Neuropathologic and neuroinflammatory activities of HIV-1-infected human astrocytes in murine brain
Abstract
The balance between astrocyte and microglia neuroprotection and neurotoxicity defines the tempo of neuronal dysfunction during HIV-1-associated dementia (HAD). Astrocytes maintain brain homeostasis and respond actively to brain damage by providing functional and nutritive neuronal support. In HAD, low-level, continuous infection of astrocytes occurs, but the functional consequences of thisinfection are poorly understood. To this end, human fetal astrocytes (HFA) and monocyte-derived macrophages (MDM) were infected with HIV-1DJV and HIV-1NL4-3 (neurotropic and lymphotropic strains respectively) and a pseudotyped Vesicular Stomatitis Virus (VSV/HIV-1NL4-3) prior to intracranial injection into the basal ganglia of severe combined immunodeficient mice. Neuropathological and immunohistochemical comparisons for inflammatory and neurotoxic activities were performed amongst the infected cell types at 7 or 14 days. HIV-1-infected MDM induced significant increases in Mac-1, glial fibrillary acidic protein, ionized calcium-binding adapter molecule 1, and proinflammatory cytokine RNA and/or protein expression when compared with HSV/HIV-1- and HIV-1-infected HFA and sham-operated mice. Levels of neuron-specific nuclear protein, microtubule-associated protein 2, and neurofilament antigens were reduced significantly in the brain regions injected with human MDM infected with HIV-1DJV or VSV/HIV-1. We conclude that HIV-1 infection of astrocytes leads to limited neurodegeneration, underscoring the early and active role of macrophage-driven neurotoxicity in disease. © 2006 Wiley-Liss, Inc.
INTRODUCTION
The neuropathogenesis of human immunodeficiency virus type 1 (HIV-1) infection was long thought to revolve solely around metabolic processes induced by virus-infected and activated mononuclear phagocytes (MP; perivascular macrophages and microglia). This is underscored by clinical manifestations that are insidious at onset and are manifested by cognitive, behavioral, and motor abnormalities and linked to the number of activated MP in affected brain tissues (Glass etal.,1995; Takahashi etal.,1996). Indeed, neurons are rarely infected, even in the later stages of HIV-1-associated neuronal injury. Astrocytes show only restricted infection, a common feature of pediatric disease (Saito etal.,1994; Takahashi etal.,1996; Tornatore etal.,1994).
Nonetheless, there is growing interest in the role of astrocytes in the neuropathogenesis of HIV-1 infection (Conant etal.,1998; Fischer-Smith and Rappaport,2005; Kang etal.,2005; Kim etal.2004; Messam and Major,2000; Power etal.,2002; Seth and Major,2005) for several reasons. First, astrocytes are the most frequent cell type of the brain and outnumber neurons by 10–50:1 (Brack-Werner,1999; Rutka etal.,1997). Second, activated astrocytes release a number of neurotransmitters and neurotrophins (Araque etal.,1998; Brack-Werner,1999; Conant etal.,1994; Dewhurst etal.,1987a,b; Gorry etal.,2003). Third, astroglial cells, once relegated to a simple supportive role in the CNS, carry out a plethora of functions and serve as indispensable partners to neurons, either in physiological or in pathological conditions. Fourth, accumulating evidence demonstrates that HIV-1 can infect CD4− astrocytes and serve as targets for virus in the infected human host (Araque etal.,1998; Parpura etal.,1994; Brack-Werner,1999; Conant etal.,1994; Dewhurst etal.,1987a,b; Gorry etal.,2003; Messam and Major,2000; Tornatore etal.,1994; Wang etal.,2004). Compared with CD4+ T lymphocytes and macrophages, low-level restricted virus replication occurs after initial astrocyte HIV-1 exposure, as the cells demonstrate prolonged expression of multiple spliced HIV-1 mRNA (Di Rienzo etal.,1998; Gorry etal.,1998; Ong etal.,2005). Astrocytes can also express Nef, Rev, and other viral proteins at low levels and can be induced to secrete infectious progeny virus for prolonged time periods (Gorry etal.,2003; Ong etal.,2005). Restricted astrocyte infection is found throughout the neuropil at frequencies of up to 1% and can increase with progressive disease severity (Tornatore etal.,1994). Fifth, laboratory studies indicate that exposure of astrocytes to progeny HIV-1 or HIV-1 gp120 induces significant physiological changes in the cells, which include global dysregulation of many cellular genes, (Galey etal.,2003; Su etal.,2002,2003; Wang etal.,2003,2004), induction of apoptosis-related proteins (Deshpande etal.,2005; Ghorpade etal.,2003), and impairment of glutamate uptake and release (Bezzi etal.,2004; Danbolt,2001; Wang etal.,2003). Such changes, if they occur in vivo, can impair the essential cell functions and contribute to the death of neurons.
