Advances and prospects of transcription-factor-based biosensors in high-throughput screening for cell factories construction
Abstract
Non/semi-rational engineering strategies can improve the performance of microbial cell factories under the situations of indistinct metabolic regulations and interactions. However, generating positive variants through these strategies is remarkably low. Using traditional screening methods to evaluate the performance of mutants, such as mass spectrometry and chromatography, is low-throughput, slow-detection, and labor-intensive. Specifically, the efficiency of high-throughput screening (HTS) for strain screening could reach even more 103−106 strains/day, bridging the gap between non/semi-rational engineering strategies and microbial cell factories construction. This review highlights transcription factor (TFs)-based biosensors in the advances in developing synthetic biosensors for HTS. We hope that this review will help take full advantage of valuable TFs to guide the HTS technology and promote its development.
1 INTRODUCTION
Biomanufacturing has advantages of green, safety, and sustainability, which is a critical way to solve some social issues, including the environmental pressure and shortage of food and resources. Therefore, it has been listed as a strategic development technology in many countries (Clomburg et al., 2017), and the challenge is obtaining robust and efficient industrial strains (Lee & Kim, 2015). Metabolic engineering provides a formidable tool to develop industrial strains from scratches, which could improve productive performances or endow cells to synthesize new chemicals (Gu & Xu, 2021). Several successful examples of metabolic engineering have been reported, such as engineering Corynebacterium glutamicum for synthesizing l-arginine (Park et al., 2014), constructing Yarrowia lipolytica for producing omega-3 eicosapentaenoic acid (EPA) (Z. X. Xue et al., 2013), and remolding Bacillus subtilis for yielding N-acetylglucosamine (Wu et al., 2020), to name a few. However, to date, developing a new industrial strain by metabolic engineering is still a huge challenge and usually takes 6−8 years, over $50 million, even though some advanced synthetic biology tools and technologies are available (Nielsen & Keasling, 2016). This can be attributed to our limited knowledge of the complex interactions and extensive metabolic regulations in microorganisms (Nielsen & Keasling, 2016). Therefore, it is of great significance to develop a general and universal strategy for constructing industrial strains (Figures 1-3).
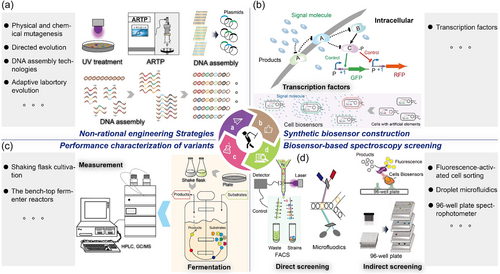
Noticeably, non/semi-rational engineering strategies, such as directed evolution, adaptive evolution, and mutation technique, can improve the performances of strains under the situations of the indistinct metabolic regulations and interactions (Packer & Liu, 2015), and have been proven to be effective strategies for strain development (Tan et al., 2019), which provide an alternative approach to broadening the scope of metabolic engineering. It should be noted that the probability of generating a positive variant by non/semi-rational engineering strategies is remarkably low, usually less than 1/105 (Zeng et al., 2020). However, evaluating the productive performances of mutants by conventional screening methods, such as mass spectrometry and chromatography, is low-throughput, slow-detection, and labor-intensive with a threshold of 100−1000 strains/day (Hossain et al., 2020; Santos-Zavaleta et al., 2018). Specifically, the efficiency of high-throughput screening (HTS) for strain screening, an emerging screening technology for the 21st century, could reach even more 103−106 strains/day (Tan et al., 2019; Zeng et al., 2020), which is based on the automated equipment to rapidly analyze the biological activities of thousands to millions of samples at the molecular, pathway, or cellular level (Hertzberg & Pope, 2000). Therefore, the development of HTS bridges the gap between non/semi-rational engineering strategies and mutants screening, which paves the way for the application of non/semi-rational engineering strategies in the construction of industrial strains (Tables 1 and 2).
