Selective blockade of astrocytic glutamate transporter GLT-1 with dihydrokainate prevents neuronal death during ouabain treatment of astrocyte/neuron cocultures
Abstract
Glutamate (Glu) is a major excitatory neurotransmitter of the mammalian central nervous system and under normal conditions plays an important role in information processing in the brain. Therefore, extracellular Glu is subject to strong homeostasis. Astrocytes in the brain have been considered to be mainly responsible for the clearance of extracellular Glu. In this study, using mixed neuron/astrocyte cultures, we investigated whether astrocytic Glu transporter GLT-1 is crucial to the survival of neurons under various conditions. Treatment of the mixed cultures with a low concentration of Glu did not produce significant death of neurons. However, cotreatment with dihydrokainate (DHK), a specific blocker of GLT-1, resulted in significant neuronal death that was suppressed by an antagonist of N-methyl-D-aspartate (NMDA) receptors. These results suggested that astrocytic GLT-1 participated in the clearance of extracellular Glu and protected neurons from NMDA receptor-mediated toxicity. When the cultures were treated with ouabain, an inhibitor of Na+/K+-ATPase, a low concentration of Glu resulted in massive neuronal death that was also suppressed by cotreatment with an antagonist of NMDA receptors. In this case, however, cotreatment with DHK significantly protected neurons from death, suggesting that GLT-1 was responsible for the death of neurons. The present study provides evidence suggesting that astrocytes use their Glu transporter GLT-1 to protect neurons from Glu toxicity, but, ironically, also use GLT-1 to kill neurons through Glu toxicity depending on their status. GLIA 40:337–349, 2002. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Extracellular L-glutamate (Glu) in the mammalian brain is subject to homeostasis because an elevated concentration results in the excessive activation of Glu receptors, thereby resulting in neuronal death (Choi, 1988). Astrocytes seem to be the cell type primarily responsible for the clearance of extracellular Glu in the brain (Rothstein et al., 1996), while the astrocytic Na+-dependent Glu transporter GLT-1 is quantitatively the dominant form (Tanaka et al., 1997; Rao et al., 2001b). The disruption of astrocytic Glu transporters such as GLT-1 may contribute to neuronal damage in stroke, trauma, Alzheimer's disease, amyotrophic lateral sclerosis, and Huntington's disease (Rothstein et al., 1994; Hazell and Butterworth, 1999; Li et al., 1999; Rao et al., 2001b). Thus, the function of astrocytic Glu transporters seems to be crucial to the survival of neurons in the brain. However, under certain conditions such as brain anoxia, the role of astrocytic Glu transporters is reversed, that is, Glu is released from astrocytes to the extracellular space (Szatkowski et al., 1990), triggering the death of neurons, thus giving rise to mental and physical handicaps (Takahashi et al., 1997). Recently, however, Rossi et al. (2000) have reported that the reversed uptake of Glu by neuronal Glu transporters is crucial in the death of neurons during ischemia. Therefore, it remains controversial as to what mechanisms are critical for the ischemia-induced rise of Glu and neuronal death.
Here we report experimental evidence obtained using mixed neuron/astrocyte cultures, suggesting that astrocytes utilize their Glu transporter GLT-1 for the protection of neurons from Glu neurotoxicity and, ironically, for the killing of neurons through Glu neurotoxicity, depending on their status.
MATERIALS AND METHODS
Cell Culture
Neurons/astrocytes were prepared from 16- to 18-day-old embryonic rat cortices and grown in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY), which was supplemented with 10% heat-inactivated fetal bovine serum (FBS), 10% Ham's F12, and 0.24% penicillin/streptomycin (culture medium). Cells were plated at a uniform density of 3.0 × 105 cells/cm2 onto poly-L-lysine (100 μg/ml)-coated plastic dishes and maintained in a 5% CO2 incubator at 37°C. The cultures were fed a filtered (0.22 μm; Millipore, Bedford, TX) conditioned medium (CM) twice a week. To obtain the CM, cells from the 16- to 18-day-old embryonic rat cortices were plated onto poly-L-lysine–coated six-well dishes and cultured for more than 2 weeks. The cultures were then fed a cooled culture medium and incubated for an additional day. The culture medium was then filtered and used as a CM. After 13–15 days, the neurons in these cultures sit on the top of a confluent monolayer of astrocytes. The experiments were performed using these cultures.