In this study, we investigated how HIV-1 infection of primary human astrocytes may affect disease using approaches analogous to what we previously used to analyze the role of macrophages in virus-associated neurodegeneration. Native and pseudotyped HIV-1 was used for infection, followed by direct injection of cells into brains of immunodeficient mice. The lack of the CD4 receptor on astrocytes was circumvented through the use of HIV-1 pseudotyped with Vesicular Stomatitis Virus (VSV)-G protein (Canki etal.,2001; Kim etal.,2004). Natural infection was reflected by direct virus exposure of astrocytes to brain-derived (DJV) and lymphotropic (NL4-3) strains. After VSV/HIV-1NL4-3 infection, the majority of astrocytes expressed HIV-1 antigens and secreted large amounts of infectious progeny virus (Canki etal.,2001). HIV-1DJV and HIV-1NL4-3 infection produced no progeny virus. Both systems permitted the direct investigation of neuropathologic outcomes of infected astrocytes versus monocyte-derived macrophages (MDM). Both VSV/HIV-1- and HIV-1-infected astrocytes elicited limited pathogenesis in brains of severe combined immunodeficient mice when compared with similarly infected MDM, independent of the levels of viral infection. Significant neurodegeneration was observed only in MDM-injected animals. This paralleled the levels of proinflammatory cytokine gene expression and murine microglial and astrocytes activation, but not growth or antiinflammatory markers. These data, taken together, demonstrate the relative importance of the virus, the astrocyte, and the macrophage in HIV-1-associated neurodegeneration.
MATERIALS AND METHODS
Cells
Human fetal astrocytes (HFA) were isolated from second trimester (14–19 weeks of gestational age) human fetal brains obtained from elective abortions in full compliance with the ethical guidelines of both the National Institutes of Health (NIH) and the University of Nebraska Medical Center, as previously described (Bencheikh etal.,1999; Canki etal.,2001; Ghorpade etal.,2003). Homogenous preparations of astrocytes were obtained using high-density culture conditions in the absence of growth factors in Dulbecco's Modified Eagle's Medium F-12 (DMEM) (GIBCO-Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS). Cells were maintained in this medium at 2–5 × 104 cells/cm2. Antibodies to glial fibrillary acidic protein (GFAP) (Dako, Carpinteria, CA) were assessed with >99% pure astrocytes.
Monocytes were purified after leukopheresis of HIV-1, 2, and hepatitis B seronegative donors by countercurrent centrifugal elutriation (Gendelman etal., 1988). Cells were cultured in DMEM with 1,000 U/mL highly purified recombinant human macrophage colony stimulating factor (MCSF) (a generous gift from Wyeth, Cambridge, MA).
HIV-1 and Envelope Expression Vectors
The HIV-1 molecular clone NL4-3 used expresses all HIV-1 proteins (Adachi etal.,1986). NL-P1, a NL4-3 derivative, carries the marker gene human placental alkaline phosphatase inserted next to Nef (Collins etal.,1998). The VSV G expression vector pL-VSV-G was obtained from M. Emerman and contains a VSV G insert in the pcDNA expression vector. This was modified by replacing the cytomegalovirus promoter with the HIV-1 long terminal repeat (Bartz etal.,1996). To generate pseudotyped virus, 1.5 × 106 293 T cells cultured in 10-cm plates were cotransfected with 10 μg of HIV-1 recombinant DNA and 15 μg of VSV. The VSV-HIV-1 viruses were stored at 80°C until use. HIV-1DJV was isolated in the same manner as stated elsewhere (Ghorpade etal.,1998; Heinzinger etal.,1995).
HIV-1 Strains and Viral Infection
HFA and MDM were infected overnight with viruses VSV/HIV-1NL4-3, HIV-1NL4-3, or HIV-1DJV at a dilution of 1:10 (virus stock/medium) and washed twice with Hank's Basic Salt Solution (HBSS; GIBCO-BRL). The DJV macrophage-tropic and CCR5 utilizing strain of HIV-1 was obtained from pathologic human brain tissue of an HIV-1-infected patient who died of dementia associated with progressive viral infection. Infected microglia were isolated and virus was recovered after co-cultivation with MDM (Ghorpade etal.,1998).
Severe Combined Immunodeficient Mice
Four-week-old male C.B-17/IcrCrl-SCID (severe combined immunodeficient) mice were purchased from Charles River Laboratory (Wilmington, WA). Animals were maintained in sterile micro isolator cages. Cells (5 × 105 cells in 5 μL) were injected intracranially (i.c.) on day 1 after viral infection. All experiments were performed with six mice per group. Each group included sham-operated, uninfected (control) HFA and MDM, and VSV/HIV-1NL4-3 or HIV-1 (DJV or NL4-3) infected HFA and MDM. All animals were killed at either 7 or 14 days for neuropathological tests.
Histopathology and Image Analysis
Brain tissue was collected and fixed with 4% phosphate-buffered paraformaldehyde and embedded in paraffin. For each mouse, 30–100 serial (5 μm thick) sections were prepared for future staining. Antibodies to vimentin (Vim) intermediate filaments (clone 3B4, Dako) were used to detect human cells (HFA and MDM) in mouse brain. Murine microglia were identified using rabbit polyclonal antibodies to ionized calcium-binding adapter molecule 1 (Iba-1, 1:500, Wako, Richmond, VA). Astrocytes were identified using antibodies to GFAP (1:2,000). Neuron-specific nuclear protein (NeuN), microtubule-associated protein 2 (MAP-2, 1:1,000), and heavy chain (200 kDa) neurofilament (NF, 1:200) antigens (Dako) detected neurons. Antibodies to HIV-1 p24 (1:10, Dako) were used to determine the number of HIV-1 infected cells. All paraffin-embedded sections were counterstained with Mayer's hematoxylin. Quantification of immunostaining was performed using Image-Pro Plus, v. 4.0, on serial coronal brain sections as performed in our previous studies (Dou etal.,2003,2005; Poluektova etal.,2002). Twenty-four photos (four-photos/two sections/mouse for six mice/group) were quantified. Each antibody's expression was quantified by determining the positive area (index) as a percentage of the total image area per microscopy field (for example % GFAP and % Iba-1) and calculated for a 0.5-mm window of tissue immediately surrounding the injection site.