| TFB | Reporter | Target molecule | Source | Biosensor host | Detection range | References |
|---|---|---|---|---|---|---|
| TetR | GFP | Anhydrotetracycline | E. coli | E. coli | 0–430nM | Rogers et al. (2015) |
| MphR | GFP | Erythromycin | E. coli | E. coli | 0–1400μM | Rogers et al. (2015) |
| AcuR | GFP | Acrylate | R. sphaeroides | E. coli | 0.016–10mM | Rogers et al. (2015) |
| TtgR | GFP | Naringenin | P. putida | E. coli | 110–9000 μM | Rogers et al. (2015) |
| AraC | GFP | Arabinose | E. coli | E. coli | 0–40mM | Rogers et al. (2015) |
| CdaR | GFP | Glucarate | E. coli | E. coli | 0–40mM | Rogers et al. (2015) |
| CsiR | RFP | Glutarate | P. putida | E. coli | 0.005–0.0195mM | M. G. Thompson et al. (2019) |
| GcdR | RFP | Glutarate | P. putida | E. coli | 0.01–2.5mM | M. G. Thompson et al. (2019) |
| AcoR | RFP | Acetoin | C. necator H16 | C. necator H16 | 0–0.078mM | Hanko et al. (2020) |
| BenM | RFP | Benzoate | C. necator H16 | C. necator H16 | 0–1.25mM | Hanko et al. (2020) |
| BadR | RFP | Cyclohexanecarboxylate | C. necator H16 | C. necator H16 | 0–1.25mM | Hanko et al. (2020) |
| FdsR | RFP | Formate | C. necator H16 | C. necator H16 | 0–2.5mM | Hanko et al. (2020) |
| HpdR | RFP | 3-Hydroxypropanoate | C. necator H16 | C. necator H16 | 0–0.2mM | Hanko et al. (2020) |
| HpdA | RFP | l-tyrosine | C. necator H16 | C. necator H16 | 0–0.156mM | Hanko et al. (2020) |
| KynR | RFP | l-kynurenine | C. necator H16 | C. necator H16 | 0–0.625mM | Hanko et al. (2020) |
| NahR | RFP | Salicylate | C. necator H16 | C. necator H16 | 0–0.156mM | Hanko et al. (2020) |
| OapR | RFP | β-alanine | C. necator H16 | C. necator H16 | 0–20mM | Hanko et al. (2020) |
| PhhR | RFP | l-phenylalanine | C. necator H16 | C. necator H16 | 0–5mM | Hanko et al. (2020) |
| PhgR | RFP | Phenylglyoxylate | C. necator H16 | C. necator H16 | 0–5mM | Hanko et al. (2020) |
| PcaQ | RFP | 3,4-Dihydroxybenzoate | C. necator H16 | C. necator H16 | 0–0.156mM | Hanko et al. (2020) |
| SauR | RFP | Sulfonatoacetate | C. necator H16 | C. necator H16 | 0–10mM | Hanko et al. (2020) |
| TtdR | RFP | Tartrate | C. necator H16 | C. necator H16 | 0–150mM | Hanko et al. (2020) |
| VanR | YFP | Vanillate | C. crescentus | E. coli | 0.01–1mM | Kunjapur and Prather (2019) |
| BetIM | GFP | Choline | E. coli | E. coli | 0–100mM | Saeki et al. (2016) |
| LuxR | GFP | 3OC6HSL | V. fischeri | E. coli | 0.5–10nM | Canton et al. (2008) |
| FadR | RFP | Fatty acids | E. coli | E. coli | 0.01–1mM | F. Zhang et al. (2012) |
| DmpR | RFP | 4-Nitrophenol | E. coli | E. coli | 1–100 mM | Chong and Ching (2016) |
| TyrR | YFP | Phenylalanine | E. coli | E. coli | 0–0.6mM | Yongfei et al. (2017) |
| Product | TFB | Reporter | Biosensor host | Outcome | References |
|---|---|---|---|---|---|
| Sugars and alcohol | |||||
| Erythritol | EryD | eGFP | Y. lipolytica | 148 g/L erythritol in bench-top reactors. | X. Qiu et al. (2020) |
| N-acetylneuraminate | NeuAc aptamer | sfGFP | E. coli | NeuAc titer increased from 1.58 to 2.61 g/L. | Yang et al. (2017) |
| Xylose | XylR | eGFP | S. cerevisiae | Xylose were shown to regulate eGFP gene expression. | Teo and Chang (2015) |
| Organic acids | |||||
| Ectoine | AraC | GFP | E. coli | Improved ectoine production 3.3-fold. | W. Chen et al. (2015) |
| p‑Coumaric acid | padR | YFP | E. coli | Rapidly sorted droplets using the fluorescent signal and microfluidic device. | Siedler et al. (2017) |
| Muconic acid | BenM | GFP | S. cerevisiae | Selected improved muconic acid-producing yeast strain. | Bian et al. (2016) |
| Oleo-chemicals | |||||
| Triacetic acid lactone | AraC | GFP | E. coli | Improved TAL synthase catalytic efficiency by 19-fold. | Tang et al. (2013) |
| Short- and medium-chain fatty acids | PPDR12 | GFP | S. cerevisiae | Detected hexanoic, heptanoic and octanoic acid over a linear range up to 2, 1.5, and 0.75 mM. | Baumann et al. (2018) |