Astrocyte-enriched cultures were prepared from cortical astrocytes of postnatal 1- to 3-day-old rat pups. Cortical hemispheres were removed, cleaned, and dissociated using papain (Roche Diagnostics, Mannheim, Germany) and mechanical trituration. Cells were placed on poly-L-lysine–coated glass coverslips and maintained in the culture medium. The medium was changed 48 h later and twice a week thereafter. The experiments described here were performed on astrocytes maintained for 13–15 days in culture.
Immunocytochemistry
Astrocytes and neurons were identified by immunostaining with antibodies against glial fibrillary acidic protein (GFAP; Sigma, St. Louis, MO) and microtubule-associated protein 2 (MAP-2; Sigma), respectively. The astrocytic glutamate transporters GLT-1 and GLAST were detected by immunostaining with anti–GLT-1 and anti-GLAST polyclonal antibodies (Chemicon, Temecula, CA), respectively. For the labeling of MAP-2, GFAP, GLAST, and GLT-1, the cortical cells were fixed with 4% paraformaldehyde for 5 min at 4°C, followed by 95% methanol in PBS for 10 min at −20°C. The cells were then incubated with a primary antibody over a 24-h period using a dilution of 1:1,000 for MAP-2, 1:400 for GFAP, 1:400 for GLT-1, and 1:5,000 for GLAST. After being washed with phosphate-buffered saline (PBS), the cells were incubated with a secondary antibody containing 1.0% goat serum for 30 min. For labeling, a 1:500 dilution of biotinylated goat antibody against mice IgG (Vector Laboratories, Burlingame, CA) was used. Bound antibodies were detected by the avidin-biotin-peroxidase complex (ABC) method using a commercial ABC kit (Vector Laboratories). Observation of peroxidase activity was made possible by incubation with 0.1% 3,3′-diaminobenzidine tetrahydrochloride (DAB) in a 50 mM Tris-HCl buffer (pH 7.4) supplemented with 0.02% H2O2. The cells were dehydrated in 70%–100% ethanol, cleared in xylene, and mounted on glass coverslips in Permount (Fisher Scientific, Fair Lawn, NJ) for light microscopic observation.
Excitotoxicity Protocol
Cultures were treated with glutamate (Glu) at various concentrations for 30 min, and the survival rate of neurons was analyzed 24 h after exchanging the Glu-containing test medium with the Glu-free conditioned medium. An Mg2+-free Earle's Balanced Salt Solution (EBSS) was used as the test medium. When dihydrokainate (DHK) was used to investigate whether Glu toxicity was enhanced by blocking GLT-1, treatment of cultures with DHK (100 μM) was started 30 min before the glutamate (10 μM) treatment and was continued throughout the treatment. When ouabain was used to block Na+/K+-ATPase, cultures were cotreated with ouabain (50 μM) and Glu (10 μM) for 15 min. To investigate whether DHK treatment would affect the neurotoxicity caused by inhibiting the activity of the Na+/K+-ATPase, treatment of cultures with DHK (200 μM) began 15 min prior to and was continued throughout the ouabain (50 μM) treatment. When DL-threo-β-benzyloxyaspartate (TBOA), a competitive blocker of GLAST and GLT-1 (Shimamoto et al., 1998; Diamond, 2001), was used, the concentration of TBOA was set at 50 μM. The experimental protocols were the same as those for DHK.
Survival Rate of Neurons
Neuronal death was analyzed following observation of the nuclear morphology by using the fluorescent DNA-binding dyes, Hoechst 33342 (H33342) and propidium iodide (PI). Cells were incubated with these dyes for 15 min at 37°C. Individual nuclei were observed using fluorescent microscopy (Olympus, IX70) and subsequently analyzed. PI was used to identify nonviable cells. More specifically, an average of 450–500 neurons from random fields were analyzed in each experiment. The survival rate of neurons—meaning the percentage of viable neurons remaining—was determined by placing images of nuclear staining on phase-contrast images and calculated as (viable neurons/total neurons before drug treatment) × 100%, since some neurons came off the dishes at the time of inspection. At least four independent experiments (n ≥ 4) were conducted and analyzed.