Real-Time PCR for Gene Expression
Brain sections (2 mm thick), which included the injection line, were collected for RNA isolation. The corresponding section of the contralateral hemisphere was used for comparison. Total RNA was extracted by the TRIzol method (Invitrogen). RNA was reverse transcribed with random hexamers, and real-time quantitative PCR was performed with cDNA using an ABI PRISM 7,000 sequence detector (Applied Biosystems, Foster City, CA). The primer pairs used were as follows: macrophage adhesion molecule (Mac-1), 5′-GCCAATGCAACAGGTGCATAT-3′ and 5′-CACACATCGGTGGCTGGTAG-3′; interleukin 1β (IL-1β), 5′-CACCCCGACTGAAGGTGACT-3′ and 5′-CTGTTCCAGAAGCGCCATTAA-3′; inducible nitric oxide synthase (iNOS), 5′-GGCAGCCTGTGAGACCTTTG-3′ and 5′-GAAGCGTTTCGGGATCTGAA-3′; brain-derived neurotrophic factor (BDNF), 5′-CTGCAGCCTTCCTTGGTGTAA-3′ and 5′-CCAAAGGCCAACTGAAGCA-3′; IL-10, 5′-GTTGCCAAGCCTTATCGGAA-3′ and 5′-CCAGGGAATTCAAATGCTCCT-3′. Primers and probe for tumor necrosis factor-α (TNF-α) were obtained from Applied Biosystems (Assays-on-demand). Level of HIV RNA was analyzed as described previously (Dou etal.,2003). A SYBR Green I detection system or Taqman system was used, and the gene expression was normalized to glycerylaldehyde phosphate dehydrogenase (GAPDH), which served as an endogenous control. All PCR reagents were obtained from Applied Biosystems.
Statistical Analysis
One-way analysis of variance was used to compare the treatment groups for each of the outcomes. Residual plots were examined to check whether the model assumptions were met. If the model assumptions were not met, then the log10 or square root transformation was applied to help with model fit. If the overall F-test was statistically significant, then pairwise comparisons were conducted, with adjustments made for multiple comparisons, using Tukey's method. P values less than 0.05 were considered to be statistically significant.
RESULTS
VSV/HIV-1NL4-3-Infection of HFA and MDM
VSV/HIV-1NL4-3 G envelope infection resulted in easily demonstrable HIV-1 infection and continuous viral replication in HFA. HFA were >99% GFAP positive. VSV/HIV-1NL4-3 infected (Figs. 1B,C) and uninfected (Fig. 1A) HFA were examined by immunostaining. HIV-1 p24 antigens showed robust viral protein expression (Figs. 1B,C, brown) in astroctyes. HIV-1p24 antigen was present in 55% of cells 7 days after viral infection. HIV-1 p24 labeled proteins were present within the cytoplasm and seen as “bleb” in cell processes (Figs. 1B,C, arrowhead). VSV/HIV-1 HFA infection induced limited cell loss but led to enlargements of astrocyte processes and reactive gliosis.
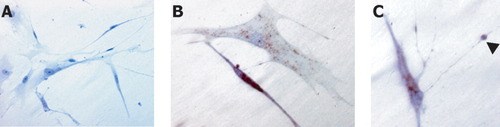
VSV/HIV-1 p24 antigens expressed on HFA after VSV/HIV-1NL4-3 infection. HFA were infected with 1 pg of VSV/HIV-1NL4-3 and immunostained at day 7 after cell injection. Uninfected HFA were used as the control (A). Infected HFA were determined by immunostaining with HIV-1 p24 antibodies (B and C, brown). Viral antigens were expressed within the cytoplasm and cell processes. HIV-1 p24 positive swelling (C, arrowhead) was observed within the processes. Magnification, ×400.
To determine the functional consequences of HIV-1-infected HFA, we injected infected and control cells into the basal ganglia of SCID mice. In mice injected with HIV-1 uninfected and infected HFA, prominent staining of GFAP (Fig. 2A, green) and Vim (Fig. 2A, red) was observed in injected brain regions. Astrogliosis was induced following human astrocyte injection on days 7 and 14 (Fig. 2A). Double-stained brain sections for human-specific Vim (for human cells) and GFAP (for both HFA and mice astrocytes) showed parallel distributions over time for both infected and uninfected HFA.
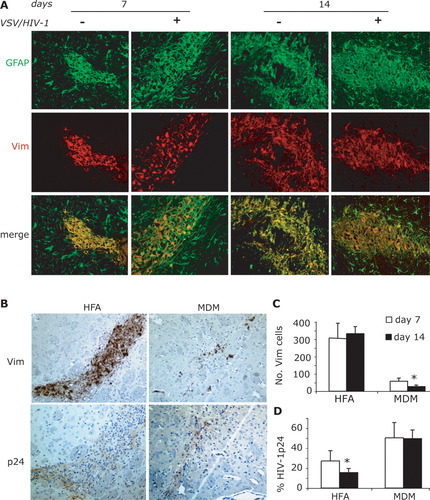
Number of HFA and MDM in brains of SCID mice. Immunohistochemistry was performed with antibodies to GFAP (green, human and mouse astrocytes) and Vim (red, HFA). Vim and GFAP double-labeled HFA are shown in the injected hemisphere at days 7 and 14 (A). Serial 5-μm brain sections were stained with antibodies to Vim and HIV-1 p24 (B). Differences in number of Vim-positive human cells between VSV/HIV-1 infected HFA and MDM are shown in (C). The levels of HIV-1 infection were analyzed by percentage of virus-positive cells (HIV-1 p24+/Vim+) at 7 and 14 days (D). Magnification, ×200, *P < 0.05.