| malonyl‑CoA | AraC | LacZ | E. coli | Selected synthesized significantly higher titers of the free fatty acids. | H. Li, Chen, et al. (2017) |
| Fatty Acyl-CoA | FadR | GFP | S. cerevisiae | Identification of gene targets improving production of fatty acids and derived products. | Dabirian, Gonçalves Teixeira, et al. (2019) |
| Fatty acids | FadR | RFP | E. coli | Increased the titer to 1.5 g/L. | F. Zhang et al. (2012) |
| Aromatic chemicals | |||||
| Benzoate | NahR | LacZ or TetA | E. coli | Isolation of benzaldehyde dehydrogenase (XylC) expressing E. coli biocatalyst cells. | Fiet et al. (2006) |
| 2,4 dinitrotoluene | XylR | LacZ | E. coli | XylR regulator to acquire a new responsiveness to 2,4 dinitrotoluene. | Galvão et al. (2007) |
| Benzamide | BenR | GFP | E. coli | Identified three novel enzymes. | Taku et al. (2010) |
| Vanillin | QacR | GFP | E. coli | Identified two QacR mutants that respond to vanillin. | Cornejo et al. (2016) |
| Salicylate | AraC | GFP | E. coli | 123% improved production of salicylate in shake-flask culture. | Qian et al. (2019) |
| S-adenosylmethionine | MetJ | YFP | S. cerevisiae | Identified a novel multicopy enhancer of SAM levels. | Umeyama et al. (2013) |
| Tyrosine | TyrR | RFP | E. coli | Increased in tyrosine production 5-fold. | Curran et al. (2013) |
| Tyrosine | ARO9 | YFP | S. cerevisiae | Improved tyrosine-derived heterologous pathway titer up to 3-fold. | Leavitt et al. (2017) |
| l-valine | Lrp | YFP | C. glutamicum | Increase in the yield of l-valine, l-leucine, and l-isoleucine. | Mustafi et al. (2012) |
| l-leucine | Lrp | YFP | C. glutamicum | ||
| l-isoleucine | Lrp | YFP | C. glutamicum | ||
| l-lysine | LysG | YFP | E. coli C. glutamicum | Increase in the yield of l-lysine, l-arginine and l-histidine. | Schendzielorz et al. (2014) |
| l-arginine | LysG | YFP | |||
| l-histidine | LysG | YFP | |||
| Methionine | Lrp | YFP | C. glutamicum | The mutant which could produce amino acids was isolated by this sensor. | Mustafi et al. (2012) |
| Terpenes and flavonoids | |||||
| Borneol | CamR | GFP | E. coli | Increased the system's signal-to-noise ratio to 150-fold. | D'oelsnitz et al. (2022) |
| Fenchol | CamR | GFP | E. coli | ||
| Eucalyptol | CamR | GFP | E. coli | ||
| Camphene | CamR | GFP | E. coli | ||
| Naringenin | FdeR | CFP | E. coli | 7-fold increase of the fluorescent signal. | Siedler et al. (2014) |
| Quercetin | QdoR | GFP | E. coli | ||
| Kaempferol | QdoR | GFP | E. coli | ||
| Naringenin | FdeR | GFP | S. cerevisiae | Selected improved naringenin producing yeast strain. | Skjoedt et al. (2016) |
| Naringenin | fdeO | GFP | S. cerevisiae | Identified with a naringenin titer of 52.0 mg/L. | Wang et al. (2019) |
In general, HTS for strain screening is based on the direct spectroscopy screening, electrochemical sensors, and synthetic biosensors (Zeng et al., 2020). Direct spectroscopy screening is based on chemicals that can generate the specific spectroscopy absorbance under special excitation conditions or coupling the enzyme-catalytic or chemical reactions (Hertzberg & Pope, 2000). The electrochemical sensor-based HTS is based on electrochemical sensors, which could sense the current changes generated by the reactions on the electrode surface (Rathee et al., 2016). In addition, the synthetic biosensor-based HTS is based on the native biological systems that can recognize the environmental signals, such as temperature, pressure, and concentration (Lim et al., 2018). To restrict the extent of the present review, we highlight advances in the development of synthetic biosensors for HTS, emphasizing transcription-factors (TFs)-based biosensors. Moreover, we provided a brief discussion of current limitation on the TFs-based biosensors for HTS.