Chemicals
Dihydrokainate (DHK), 1β,3β,5β,11α,14,19-hexahydroxycard-20(22)-enolide 3-(6-deoxy-α-L-mannopyranoside) (ouabain), bisbenzimide (Hoechst 33342), DL-2-amino-5-phosphonopentanoic acid (AP5), and PI were obtained from Sigma. TBOA was purchased from Tocris (Avonmouth Bristol, U.K.). All other compounds were obtained from Wako Chemical (Tokyo, Japan).
Statistics
Data are presented as mean ± SD. Statistical comparison of the control group with treated groups was carried out using an unpaired t-test. Differences with a value of P < 0.05 were considered significant.
RESULTS
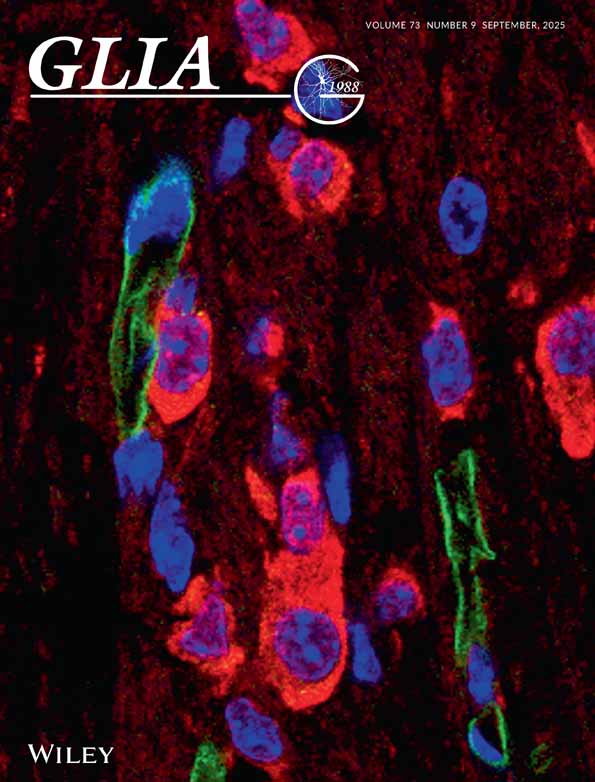
We analyzed the functional role of the astrocytic glutamate (Glu) transporter GLT-1 in mixed cultures of neurons and astrocytes (Fig. 1, A1 and A2) from fetal rat brain (embryonic day 17). During this process, we first tried to clarify the dose-dependent neurotoxicity of Glu in the cultures. Exposure to a relatively low concentration of Glu (5 and 10 μM) did not result in significant neuronal death in our mixed cultures (Fig. 1C and E). However, a high concentration of Glu induced significant neuronal death (Fig. 1D and E).
Glutamate-induced neurotoxicity in mixed cortical neuron/astrocyte cultures. A: Immunocytochemical analysis using anti–MAP-2 (A1) and anti-GFAP (A2) antibodies indicates that the cultures were mixed. Photomicrographs B, C, and D show glutamate (Glu)-induced neuronal death in the sham-, 10 μM Glu-, and 50 μM Glu-treated cultures, respectively. Photomicrographs C1 and D1 show the cultures before treatment, whereas C2 and D2 illustrate their state 24 h after Glu treatment for 30 min. Cell nuclei were stained with bisbenzimide (Hoechst 33342) and propidium iodide (PI; B3, C3, and D3). Red nuclei in D3 indicate dead PI-positive neurons. Treatment of mixed cultures with Glu resulted in dose-dependent neuronal death (E). Note that low concentrations of Glu (5 and 10 μM) did not induce a significant death of neurons in these cultures. Scale bars indicate 250 μm. Data are expressed as mean ± SD (n > 4). Asterisk, P < 0.05.