Number and levels of infection of HFA and MDM in SCID mice were determined by immunostaining of brain tissue sections 7 and 14 days after cell injection. Serial 5-μm brain sections were cut through the needle track, then stained with antibodies to Vim and HIV-1 p24 (Fig. 2B). Decreased number of MDM was seen when compared with HFA (Fig. 2C, P < 0.01). The number of MDM was 55.14 ± 9.6 at day 7 and reduced to 28.14 ± 6.5 at day 14 (Fig. 2C, P < 0.02). In contrast, increased number of HFA was observed at day 14 when compared with day 7 (Figs. 2B,C). Nonetheless, infected HIV-1 p24 immunopositive HFA decreased from 27.23% ± 10.27% at day 7 to 15.55% ± 3.9% at day 14 (HIV-1 p24/Vim immunopositive cells) (Fig. 2C, P < 0.05). In contrast, the ratio of HIV-1 p24 immunopositive MDM (HIV-1p24/Vim) at days 7 (51.85% ± 14.56%) and 14 (48.86% ± 8.68%) was significantly higher than HFA at day 14 (Fig. 2D).
VSV/HIV-1 Infection of HFA and MDM and Neurotoxicity
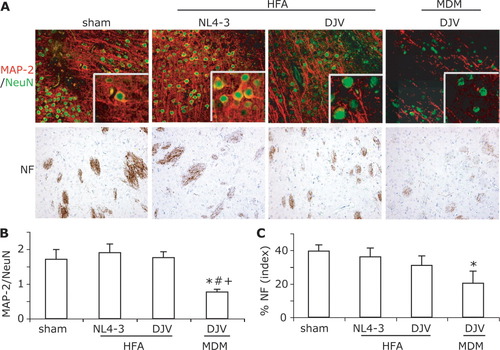
To examine changes in neurotoxicity seen in brains after MDM and HFA cell injection, immunostaining for MAP-2 (dendrites), NeuN (neurons), and NF (axon) were performed in areas of pathology (Fig. 3A). In sham-operated injected brains, MAP-2 immunopositive staining was localized in neuronal perikarya, proximal dendrites, and neuropil. No reductions in MAP-2 immunoreactivity were visually discernible around the injection line in sham-operated mice. Brain regions with injected cells resulted in demarcated reductions in MAP-2 immunostaining. In the regions surrounding HIV-1 infected HFA and MDM, quantitative measurements of MAP-2 expression (immunopositive region of MAP-2/NeuN staining as a percent of total brain region examined) were analyzed at day 14 after injection (Fig. 3B). Diminished MAP-2 staining was observed in brains injected with VSV/HIV-1 infected MDM when compared with that in sham-operated (P < 0.05) and control HFA (P < 0.05) and MDM (P < 0.05) groups. No significantly decreasing levels of MAP-2 were shown in VSV/HIV-1 infected HFA treated mice when compared with all experimental groups. Quantitative immunostaining for NF showed significant neuronal losses in brains of mice injected with VSV/HIV-1 infected MDM when compared with that in sham-operated (P < 0.01) and control HFA (P < 0.01) and MDM (P < 0.05) groups (Fig. 3C). Minimal, but detectable, neuronal degeneration was present in brains of mice injected with VSV/HIV-1 infected HFA when compared with sham-operated mice (P < 0.05). In and around the needle track area, VSV/HIV-1 infected HFA showed low levels of MAP-2, NeuN, and NF staining. However, neuronal loss was prominently demonstrated by an assay of NF antigens. In contrast, mice injected with VSV/HIV-1 infected MDM showed extensive losses of neuronal nuclei, dendrites, and synaptic clefts.
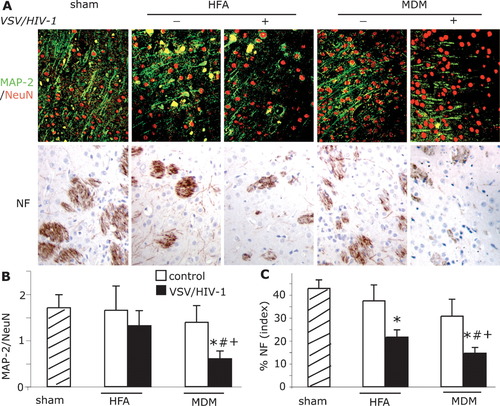
Neurotoxicity of VSV/HIV-1 infected MDM and HFA in SCID mouse brains. Uninfected HFA and MDM served as controls. Serial 5-μm paraffin-embedded brain tissue sections were stained with antibodies to MAP-2 (green), NeuN (red), and NF (brown) (A). Quantitative image was used to analyze immunostaining of MAP-2 dendrites/NeuN (B), and NF (C). Magnification, ×200. *denotes statistical differences when compared with sham-operated mice; #denotes statistical difference when compared with control HFA group; + denotes statistical difference when compared with control MDM mice.