2 ADVANTAGES OF THE TFs-BASED BIOSENSORS FOR HTS
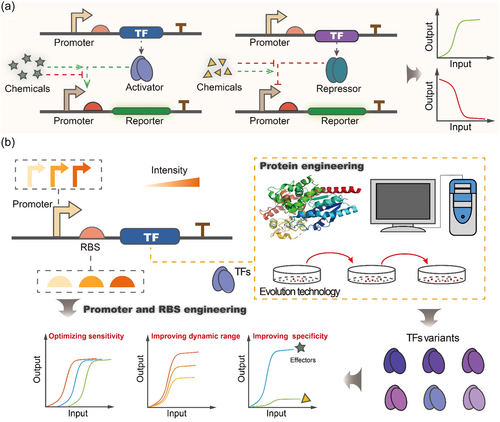
What is the TFs-based biosensor? In electronics, a sensor is an artificial device used to detect electrical signals and transform them into output signals, consisting of the signal identification part, signal transducers, and reader devices. Herein, the TFs-based biosensor performs a similar function to the electronic sensor but is composed of biological modules, including TFs, TFs-cognate promoters, and report genes (C. Qiu et al., 2019). TFs are a series of proteins that can sense environmental effectors to control the transcriptions of genes by binding or dissociating to specific DNA sequences in the cis-acting element promoters (Hartline et al., 2020; Lalwani et al., 2018), which can directly turn gene expression on and off in response to environmental changes (Lalwani et al., 2018). Specifically, TFs-based biosensors have been identified as the attractive tools in metabolic engineering for dynamically maintaining cellular metabolism to avoid the toxicity of accumulated metabolites and promote the production of desired products (Castillo-Hair et al., 2019; Lu et al., 2021; Lv et al., 2020). For example, P. Xu et al. (2014) applied the transcription factor FapR to construct the malonyl-CoA-activated or inhibited synthetic biosensors, which were used to dynamically balance the synthesis and consumption of intracellular malonyl-CoA in Escherichia coli to increase fatty acid titer. Wu et al. (2020) combined CRISPRi with the glucosamine-6-phosphate (GlcN6P) biosensor to create an autonomous dual-control (ADC) system for optimizing the production of nutraceutical N-acetylglucosamine in B. subtilis, which self-adjusted the expression of target genes without requiring external control of fermentation conditions. Moreover, N-acetylglucosamine titer in their study increased from 81.7 to 131.6 g/L without byproduct acetoin production, indicating the stability and robustness of the TFs-based biosensor-mediated pathway circuits in the bioreactor system (Wu et al., 2020). Therefore, the great success of TFs-based biosensors in metabolic engineering promotes its application in strains HTS.
On the other hand, not all chemicals have the intrinsic properties of generating the specific spectroscopy absorbance under special excitation conditions or coupling the enzyme-catalytic or chemical reactions. Thus, the applicative scenarios of spectroscopy screening for strains HTS are greatly limited. Moreover, the electrochemical sensor applied for strains HTS needs to be customized, making it difficult to popularize the technology. Compared to the spectroscopy screening and electrochemical sensors, TFs-based biosensors are constructed by engineering the native TFs that widely exist in living organisms, including microbes, plants, and animals (J. Zhang et al., 2015). Specifically, the process of TFs-mediated gene regulation includes (i) the ligand-binding domains of TFs selectively binding effector molecules, resulting in the conformational changes of TFs, and (ii) that binds the cognate DNA operator sequence to achieve gene transcription regulation (Shi et al., 2018). Coupled with the ability to design TFs with exquisite effector selectivity, TFs offer a unique opportunity for various natural chemicals to develop TFs-based biosensors with sufficient molecular precision to guide the regulation and monitoring of complex biosynthetic systems (Ding et al., 2021).
Moreover, the diversity of microorganisms and genomic sequences offers excellent potential for developing TFs-based biosensors for HTS. However, eukaryotic transcription mechanisms are very complex and require DNA sequence-specific transcription factors and transcription coactivators to regulate transcription initiation. Therefore, owing to the simple transcriptional mechanism and genetic manipulation, prokaryotic transcriptional regulators have attracted more and more attention (Lim et al., 2018). Specifically, recent studies have suggested that prokaryotic TFs can function in eukaryotes (C. Qiu et al., 2019; Wan et al., 2019), and some cases have successfully developed biosensors in eukaryotes using prokaryotic TFs (Y. Zhang & Shi, 2021). For example, Dabirian, Gonçalves Teixeira, et al. (2019) took the advantage of the fatty acyl-CoA responsive TF FadR from E. coli to design a acyl-CoA-specific screening system in S. cerevisiae, which was used to screen for genes that can increase acyl-CoA synthesis by employing fluorescence-activated cell sorting (FACS). Therefore, the TFs-based biosensor is ideal for constructing HTS method for strain screening.
3 DEVELOPING A TFs-BASED BIOSENSOR FOR HTS
TFs-based biosensors have been particularly successful in metabolic engineering; however, finding relevant metabolite-responsive TFs elements for HTS applications remains a challenge. It should be noted that the most straightforward and feasible way to obtain a sensor for the target metabolite is to utilize natural elements, such as TFs and the cognate DNA sequences. However, due to the unclear interactions between native TFs and operator sites in their cognate promoters, synthetic TFs-based biosensors may suffer some defects, such as background noise and poor orthogonality (Cui et al., 2021). Therefore, engineering TFs and cognate promoters is required to low background noise, optimize correlation and specificity, adjust dynamic ranges, and finally meet the needs of practical applications (Ding et al., 2021).