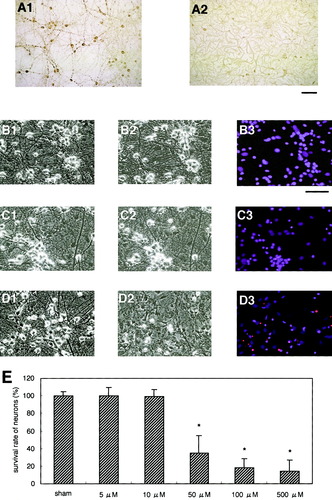
The Glu toxicity observed in this study seemed rather mild compared to that found in pure neuronal cultures reported previously (Cheung et al., 1998). Therefore, there is a possibility that astrocytic Glu transporters play some neuro-protective role in this situation. Next, we confirmed whether or not the astrocytes in our cultures expressed the astrocytic Glu transporter GLT-1 that is crucial to Glu neurotoxicity. A previous study has revealed that astrocytes cocultured with neurons change from a polygonal to a process-bearing morphology that is more characteristic of astrocytes in situ (Swanson et al., 1997). The expression of both GLT-1 and GLAST increases with such morphological changes (Perego et al., 2000). Flat polygonal astrocytes preferentially express GLAST, while the process-bearing cells express GLT-1 (Perego et al., 2000). These results suggest that neurons are directly involved in regulating the expression of astrocytic Glu transporters (Gegelachvilli et al., 1997; Schlag et al., 1998; Perego et al., 2000). Most of the GFAP-positive astrocytes in our cultures showed a process-bearing but not a flat polygonal morphology (Fig. 1, A2). GLT-1 was actually expressed on such process-bearing astrocytes in the mixed neuron-astrocyte cultures (Fig. 2A). Although GLT-1 is believed to be present only on astrocytes in the brain (Lehre et al., 1995), there are reports that GLT-1 is also present on neurons under certain conditions (Martin et al., 1997; Mennerick et al., 1998). However, we observed hardly any GLT-1–positive neurons in our mixed cultures, despite careful inspection of the immunocytochemical data. We then analyzed the change in neurotoxicity caused by the exposure to Glu while blocking GLT-1 with dihydrokainate (DHK), a selective nontransportable inhibitor of GLT-1 (Levy et al., 1998; Robinson, 1998; Rao et al., 2001b).
Neuroprotective role of the astrocytic glutamate transporter GLT-1 against Glu toxicity in mixed cultures. Immunostaining of cultures with an anti–GLT-1 antibody shows the existence of GLT-1–positive astrocytes in the mixed neuron/astrocyte cultures (A). GLT-1–positive neurons were not detected. Most GLT-1–positive astrocytes were observed under aggregates of neurons. B and C: Photomicrographs B1 and C1 show the cultures prior to Glu treatment; B2 and C2 illustrate the cultures 24 h after 30-min cotreatments with Glu (10 μM) and DHK (100 μM); and with Glu (10 μM), DHK (100 μM), and AP5 (100 μM), respectively. Although treatment of cultures with only Glu (10 μM) did not induce significant neuronal death (D), cotreatment with DHK (100 μM) did (B3 and D). DHK-induced neuronal death was markedly suppressed by AP5, suggesting that NMDA receptor-mediated mechanisms were involved in such neurotoxicity. Treatment of cultures with only DHK for 30 min did not result in the significant death of neurons (D). Scale bars show 250 μm. Data are expressed as mean ± SD (n > 4). Asterisk, P < 0.05.
A previously ineffective concentration of Glu (10 μM) caused significant neuronal death when astrocytic GLT-1 was blocked with DHK (Fig. 2B and D). However, cell death was significantly reduced by cotreatment with DL-2-amino-5-phosphonopentanoic acid (AP5), a specific inhibitor of N-methyl-D-aspartate (NMDA)-type Glu receptors (Fig. 2C and D). Cultures treated with only DHK (100 μM) for 30 min did not result in the significant death of neurons (Fig. 2D). Almost all the astrocytes, however, seemed to remain intact under these experimental conditions. These results suggest that the astrocytic Glu transporter GLT-1 actively participated in the clearance of extracellular Glu and protected neurons from NMDA receptor-mediated excitotoxic stress. Thus, the functional operation of GLT-1 seems crucial to the survival of neurons.
The sodium-dependent astrocytic Glu transporter GLT-1 transports one Glu anion coupled to the cotransport of three Na+ and one H+, as well as to the countertransport of one K+ (Levy et al., 1998). GLT-1 uses steep ionic gradients across the membrane to accumulate a high intracellular concentration of Glu in astrocytes. The ionic gradients are mainly maintained by Na+/K+-ATPase, which excludes Na+ in exchange for extracellular K+, in turn energizing other secondary ion transporters (e.g., Na+-Ca2+ exchanger). We then tried to block GLT-1 by inhibiting Na+/K+-ATPase with ouabain, a specific inhibitor of such ATPases, instead of by using DHK. Inhibition of Na+/K+-ATPase with ouabain resulted in a gradual increase of intracellular Na+ in astrocytes (Rose et al., 1998). Thus, the treatment of cultures with this drug is expected to disrupt the Na+ gradient across membranes and effectively block the Na+-dependent glutamate transporter GLT-1, an effect that is similar to DHK, although the mechanism of inhibition is different.