HIV-1 Infection of HFA and MDM
To investigate the effects of infected astrocytes and macrophages in a “natural” setting, infection with HIV-1DJV and HIV-1NL4-3 were used. Immunostaining with Vim (Fig. 4A, red) and HIV-1 p24 (Fig. 4A, green) were performed in HFA and MDM (Fig. 4A). In laboratory studies, neither viral strain resulted in productive HFA replication. HFA infection by HIV-1NL4-3 and the neurotrophic HIV-1DJV strain led to abortive outcomes (data not shown). In contrast, similar number of human MDM exposed to the DJV strain of virus led to the formation of typical cytopathicity and the formation of multinucleated giant cells (Fig. 4A). Neither cytopathicity norvirus production was observed in MDM exposed to NL4-3 (data not shown).
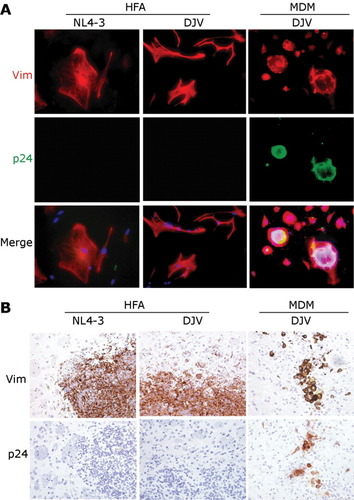
Cellular HIV-1 p24 levels in viral infected HFA and MDM. After cell exposure with 1 pg of HIV-1NL4-3 or HIV-1DJV, cultures were immunostained by Vim (red) and HIV-1 p24 (green) (A). Serial 5-μm brain sections were stained with antibodies to Vim and HIV-1 p24 (B). In all cases, Vim stained HFA and MDM were located in injection sites, including basal ganglia, caudate, and cortex. Magnification is ×400 (A) and ×200 (B).
HIV-1 Infection of HFA and MDM and Neurotoxicity
Following injection of HIV-1DJV and HIV-1NL4-3 infected HFA and/or MDM into the basal ganglia of SCID mice, serial 5-μm brain sections were cut and examined by immunohistochemistry. HFA and MDM were determined by counting the number of Vim-positive cells (Fig. 4B). The number of HIV-1 p24 positive MDM was readily detected in mice injected with infected MDM but not similarly infected HFA. SCID mice were injected with HIV-1DJV infected HFA or MDM and then killed at day 14. The HIV-1NL4-3 was used for comparison in the earlier VSV/ HIV-1NL4-3 studies. The neuronal perikarya, proximal dendrites, and neuropil were stained with antibodies to MAP-2 (Fig. 5A, red) and NeuN (Fig. 5A, green). Axons were stained with antibodies to NF (Fig. 5A, brown). Visual examination of immunofluorescent-stained sections showed that HIV-1DJV infected MDM significantly reduced the number of neurons and their dendritic processes in the basal ganglia and cortex when compared with infected HFA and sham-operated mice. Quantitative image analysis of MAP-2 expression (MAP-2/NeuN immunostained areas as a percent of total brain areas scanned) showed significant losses in mice injected with HIV-1 infected MDM when compared with sham (P < 0.05) and HIV-1DJV (P< 0.05) and HIV-1NL4-3 (P < 0.05) infected HFA treated animals (Figs. 5B,C). Neurotoxicity observed in brains of mice injected with HIV-1 (both DJV and NL4-3) infected HFA was not significantly different from that in sham-operated animals at day 14. Diminished NF antigen expression was detected in mice injected with HIV-1DJV infected MDM when compared with that in sham animals (P < 0.05). Mice injected with HIV-1 infected MDM showed dramatic neurodegeneration with typical pathological features of encephalitis reminiscent of human disease, including reactive astrocytosis, microgliosis, and formation of multinucleated giant cells. The areas of HIV-1 infected MDM-induced neuronal injury extended beyond the presence of human cells and were found throughout the injection site.
Neurotoxicity of HIV-1 infected MDM and HFA in SCID mouse brains. Serial 5-μm paraffin-embedded brain tissue sections were stained with antibodies to MAP-2 (green), NeuN (red), and NF (brown) (A). Quantitative image analysis was performed with the positive staining of MAP-2 dendrites/NeuN (B) and NF (C). Magnification, ×200. *denotes statistical differences when compared with sham-operated mice; # denotes a statistical difference when compared with HIV-1NL4-3 HFA mice; + denotes statistical difference when compared with HIV-1DJV HFA mice.
VSV/HIV-1 or HIV-1 Infection of HFA and MDM and Murine Astrocyte and Microglial Responses
Astrocytosis and microglia activation were observed in brains of mice injected with VSV/HIV-1 infected HFA or MDM. Serial 5-μm brain tissue sections from mice injected with uninfected HFA, and VSV/HIV-1 infected HFA and MDM were cut and double immunolabeled for Vim (red), GFAP (green), and Iba-1 (green) (Fig. 6A). Increased GFAP staining was observed within hours after cell injection (data not shown). Injection of VSV/HIV-1 infected human MDM into SCID mice showed formation of multinucleated giant cells, astrocytosis, and microgliosis similar to those seen with native infection (Dou etal.,2003,2005; Persidsky etal.,1995). Typically, hypertrophy of reactive mouse astrocytes (green) surrounding the site of cell injection was observed in brain regions containing uninfected and infected HFA (yellow) and MDM (red). Murine microglia were identified by Iba-1 (Fig. 6A). Microglial responses were detected in and around Vim (red) immunopositive HFA and MDM (double labeling to Iba-1 and Vim, yellow).