3.1 Literature and omics mining
Transcriptional regulation is the most common form of gene expression regulation in organisms, enabling genes to be uniquely expressed in response to environmental changes (Mitchler et al., 2021). Up till now, more and more transcriptional regulation elements, including the molecule-binding TFs and their cognate promoters, have been studied and reported (Ambri et al., 2020). Hence, literature mining is crucial for obtaining the potentially transcriptional elements for HTS. For example, X. Qiu et al. (2020) developed a synthetic microbial biosensor system (SMB) for erythritol overproducers screening using Ery operon originating from Brucella species, reported by J. Zhang et al. (2014). In detail, X. Qiu et al. (2020) firstly demonstrated the effective interactions between the transcriptional factor EryD, promoter PEry, and erythritol in vitro by Isothermal Titration Calorimetry (ITC) assay. However, direct introduction of EryD and promoter PEry into E. coli showed no functional activity. They speculated that this result was attributed to the distinct transcription mechanism between E. coli and Brucella abortus. Therefore, they turned to construct a hybrid promoter by inserting truncated nucleotide sequences of PEry (suppositional EryD-binding region) into T7 promoter. As a result, a hybrid promoter with inserting 104 bp into T7 gave a strong eGFP expression, which was named as SMB. Further, the obtained SMB was used to characterize 1152 mutants obtained by UV and ARTP, and the optimized strain produced 148 g/L erythritol in the 3-L fermenter (X. Qiu et al., 2020).
Besides, omics technologies such as genomics and transcriptomics are also important ways to obtain potential transcription elements, so as to comprehensively analyze cell metabolism to find new transcription elements (Alvarez-Gonzalez & Dixon, 2019; Zeng et al., 2020). For example, Grazon et al. (2020) designed a combination approach of genomic screening and functional analysis to identify and isolate biosensing TFs and used this approach to identify a progesterone-sensing TF and further developed it into a progesterone optical sensor. Sun et al. (2020) discovered a new styrogen-responsive biosensor by mining Novosphingobium Aromaticivorans DSM 12444 genome, which can distinguish resveratrol from p-coumaric acid and trans-cinnamic acid. Moreover, this biosensor showed a 667-fold enrichment in FACS by sensing the intracellular resveratrol concentration.
3.2 Promoter engineering
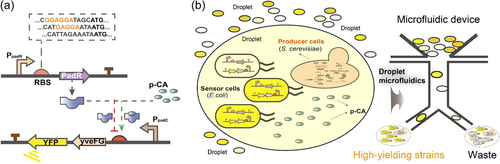
Optimization of the expression level of TFs is a common strategy to low background noises and optimize dynamic ranges of synthetic biosensors (Cui et al., 2021). Several attempts at promoter engineering have shown great success in fine-tuning the expression level of TFs to optimize TFs-based biosensors for HTS. For example, engineering promoter properties have been used to construct a highly sensitive biosensor to optimize caprolactam production, an important polymer precursor to nylon (M. G. Thompson et al., 2020). In this case, a lactam-sensitive transcription factor OplR was found in Pseudomonas putida KT2440 through leveraging proteomics data, which is more sensitive than the previously reported caprolactam sensors. Furthermore, control of OplR expression significantly affected the detection range of caprolactam, ranging from 700 nM to 1.23 mM, suggesting that the sensing parameters of transcription factors can be modulated by changing the expression level of transcription factors (M. G. Thompson et al., 2020). Besides, customizing the ribosome binding sites (RBS) could optimize the protein translation efficiency of TFs (Ding et al., 2021). For example, Siedler et al. (2017) randomly mutated the RBS of PadR, a coumaric acid transcription repressor, and increased its response range to 130-fold.
In addition to optimizing the expression level of TFs, the numbers and location of TFs binding sites in promoters also play a significant role in the dose–response of TFs-based biosensors (Ding et al., 2021). Cui et al. (2019) found that mutation or deletion of the “0A box” sequences, the phosphorylation transcription factor spo0A-P binding sites, in the native PspoIIA promoter can generate different affinities promoters for Spo0A-P. By altering the location, number, and sequences of Spo0A-P binding sites, they obtained a series of synthetic promoters with different regulation levels by Spo0A-P. Moreover, designing a hybrid promoter using candidate TFs binding sites is an efficient method for developing a novel biosensor (Hossain et al., 2020). Wu et al. (2020) constructed 14 inducible hybrid promoters by inserting the glucosamine-6-phosphate (GlcN-6P) TF GamR binding sites, gamO2, into two sides or middle position of the −10 and −35 regions of Pveg promoter in B. subtilis. Furthermore, a GlcN-6P responsive biosensor was obtained by using these constructed hybrid promoters, which showed a 13.4-fold improved dynamic range (Wu et al., 2020). Specifically, inserting two and more TFs binding sites in the promoters driving the reported endows TFs-based biosensors with a multiligand input. For example, an artificial biosensor with a multiligand input that strictly controls gene expression was constructed by inserting two TFs binding sites in the upstream of the −35 regions and between the −10 and −35 regions in the cognate promoter, which exhibit high-efficiency induction only in the presence of two inducers, including xylose and IPTG (Y. Chen et al., 2018).
