain development. This finding is not surprising, since it is unlikely that initial tissue loss directly controls the development of chronic pain, which appears to be regulated by different processes, such as long-term glial-neuron interactions or the formation of aberrant nociceptive synapses, following nonlaminal-specific axonal regeneration (Tang et al.,2007). Follow-up studies on human SCI have also shown no correlation between injury completeness and chronic pain (Siddall et al.,2003) [82]. Although Cx43 inhibition, or expression interference, has been observed to promote tissue sparing (Cronin et al.,2008; O'Carroll et al.,2008), this may be indirectly due to the disruption of P2X-mediated cytokine release, or more directly due to the effective uncoupling of astrocytic networks. Regardless, it is important to note that Cx43 itself is not likely a potential target for neuroprotection, since (1) no BBB-permeable connexin inhibitors exist and all existing connexin inhibitors are nonspecific; and (2) Cx43 is widely expressed outside the CNS, especially in the heart (Rohr,2004). Better candidates for neuroprotection, as well as for chronic pain, are the recently described P2 receptor antagonists (Donnelly-Roberts and Jarvis,2007; Matasi et al.,2011), which are presently undergoing clinical trials for rheumatoid arthritis and chronic pain (Friedle et al.,2010).
Preliminary findings have also implicated P2Y6 and P2Y12 in the facilitation of chronic pain (Koizumi et al.,2007), although further investigations are needed to define the full extent of their involvement. P2Y receptors expressed by activated spinal cord microglia may contribute to microgliosis, since ATP is known to cause microgliosis in the spinal cord (Chen et al.,2010). Similarly, our data suggest that ATP release is further required for astrogliosis in the spinal cord and that gliosis could be associated with inflammatory mediator production and chronic pain states.
The present findings demonstrate that Cx43 gap junctions and hemichannels are critically involved in the development and maintenance of chronic neuropathic pain. Following acute SCI, heat hyperalgesia and mechanical allodynia were significantly attenuated in mice with Cx43/Cx30 deletions, and this attenuation was found to be greater than that of mice treated with minocycline. The antinociceptive effects of Cx43/Cx30 deletions are consistent with a reduction in ATP release, and thus, the disruption of downstream pathways previously implicated in chronic pain. Taken together, our finding demonstrates that chronic pain is strongly regulated by Cx43 gap junctions and hemichannels and those future therapeutic solutions might be made more effective by targeting downstream pathways, such as the ATP-induced activation of glial P2 receptors.
Acknowledgements
The authors thank Justin Chan for custom Matlab software and Klaus Willecke for providing transgenic mice. W.P. carried out the surgeries and analysis of the atrophy volume, whereas W.P., B.K., and M.C. collected behavioral data. K.M. carried out the immunohistological staining of the spinal cords. M.C., B.K., R.R.J., and M.N. conceived the study and drafted the manuscript.