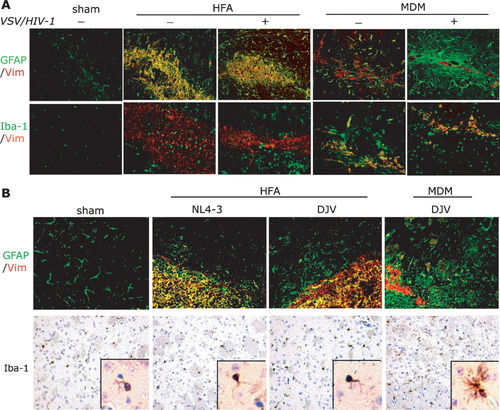
Neuroinflammatory phenotypes of VSV/HIV-1 infected HFA and MDM in SCID mouse brains. Immunohistochemical analyses were performed from 5-μm brain tissue sections stained with antibodies to GFAP/Vim and Iba-1/Vim. These sections were derived from sham-operated, control (uninfected) HFA and MDM, and viral infected HFA and MDM. Morphological analyses showed that GFAP and Iba-1 labeled cells surrounded HFA (yellow) and Vim (red) MDM in the brain sections with VSV/HIV-1 infection at day 14 (A). In native virus HIV-1NL4-3 and HIV-1DJV infected HFA and MDM in SCID mouse brains, immunohistochemical analyses of GFAP (green) and Vim (red), and Iba-1 (brown) were performed at day 14 (B). Widespread astrogliosis (green) was seen surrounding the injection site in infected MDM mice. Viral infected HFA and MDM groups showed higher activation of microglia. Activated phenotype of microglia occurred in infected MDM mice (B). Magnification, ×200.
Immune responses to HIV-1NL-4-3 and HIV-1DJV infection to HFA and MDM were determined with antibodies to Vim (red), GFAP (green), and Iba-1 (brown) (Fig. 6B). Activation of murine astrocytes (GFAP) and microglia (Iba-1) was found associated with HFA (double labeling to Vim and GFAP as yellow) and MDM (Vim+, red) (Fig. 6B). We observed GFAP-positive activated astrocytes clustered extensively around injection sites (Fig. 6B). On GFAP immunostained sections, activation of astrocytes only occurred near the injection site of animals that received native virus treated HFA and uninfected MDM. HIV-1DJV infected MDM caused extensive astrogliosis and microglial reactions, which was widespread in the injected hemisphere (Fig. 6B). Low levels of Iba-1 expression and nonreactive microglia were observed surrounding the injection lines in sham-operated animals.
Quantitative immunostaining showed changes in GFAP staining in mice injected with VSV/HIV-1, HIV-1NL4-3 and HIV-1DJV infected and uninfected HFA and MDM (Fig. 7A). At day 14, increased numbers of murine astrocyte responses followed VSV/HIV-1 infected HFA injection when compared with sham-operated animals (P < 0.05). In contrast, astrogliosis was significantly increased in animals injected with VSV/HIV-1 infected MDM than insham-operated (P < 0.01), HIV-1DJV infected HFA (P < 0.05), and HIV-1NL4-3 infected HFA (P < 0.01) groups. However, uninfected MDM and HFA injected mice did not showed significantly high levels of GFAP expression when compared with sham-operated animals.
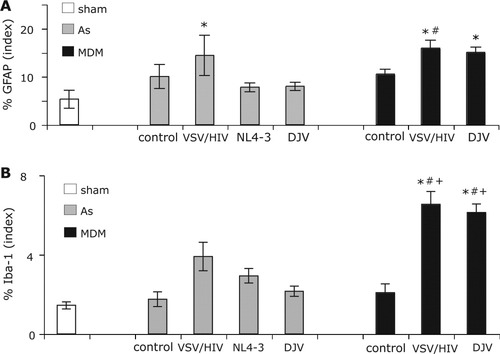
Quantitative immunohistochemistry was used to analyze signs of astrocyte and microglial activation. The levels of GFAP (A) and Iba-1 (B) expression were analyzed by quantitative immunostaining. *denotes statistical difference when compared with sham-operated mice; #denotesstatisticaldifferences when compared with HFADJV and HFANL4-3 mice; + denotes a statistical difference when compared with control HFA and MDM groups.
Murine microglia were identified by immunostaining with antibodies to Iba-1. Microglial responses were detected in and around Vim immunopositive HFA and MDM. Quantitative image analysis of Iba-1 (positive area/field of examination) showed no difference between infected and uninfected HFA injected groups at day 14 (Fig. 7B). Moreover, Iba-1 expression in infected HFA groups also was not statistically significant when compared with that in sham-operated animals. In mice injected with VSV/HIV-1 and native HIV-1 infected MDM, the number of Iba-1 stained cells increased significantly when compared with native HIV-1 infected HFA groups (P < 0.05 and P < 0.05), control HFA (P < 0.01 and P < 0.01) and MDM (P < 0.05), and sham-operated (P < 0.01 and P < 0.01) groups at day 14 (Fig. 7B). Moreover, there was no difference between the two viral infected MDM groups. Analysis of the injection area showed that the sham-operated and HFA groups had a slightly increased the Iba-1 immunostaining when compared with control hemisphere (data not show).