3.3 Protein engineering
Although many TFs have been reported, the number of known TFs is still limited, making it sometimes challenging to find relevant TFs for the desired products in HTS applications (C. Qiu et al., 2019). Moreover, microbial cell factories are often used to synthesize some unnatural chemicals that may not be directly sensed by any characterized TFs (Lim et al., 2018). Therefore, expanding the specificity of characterized TFs for more chemicals is very meaningful and desirable. For this, much effort has focused on rational and random protein engineering. Notably, TFs bind to target effector molecules and generate conformation changes, which allows them to bind in (or release from) the specific sequences in promoters to regulate the expression of genes (Mitchler et al., 2021). According to this mechanism, adjusting the protein structure of TFs shows excellent potential. X. Zhang et al. (2021) altered the effector specificity of the regulatory protein VanR from vanillic acid to vanillin by simultaneous site-saturation mutagenesis of VanR's amino acid residues Y103, R148, and Y168. H. Li, Liang, et al. (2017) developed a whole-cell biosensor that responds explicitly to shikimate by introducing saturated mutations that affect ligand binding to pocket residues of a uric acid-responsive TF, indicating saturated mutagenesis is an efficient strategy to change the TFs specificity. Besides, the evolution technology also provides a new way to evolve the ligand specificity of TFs. A good example of evolving the ligand specificity of TFs through evolution technology is vanillin production (Machado et al., 2019). In this case, the MarR TFs family member PcaV, responsive to protocatechuic acid (PCA), underwent directed evolution to alter its ligand specificity from PCA to vanillin and other close aromatic aldehydes (Machado et al., 2019). Further, amino acid sequencing and mutational analysis revealed that M113S and N114A of PcaV are necessary to alter the specificity to vanillin, and I110V could reduce the background noise of PcaV (Machado et al., 2019), suggesting that TFs are plastic to alter its ligand specificity by the evolution technology.
On the other hand, TFs usually contain a metabolite-binding domain (MBD), a DNA binding domain (DBD), and at least a domain of recruiting transcriptional machinery (J. Zhang et al., 2015). The high modularity of TFs makes it possible to endow novel properties with high selectivity and affinity to a new molecule by rational domain swapping (J. Zhang et al., 2015). Umeyama et al. (2013) fused the DBD and MBD of the E. coli MetJ repressor to develop a S-adenosylmethionine biosensor in Saccharomyces cerevisiae, which can sense S-adenosylmethionine to binding DBD-specific nucleotide sequences, metO, in the upstream of the TATA box. De Paepe et al. (2019) explored a variety of chimeric detector−effector pairs by fusing a donor regulatory circuit, from Sinorhizobium meliloti, with a nonspecific biosensor chassis, from Herbaspirillum seropedicae. The generated biosensors can selectively respond to luteolin from three closely related flavonoids, including apigenin, naringenin, and luteolin, which is the first reported luteolin-specific TFs-based biosensor in E. coli (De Paepe et al., 2019). Moreover, Chou and Keasling (2013) developed a new transcription factor that can respond to IPP, isopentenyl diphosphate, by fusing the IPP isomerase with the DBD of the arabinose-responsive transcription factor AraC.
4 APPLICATIONS OF TFs-BASED BIOSENSORS FOR HTS
TFs-based biosensors have attracted extensive attention owing to their potential for strain evolution, enzyme engineering, and real-time monitoring of chemicals (J. W. Li et al., 2020; M. Xu et al., 2020). Moreover, much effort has been put into developing TFs-based biosensors for the construction of microbial cell factories, including identifying rate-limiting genes, optimizing biosynthetic pathways, and screening high-yielding strains (Lim et al., 2018; Yu et al., 2019). There are many examples of using TFs-based biosensors to screen high-yielding strains in the production of nutritional chemical products, including functional sugars, alcohols, organic acids, amino acids, flavonoids, and more (Cui et al., 2021; Lim et al., 2018; J. Z. Xu et al., 2018). For a clearer presentation, we just take flavonoids, aromatic chemicals, amino acids, and lipid chemicals as examples.
4.1 Flavonoids
Flavonoids, a class of important secondary metabolites, generally have biological activities such as free radical scavenging, antioxidant, anticancer, and anti-inflammatory, and have essential health-care and medicinal value (Gu & Xu, 2021; Lv et al., 2020). Due to the continuous increase of flavonoid applications in medicine, agriculture, and other fields, the market demand has continued to expand, and their sustainable production and manufacturing issues have attracted widespread attention (Lv et al., 2019). With the development of technologies in various omics, bioinformatics, molecular biology, and other related fields, breakthroughs have been made in natural product biosynthesis and synthetic biology (Gu & Xu, 2021). Furthermore, it has become possible to construct the flavonoid biosynthetic pathway in microorganisms (Lv et al., 2019). Mainly, biosensors based on TFs combined with fluorescent protein reporter systems have been applied to enhance the microbial production of flavonoids. For example, Siedler et al. (2014) constructed two flavonoid biosensors in E. coli by using the transcription activator FdeR from H. seropedicae and the repressor QdoR from B. subtilis. The experimental results showed that FdeR was responsive to naringenin, whereas QdoR was responsive to quercetin and kaempferol. After adding effectors, the fluorescence signal of both biosensors increased by more than sevenfold, and the fluorescence intensity was linearly related to the concentration of effectors. In this case, the QdoR biosensor was successfully applied to enhance the production of kaempferol by combining FACS (Siedler et al., 2014). Besides, Raman et al. (2014) constructed a TtgR-TolC biosensor in E. coli based on the transcriptional repressor TtgR, which responds to naringenin. Combined with targeted genome-wide mutagenesis to alter the expression of naringenin production pathway genes, the production of naringenin increased 36-fold to 61 mg/L, more than twice the highest titer reported (Raman et al., 2014).