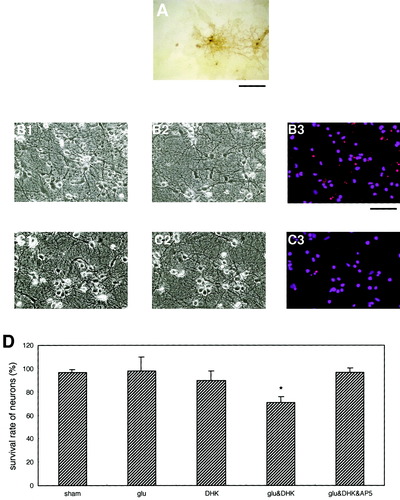
The treatment using a low concentration of Glu (10 μM) for only 15 min resulted in massive neuronal death when the cultures were cotreated with 50 μM ouabain (Fig. 3A and D). The survival rate of neurons was markedly reduced on cotreatment with ouabain compared with DHK (Fig. 2D). The reason for this difference in the survival rate is unclear, although one possibility is that the ouabain treatment depressed the Na+/K+-ATPase of neurons as well as astrocytes. Inhibition of the Na+/K+-ATPase of neurons may result in the disruption of neuronal Na+-dependent Glu transporters, possibly contributing to neuronal death. In fact, a recent study has demonstrated that the dysfunction of neuronal Glu transporters is involved in the ischemia-induced rise in Glu and neuronal death (Rossi et al., 2000). Another possibility is that the ouabain treatment reverses the role of the astrocytic Na+-dependent Glu transporter GLT-1. The reversed uptake of Glu also contributes to the marked rise in extracellular Glu and neuronal death (Szatkowski et al., 1990, 1994). We then tested these possibilities.
The astrocytic glutamate transporter GLT-1 responsible for the killing of neurons in mixed neuron/astrocyte cultures. A–C: Photomicrographs A1, B1, and C1 indicate the mixed cultures before treatment; A2, B2, and C2 show the cultures 24 h following 15-min cotreatments with Glu (10 μM) and ouabain (50 μM); with Glu (10 μM), ouabain (50 μM), and DHK (200 μM); and with Glu (10 μM), ouabain (50 μM), and AP5 (100 μM), respectively. Cotreatment of mixed cultures with ouabain resulted in massive neuronal death (A3 and D), whereas DHK cotreatment markedly suppressed the ouabain-induced neuronal toxicity (B3 and D). Ouabain-induced neuronal death was also suppressed by cotreatment with AP5 (C3 and D), suggesting that NMDA receptor-mediated mechanisms were involved in the neurotoxicity. The scale bar is 200 μm. Data are expressed as mean ± SD (n > 4). Asterisk, P < 0.05.
Cotreatment of cultures with DHK, which caused neuronal death in Figure 2, significantly protected neurons from death when the activity of Na+/K+-ATPase was inhibited with ouabain (Fig. 3B and D). This result seems to exclude the possible involvement of dysfunctional neuronal Glu transporters in massive neuronal death caused by the inhibition of the activity of neuronal Na+/K+-ATPase, since the neurons in our cultures did not express GLT-1 and because DHK has been known to block GLT-1 specifically. Therefore, this result may favor the second possibility that the role of astrocytic Glu transporter GLT-1 was reversed by inhibiting astrocytic Na+/K+-ATPase with ouabain, that is, astrocytes took part in the killing of neurons during this experimental condition. This idea logically leads to the assumption that the neuronal death caused by ouabain treatment was due to Glu toxicity resulting from the rise in extracellular Glu released from astrocytes. We then tested this possibility. Neuronal death induced by ouabain treatment was markedly depressed by cotreatment with AP5, a blocker of NMDA-type Glu receptors (Fig. 3C and D). Thus, this result supported our assumption that the neuronal death induced by ouabain treatment was in fact due to Glu-induced excitotoxicity.