VSV/HIV-1 or HIV-1 Infection of HFA and MDM and Glial Inflammatory Gene Expression
RNA levels of Mac-1, TNF-α, IL-1β, and neurotrophins were analyzed in SCID mice injected with HIV-1 or VSV/HIV-1 infected HFA and MDM (Fig. 8). Real-time PCR analysis for Mac-1, a cell-surface receptor for a complement that is upregulated by activated microglia, showed significant increases in the brains of HIV-1 infected MDM injected mice when compared with replicate brains injected with HIV-1 infected HFA (P < 0.05) and also with sham and uninfected HFA groups (P < 0.01) (Fig. 8). These results are consistent with the morphological observations. In addition, RNA levels of proinflammatory cytokines, including TNF-α and IL-1β, were significantly increased (P < 0.01) in HIV-1 infected MDM injected mice than in all experimental groups. Incontrast, HFA injected mice, with HIV-1 treatment and VSV/HIV-1 infection, showed no significant changes in the levels of TNF-α and IL-1β when compared withsham-operated animals. Levels of iNOS were found to be increased in HIV-1 infected MDM injected animals than in sham-operated (P < 0.01), HIV-1 infected (P < 0.01) and VSV/HIV-1 infected HFA (P < 0.05) injected mice. The levels of antiinflammatory cytokine IL-10 andBDNF were similar in all animal groups (P > 0.05) (Fig. 8).
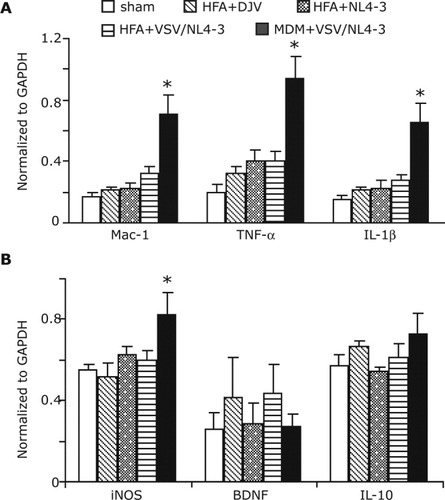
Real-time PCR for proinflammatory cytokines and iNOS in brain tissues of SCID mice. RNA was isolated from HIV-1 HFA or HIV-1 MDM injected brains of SCID mice, then analyzed for Mac-1 (CD11b/CD18), TNF-α and IL-1β (proinflammatory cytokines), iNOS, BDNF (neurotrophins), and IL-10 (antiinflammatory cytokine) expression by quantitative real-time PCR. Gene expression normalized to GAPDH was used as a control. Results are expressed as mean ± SEM of four animals. *denotes statistical differences when compared with sham-operated, VSV/HIV-1-HFA, HIV-1DJV HFA, and HIV-1NL-4-3 HFA injected mice.
DISCUSSION
HIV-1 associated cognitive impairments occur late in the course of disease and are linked to progressive immune suppression. A pathological correlate of disease is amultinucleated giant cell encephalitis characterized by macrophage brain infiltration, astrogliosis, myelin pallor, and neuronal dropout (Navia etal.,1986). Productive HIV-1 replication occurs exclusively in brain MP (Gartner etal.,1986; Koenig etal.,1986; Wiley etal.,1986). However, infection or interactions between progeny HIV-1 and viral proteins with astrocytes may also play prominent roles in disease (Hao and Lyman,1999; Ma etal.,1994). Astrocytes are essential for brain homeostasis and play critical roles in neuronal function and survival by (1) serving as structural and functional components of glial–neuronal networks, (2) maintaining low levels of extracellular glutamate and thereby preventing excitotoxicity, (3) controlling synaptogenesis and synaptic integrity, (4) contributing to neurogenesis (Araque etal.,1998; Gray and Patel,1995; Parpura etal.,1994), and (5) producing neurotrophic factors (Patel etal.,1996; Schwartz and Nishiyama,1994). Astrocytes are also among the first cells to respond directly to brain injury as a consequence of HIV-1 infection (He etal.,1997; Persidsky etal.,1996; Zheng etal.,1999). Their participation in the disease process can occur through alterations in the cells' innate immune function, via the Fas ligand, and by affecting direct mechanisms of neuronal destruction, such as glutamate uptake and excitotoxicity, or by neurotrophic factor withdrawal (Danbolt,2001; Ghorpade etal.,2003; Kaul etal.,2001; Lee etal.,2000; Oliet etal.,2001). Progeny virions or HIV-1 proteins can engage surface receptors on astrocytes and alter intracellular signaling and regulatory cell functions (Koller etal.,2002; Lee etal.,2003; Romero etal.,1998). Importantly, astrocytes stimulated by proinflammatory cytokines are a major source of chemokines, as well as other bioactive molecules, that can affect blood–brain barrier integrity, macrophage migration into the brain, and neuronal function (Epstein,1998; Ge and Pachter,2004). Astrogliosis is a prominent feature of early HIV-1 infection in the brain (Masliah etal.,1996), and likely serves in the capacity of maintaining neuroprotective functions during disease (Struzynska etal.,2001). Taken together, astrocyte dysfunction may seriously affect neuronal function and viability.