Meanwhile, Skjoedt et al. (2016) verified the functional activity of prokaryotic lysr-type transcriptional regulators LTTRs in S. cerevisiae, and determined that LTTR-dependent reporter gene expression was inhibited in the presence of cognate small effectors. A FdeR-based biosensor with output related to the naringenin yield was constructed based on the TF, and it was used to screen S. cerevisiae with producing different levels of naringenin (Skjoedt et al., 2016). In addition, Wang et al. (2019) also designed a naringenin-responsive biosensor in S. cerevisiae, but using a multiparameter engineering strategy. In this study, the detection range and sensitivity of naringenin-responsive biosensor were further optimized by the engineering detector and effector module before screening mutants with the high naringenin production (Wang et al., 2019).
4.2 Amino acids
Due to the importance of amino acids in cosmetic, medical, food, and other industrial applications, improving their yield, and production efficiency by microbial fermentation has attracted widespread attention (Lim et al., 2018). Strain is the core of industrial microbial fermentation (Clomburg et al., 2017), thus obtaining high-yielding strain is the key to further improving amino acid production. For this, Han et al. (2020) designed a biosensor capable of detecting the concentration of l-valine based on the transcriptional regulator LRP from C. glutamicum. In their work, gene gfp was inserted behind LRP-cognate promoter PbrnF to transform l-valine concentrations into the fluorescence response value. On the basis of this biosensor, 900 mutants generated by ARTP were screened, and the strain HL2-7 was obtained, which produced 3.20 g/L of l-valine with a 21.47% increase compared to the starting strain HL104 (Han et al., 2020). Wang et al. (2016) achieved the coupling of lysine titer and fluorescent protein intensity by promoting the expression of green fluorescent protein (EGFP) through an l-lysine-inducible promoter. Based on this, from a library of 10 million mutants, two strains with high l-lysine production, namely MU-1 and MU-2, were screened by FACS. Furthermore, MU-1 and MU-2 produced 136.51 ± 1.55 and 133.29 ± 1.42 g/L of lysine in the 5-L fermenters, which were 9.05% and 10.41% higher than the starting strain, respectively (Liu et al., 2015). However, for most other amino acids, such as threonine, the HTS system has not been established due to the lack of relevant TFs. Under this scenario, proteomic analysis is a beneficial technique to screen useful TFs and can be applied to the development of HTS methods. For example, two novel threonine-sensing promoters in E. coli, including cysJp and cysHp, were discovered by proteomic analysis (Liu et al., 2015). Subsequently, an HTS method for FACS was constructed based on the fusion of cysJp and cysHp promoter and green fluorescent protein reporter gene, which completed the screening library of 20 million mutants within 1 week. The threonine titer of obtained 34 mutants was significantly higher than the control strain, and the highest yield increased by 17.95% in the 5-L fermenter (Liu et al., 2015).
4.3 Aromatic chemicals
Aromatic chemicals are widely used in the fields of medicine, food, and the chemical industry (Shen et al., 2020). However, the titer of aromatic chemicals produced by microorganisms is very low under natural conditions, making it challenging to meet the requirement of industrial production (B. Thompson et al., 2015). In microorganisms, 3-deoxy-d-arabinoheptulose-7-phosphate (DAHP) is first condensed with phosphoenolpyruvate and erythrose-4-phosphate as precursors; then, DAHP is transformed to chorismate through six-step catalytic reactions in the shikimate pathway; and further, chorismate is used as a precursor to synthesize a variety of aromatic derivatives (Gu, Ma, Zhu, Ding, et al., 2020; Gu, Ma, Zhu, Xu, 2020). However, redirecting carbon fluxes toward the synthesis of aromatic chemicals is difficult, which requires disrupting signals that interfere with the feedback regulation (Gu, Ma, Zhu, Xu, 2020; Nielsen & Keasling, 2016). Therefore, HTS provides an available solution to promote the microbial synthesis of aromatic derivatives. Qian et al. (2019) designed and constructed a biosensor based on the salicylic acid-responsive variant AraC-SA to screen the optimal combination of expression intensities of six genes in the salicylic acid pathway. As a result, the salicylic acid titer of obtained strain was 1.23-fold of that in the starting strain cultured in the shake flasks (Qian et al., 2019). Specifically, the cross-integration of computer simulation, sequence analysis, and synthetic biology technologies has brought protein engineering into the era of rational design and de novo design. Moreover, using bioinformatics means to analyze the information of target TFs could lock the mutation sites and significantly improve the effective mutation rate. For example, De Los Santos et al. (2016) used the computational protein design tool Phoenix Match to simulate the potential binding sites of vanillin and designed vanillin-responsive mutants based on the TF protein QacR from Staphylococcus aureus.