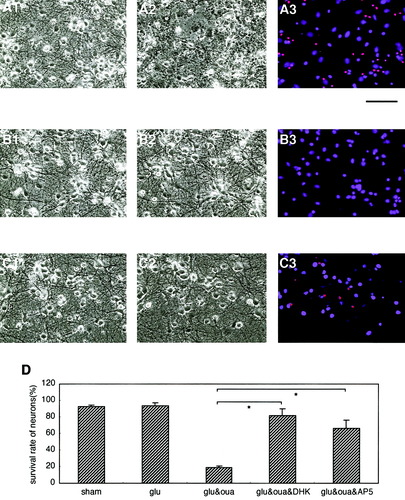
To confirm the involvement of astrocytic Glu transporters in the killing of neurons when the activity of Na+/K+-ATPase was inhibited, we treated the mixed neuron/astrocyte cultures with DL-threo-β-benzyloxyaspartate (TBOA), a broader nontransportable blocker of both GLT-1 and GLAST (Shimamoto et al., 1998; Diamond, 2001). Cotreatment with a low concentration of both Glu (10 μM) and ouabain (50 μM) for 15 min resulted in massive neuronal death (Fig. 4B and D). Cotreatment of cultures with TBOA (50 μM) significantly protected neurons from death when the activity of Na+/K+-ATPase was inhibited with ouabain (Fig. 4C and D). The survival rate of neurons in the TBOA treatment was not statistically different from that in the DHK treatment (Fig. 4D). An immunocytochemical study using anti-GLAST antibodies showed that there were GLAST-positive astrocytes in the mixed neuron/astrocyte cultures (Fig. 4E). However, the immunoreactivity seemed to be weak and the rate of GLAST-positive astrocytes was low in the cultures tested (n = 3 cultures).
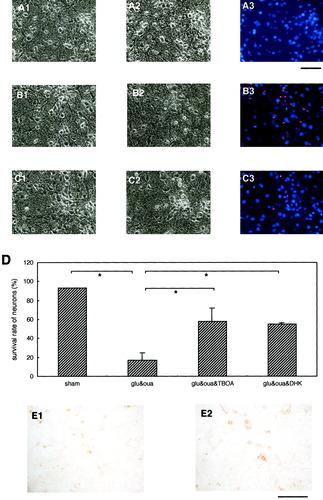
Astrocytic glutamate transporters and neuronal death in mixed neuron/astrocyte cultures. A–C: Photomicrographs A1, B1, and C1 indicate the mixed cultures before treatment; A2 shows the sham-treated control cultures, whereas B2 and C2 indicate the cultures 24 h after 15-min cotreatments with Glu (10 μM) and ouabain (50 μM); and with Glu (10 μM), ouabain (50 μM), and TBOA (50 μM), respectively. Cotreatment of mixed cultures with ouabain resulted in massive neuronal death (B3 and D), whereas TBOA cotreatment markedly suppressed the ouabain-induced neuronal toxicity (C3 and D). Ouabain-induced neuronal death was also suppressed by cotreatment with 200 μM DHK (D). Immunocytochemical analysis of cultures using an anti-GLAST antibody revealed that GLAST-positive astrocytes could be identified in the mixed neuron/astrocyte cultures (E1 and E2). Scale bars show either 250 μm for A, B, C, and E1, or 125 μm for E2. Data are expressed as mean ± SD (n = 4). Asterisk, P < 0.05.
Although ouabain treatment produced massive death of neurons, almost all of the astrocytes seemed to remain intact under these experimental conditions. We then tried to clarify whether or not the experimental conditions toxic to neurons observed in this study were also toxic to astrocytes in astrocyte-enriched cultures (Fig. 5A). Cotreatment of astrocytes with Glu and DHK, as well as with Glu and ouabain, did not induce significant cell death among astrocytes (Fig. 5B–E). These results suggested that the experimental conditions toxic to neurons were not toxic to astrocytes.