In the current report, a mouse model of HIV-1 infection was established for the evaluation of infected astrocytes in disease pathogenesis using implantation systems previously developed in our laboratories (Persidsky etal.,1996). To accomplish this task, restricted infection of astrocytes was overcome by utilizing HIV-1 pseudotyped with the VSV G envelope glycoprotein. VSV G protein mediates entry through an endocytic pathway permitting HIV-1 into cells (Canki etal.,2001; Stitz etal.,2001). Here, HIV-1NL4-3 was pseudotyped with VSV by co-transfection of intact NL4-3 DNA and a VSV envelope expression vector, yielding VSV/HIV-1NL4-3 (Canki etal.,2001; Kim etal.,2004). Astrocytes were susceptible to productive infection by VSV/HIV-1NL4-3 and revealed the morphology of productive viral infection, including production of p24 and Tat proteins, viral protease-mediated processing of Gag, appropriately spliced viral RNAs, and release of progeny virus. Thus, the membrane barriers to HIV-1 entry and replication in HFA were overcome to create a model for continuous astrocyte infection during disease (Wang etal.,2004).
Our study demonstrates that (1) pathological changes seen in SCID mice injected with HIV-1 infected macrophages, but not astrocytes, mirror features of human disease (Gendelman etal.,1994,1997,2003; Gray etal.,1996; Lawrence and Major,2002; Persidsky etal.,1995); (2) HFA remain viable in mouse brain tissue for periods of up to 14 days; (3) viral infection affects the morphology of astrocytes, but does not induce cytopathicity; (4) neuroinflammatory processes (murine microgliosis and astrogliosis) are induced, at low levels, by HFA; and (5) perhaps most importantly, neuronal damage is limited by infected HFA, when compared with macrophages, and observed despite demonstrable viral infection. This suggested that the cellular inflammatory or neurotoxic products generated during viral replication affect pathological outcomes and also that infected MDM are intrinsically more neurotoxic than infected HFA. Nonetheless, it should be emphasized that the current model is limited to “acute effects” of secretory products of implanted astrocytes and not on the long-term interactions between astrocytes and other cell types or astrocyte-interactions with progeny virions, soluble viral proteins, and other as yet undefined cellular processes to be determined. What is clear, though, is that the secreted products of infected HFA are significantly less neurotoxic than those of infected MDM and provide unique insights into cellular roles for disease. Future investigations on infecting native (murine) astrocytes, coupled with their long-term abilities to interact with, support, and buffer native neurons, need be addressed in other model systems from our laboratories (Potash etal.,2005).
It is now well accepted that both HIV-1 infected MP and astrocytes contribute to brain disease through secretion of neurotoxins and viral proteins (Dreyer etal.,1990; Jiang etal.,2001; Krebs etal.,2000; Smith etal.,2001; Westmoreland etal.,1996; Yoshioka etal.,1995). However, such toxic secretions are tightly regulated through the interactions between macrophages and astrocytes. Nonetheless, levels of infection in astrocytes and brain macrophages or microglia are distinct. How such low-level astrocyte infection alters the tempo of disease is not yet known, but is likely to revolve around its own innate immune properties as well as its abilities to affect neighboring macrophage and microglial functions and neurotoxic activities. One unique feature of astroctye infection is that it is likely to continue for long periods of time before the start of productive viral replication inMP.
A mouse model was developed from our previous works and reflects aspects of human HIV-1 associated neurodegeneration (Anderson etal.,2003; Limoges etal.,2000; Persidsky and Gendelman,2002; Persidsky etal.,1995,1996). Indeed, activated and HIV-1 infected MP contribute to brain disease through secretion of neurotoxins, such as arachidonic acid and its metabolites, platelet-activating factor, proinflammatory cytokines (TNF-α or IL-1β), quinolinic acid, NTox, and nitric oxide, as well as viral proteins (Dreyer etal.,1990; Jiang etal.,2001; Krebs etal.,2000; Smith etal.,2001; Westmoreland etal.,1996; Yoshioka etal.,1995). Interestingly, both HIV-1 infected MDM showed typical pathological features of HIV-1 infected brains, including significant astrogliosis, microgliosis, and neuronal injury. HIV-1 infected astrocytes may also affect neuronal damage (Brack-Werner,1999; Nath and Geiger,1998) but this is at much lower levels than that observed for macrophages.
Nonetheless, previous works showed that viral proteins (such as gp120, Tat, Rev, and Nef) can be expressed in HIV-1 infected astrocytes and potentially alter disease outcomes and neurotoxicity (Canki etal.,2001). Such viral proteins are important candidates for viral mediators of astrocyte-directed impairment of neuronal functions. However, our data support a predominant neurotoxic effect from secretory factors produced by macrophages. Cytokines, such as TNF-α and IL-1β, contribute to neuronal loss (Nuovo etal.,1994), and infected macrophages secreted significantly more Mac-1, TNF-α, and IL-1β than did astrocytes. HIV-1 infected MDM elicited significant levels of Mac-1, which may help to explain higher astrocytosis and microgliosis in HIV-1 infected MDM injected mice, when compared with brains with infected astrocytes.
It is well accepted that HIV-1 infected MDM induce profound astrogliosis and microgliosis along with significant losses in the volume and intensity of MAP-2, and NF immunoreactivity. In contrast, HIV-1 infected astrocytes, while also demonstrating activation of astrocytes and microglia, show minimal alterations in MAP-2 immunostaining. Nevertheless, it is likely that HIV-1 infection of astrocytes affects the balance between harmful and beneficial effects of the cells during continuous low-level infection of the nervous system. It is the subtle immunoregulatory and neurotoxic roles of astrocytes that may define the chronic stages of disease.
Acknowledgements
We thank Robin Taylor for excellent editorial and graphic assistance and Dr. Larisa Poluektova for critical review of the manuscript. We thank Drs. James Anderson and Fred Ullrich of the University of Nebraska Medical Center for statistical support and lively discussions.