4.4 Oleo-chemicals
Lipid chemicals are mainly composed of long carbon chains and hydrogen and oxygen elements and have critical applications in many fields, especially in pharmaceuticals and functional foods. In particular, sustainable and efficient production of lipid compounds through microbial fermentation can break through the bottleneck problem of natural product development and utilization (Jia et al., 2021). In microorganisms, extracellular carbon feedstock, such as glucose, is firstly internalized and converted into cytosolic pyruvate by the glycolytic pathway (Qiao et al., 2017). Then, pyruvate is converted to acetyl-CoA, which is a basic building block for lipid synthesis. However, lipids are the highly reduced metabolites, which need to consume the cytosolic NADPH to reduce acetyl groups (CH3−CO−) to acyl groups (−CH2−CH2−) when growing fatty acid carbon backbone (J. Xue et al., 2017). To improve the production of lipids, Baumann et al. developed a biosensor that responds to short and medium-chain fatty acids (SMCFA), to screen high-producing strains of SMCFA in S. cerevisiae. The biosensor contains a PDR12 promoter that can be activated by SMCFA and a coupled reporter gene gfp, and the function was verified by adding caprylic acid and its derivatives in the culture (Baumann et al., 2018). This biosensor enables HTS of SMCFA producers and has the potential to significantly accelerate the process of constructing the SMCFA microbial cell factories.
On the other hand, designing sensors of precursors in lipid synthesis, such as acetyl-CoA, can also improve lipid synthesis by microorganisms. For example, an acyl-CoA screening system was established to screen for genes that increase the acyl-CoA levels by using FadR, a TF from E. coli (Dabirian, Gonçalves Teixeira, et al., 2019). Using this constructed biosensor, genes RTC3, GGA2, and LPP1 were identified and overexpressed, resulting in a 1.80-fold increase in fatty alcohol titer. This is the first time acyl-CoA-based biosensor has been used to find genes that enhance the synthesis of fatty acids and derivatives in S. cerevisiae (Dabirian, Gonçalves Teixeira, et al., 2019). In addition, a whole-cell E. coli biosensor was developed for rapidly reporting intracellular malonyl-CoA concentrations, which was applied for identifying targets that are critical for improving the malonyl-CoA biosynthesis at a genome-wide scale (H. Li, Chen, et al., 2017). The titers of phloroglucinol, free fatty acids, and polyketide triacetic acid lactone (TAL) produced by obtained mutant synthesized were significantly increased (H. Li, Chen, et al., 2017).
5 CONCLUSIONS
TFs have been successfully used for HTS of high-yielding strains, avoiding tedious and time-consuming detection (Ding et al., 2021; Lim et al., 2018). However, despite the significant success in improving the performances of TFs-based biosensors, predicting and developing novel TFs-based biosensors for HTS applications remain challenging (Cui et al., 2021). Understanding the complex interactions and regulatory mechanisms among promoters, TFs and reporters will provide a solid foundation for the rational design of TFs-based biosensors. Specifically, biological-based data mining combined with deep learning and machine learning will facilitate rational design (Dabirian, Li, et al., 2019; Ding et al., 2021). Moreover, Baker et al. developed the ROSETTA technology based on the principle of protein folding to the lowest free energy state, which aimed at pursuing completely new functional proteins (Koepnick et al., 2019). Langan et al. designed a unique switchable system based on ROSETTA that is a static five-helix “cage” with a single interface interact either intramolecularly with a terminal “latch” helix or intermolecularly with a peptide “key”. If the key displaces the latch from the cage, the latch will output order control binding, degradation, or nuclear export (Langan et al., 2019). It is a promising strategy to fuse metabolite-binding protein with a specific DNA-binding domain to produce a synthetic TF by designing switchable protein functions that are controlled by induced conformational change. Therefore, the ROSETTA technology can be used to design new TFs. In addition, the continuous development of synthetic biology also has strengthened the dominant position of TFs-based biosensors in basic and applied research. Hence, we hope this review will help take full advantage of valuable TFs to guide HTS technology and further promote the development of TFs-based biosensors in HTS applications.
AUTHOR CONTRIBUTIONS
Changfan Li: Writing—original draft; writing—review and editing. Chang Wang: Writing—review and editing. Jiang Zhu: Writing—review and editing. Feng Xue: Writing—review and editing. Xiaoman Sun: Writing—review and editing. Yang Gu: Writing—original draft; writing—review and editing.
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (22108126), and the Natural Science Foundation of Jiangsu Province (BK20202002 and BK20210572).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
None declared.