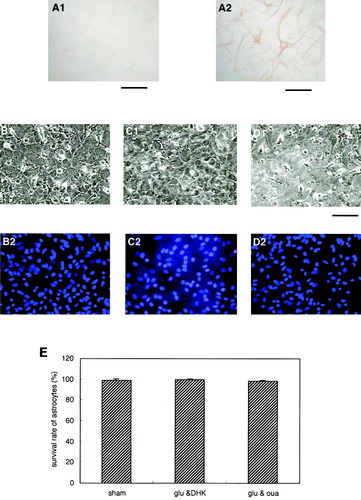
Astrocytes survived neurotoxic treatment in astrocyte-enriched cultures. A: Immunocytochemical analysis of cultures using anti–MAP-2 (A1) and anti-GFAP (A2) antibodies revealed that MAP-2–positive neurons could not be clearly identified in these cultures. B–D: Photomicrograph B1 shows the sham-treated control cultures, whereas photomicrographs C1 and D1 indicate the state of the cultures 24 h after 30-min treatments with Glu (10 μM) and DHK (100 μM); and with Glu (10 μM) and ouabain (50 μM), respectively. Cotreatment of astrocyte-enriched cultures with Glu and either DHK or ouabain did not induce significant death of astrocytes (E). Scale bars show 250 μm. Data are expressed as mean ± SD (n = 4).
To confirm that the astrocytic Glu transporter GLT-1 is crucial for both the survival and the death of neurons, we produced mixed neuron-astrocyte cultures with little GLT-1 expression. Previous studies have revealed that astrocytic GLT-1 expression is regulated by small neuron-derived peptides (Brown, 2000; Figiel and Engele, 2000). Therefore, neuronal density was reduced to the extent that astrocytic GLT-1 expression could not be clearly detected (Fig. 6E and F). In these cultures, treatment with 10 μM Glu, which was not toxic to neurons in the cultures with astrocytic GLT-1 (Figs. 1 and 2), resulted in slight but significant neuronal death (Fig. 6B and D). However, cotreatment of these cultures with ouabain did not further facilitate glutamate excitotoxicity (Fig. 6C and D). In contrast, ouabain treatment resulted in massive neuronal death in the cultures with astrocytic GLT-1 (Fig. 3A and D).
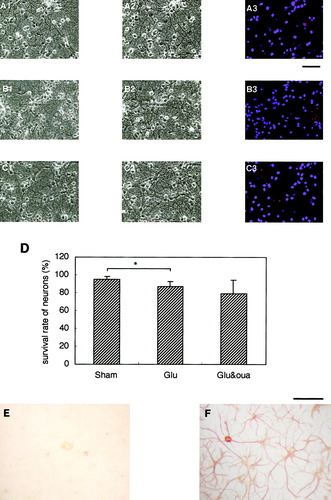
Inhibition of the activity of Na+/K+-ATPase did not induce neuronal death in mixed neuron/astrocyte cultures with little astrocytic GLT-1. A–C: Photomicrographs A1, B1, and C1 show the mixed cultures before treatment; A2, B2 and C2 indicate the cultures 24 h after sham treatment, treatment with Glu (10 μM), and cotreatment with Glu (10 μM) and ouabain (50 μM), respectively. In these cultures, treatment with only Glu (10 μM) resulted in the slight but significant death of neurons (B3 and D). This was probably because of a deficiency of the astrocytic glutamate transporter GLT-1. However, cotreatment with ouabain did not further facilitate glutamate excitotoxicity (C3 and D). Immunocytochemical analysis of these cultures revealed that astrocytes did not have detectable levels of GLT-1 (E), although the morphology of astrocytes (F) was not so different from that of the cells with GLT-1 in Figure 2A. Scale bars show 250 μm. Data are expressed as mean ± SD (n = 4). Asterisk, P < 0.05.
DISCUSSION
The present study has provided evidence that the astrocytic Glu transporter GLT-1 is closely involved in protecting neurons from NMDA-mediated neurotoxicity, as well as in killing neurons through NMDA-mediated neurotoxicity. Treatment of cultures with DHK, a nontransportable inhibitor of astrocytic GLT-1, resulted in the significant death of neurons. However, the same DHK treatment resulted in the significant protection of neurons when the activity of astrocytic Na+/K+-ATPase was inhibited with ouabain.
In the brain, at least 40% of the energy released by respiration is required by Na+/K+-ATPase in order to maintain the ionic gradients of sodium and potassium across cell membranes (Astrup et al., 1981; Hansen, 1985). Therefore, the sodium pump in the brain requires an enormous expenditure of energy, indicating that the activity of Na+/K+-ATPase is markedly suppressed during ischemia due to a decreased availability of glucose and oxygen (Lees, 1991). In the present study, ouabain treatment might have reduced the activity of Na+/K+-ATPase in both neurons and astrocytes. However, the reduction in the Na+/K+-ATPase activity of neurons itself did not appear to be crucial for neuronal death. Rather, the suppression of astrocytic Na+/K+-ATPase proved crucial for neuronal death, provided that astrocytes expressed GLT-1.
Previous studies have shown that DHK directly activates ionotropic excitatory amino acid (EAA) receptors, including the NMDA receptor of neurons (Maki et al., 1994; Wang et al., 1998). These results do raise the possibility that DHK-induced effects on the survival of neurons observed in this study could be caused by such a direct action of DHK on the NMDA receptor—not by the inhibition of astrocytic GLT-1. However, this is unlikely to be the case, since cotreatment of cultures with DHK and ouabain resulted in the significant protection of neurons from cell death (Fig. 3). In addition, treatment with AP5, an antagonist of the NMDA receptor, suppressed the ouabain-induced death of neurons (Fig. 3). Provided that the direct action of DHK on the NMDA receptor was involved in the protection of neurons from ouabain-induced neurotoxicity, AP5 treatment could result in the exacerbation of neuronal injury—not in the neuroprotection observed in the present study.
In the neuron/glia mixed cultures used in this study, GLT-1–positive neurons could hardly be identified despite careful immunocytochemical inspection. However, a recent study has demonstrated the presence of GLT-1 variants in cultured neurons as well as in neurons of the rat brain (Chen et al., 2002). They have postulated that such GLT-1 variants in neurons uptake glutamate at presynaptic terminals and are responsible for the preservation of input specificity at excitatory synapses. However, the functional significance of GLT-1 expressed by neurons in the pathophysiology of excitotoxicity in the brain remains unclear at present.
GLAST-positive astrocytes were identified in the mixed neuron/astrocyte cultures (Fig. 4E). However, the immunoreactivity seemed weak and the rate of GLAST-positive astrocytes was low in the cultures. Treatment of the cultures with TBOA, a blocker of both GLT-1 and GLAST (Shimamoto et al., 1998; Diamond, 2001), produced similar results to the DHK treatment. Treatment of cultures with 50 μM TBOA resulted in the significant death of neurons in the presence of a low concentration of Glu (10 μM; data not shown). TBOA treatment at the same concentration of 50 μM, however, significantly protected neurons from death when the cultures were cotreated with 50 μM ouabain and 10 μM Glu (Fig. 4D). Dose dependency of TBOA-induced protective effects on the survival rate of neurons was investigated (data not shown). The near-maximum protection was obtained when the concentration of TBOA was approximately 50 μM. The TBOA-induced protective effect on neurons was almost comparable to that induced by DHK (Fig. 4D). Thus, blocking of the activity of GLAST by TBOA was probably not the primary contributor to the protection of neurons under the experimental condition in our cultures.
Previous studies have demonstrated that brain ischemia caused a reduced expression of astrocytic GLT-1 in the postischemic phase (Pines et al., 1992; Torp et al., 1995). Moreover, this reduction is considered to be a contributing factor to the delayed neuronal death following brain ischemia (Rao et al., 2000, 2001a). Astrocytic GLT-1 expression is also downregulated after traumatic brain injury in the rat (Rao et al., 1998; Samuelsson et al., 2000). A persistently reduced GLT-1 expression by astrocytes is in fact toxic to neurons because of the reduced capability for the clearance of extracellular Glu by astrocytes. However, another interpretation of the ischemia-induced postischemic reduction of astrocytic GLT-1 would be that neurons participate in the downregulation of the expression during postischemic periods in order to protect neurons themselves from Glu-induced excitotoxicity. A recent finding that seems to support the idea of neuronal self-defense in response to ischemia is that preconditioning with cortical-spreading depression results in a transient reduction in the expression of astrocytic GLT-1 (Douen et al., 2000). Neurons could respond to a brief ischemic attack by downregulating astrocytic GLT-1 expression to protect themselves temporarily from the Glu-induced toxicity caused by any subsequent severe ischemic insult, despite the fact that the reduced level of astrocytic GLT-1 is itself dangerous. This possibility is now being investigated in a rat model of brain ischemic preconditioning.
Acknowledgements
The authors thank Mr. Junji Hori and Mr. Junji Yanoma of the Research Institute for Electronic Science, Hokkaido University, for their help in establishing neuron/astrocyte cocultures.