Multilevel effects of sublethal fenitrothion exposure in Chironomus riparius Mg. (Diptera, Chironomidae) larvae
Abstract
The effects of fenitrothion exposure on fourth-instar Chironomus riparius larvae were investigated on biochemical, physiological, and population-level parameters. Biochemical effects were investigated through measurements of acetylcholinesterase and cytosolic superoxide dismutase activities. Water content and dry weight of the larvae were used as physiological parameters, and the emergence rate of adults was used as a descriptor of population-level effects. Results showed that the response of most parameters exhibited a concentration-dependent relationship. Although biochemical parameters proved to be very sensitive, no direct relation was observed with effects at a higher level of biological organization. Perturbations of osmoregulation, as reflected by changes in water content of the larvae, were more directly related with emergence failure. This study demonstrates that the use of several biological parameters can provide complementary information about the effects of chemical exposure. Therefore, use of a multilevel approach in C. riparius seems to be a promising way to diagnose environmental quality.
INTRODUCTION
Midge larvae of the genus Chironomus are frequently able to survive and develop in hypoxic or polluted environments [1, 2]. Considering the worldwide distribution of Chironomus larvae and their potential as sentinel organisms in environmental monitoring [3-7], information regarding correlations between physiological condition and demographic parameters in the presence of toxicants seems to be relevant for monitoring the effects of environmental pollutants. The effects of various physical and chemical stresses on antioxidant enzyme activities and energy metabolism in Chironomus riparius Mg. (Diptera, Chironomidae) larvae have already been studied [8-11]. Responses of these parameters could be used as an early warning system for environmental monitoring, and the early detection of sublethal effects may be used to identify the need for remedial action at a study location [12-17]. Pollutant-induced biochemical effects may potentially have consequences at higher levels of biological organization, such as changes in population dynamics and in biological diversity at both the intra- and interspecific levels [18, 19]. Moreover, such changes may have adverse ecological consequences [18]. Therefore, biochemical endpoints alone do not seem to be sufficient to diagnose environmental quality, and multilevel as well as multibiomarker approaches would be more prudent for useful environmental monitoring [13-17, 20].
The chronic effects of environmental pollutants on the population dynamics of midges of the genus Chironomus have been widely studied [21-25]. However, effects at the biochemical or physiological levels and consequences on population have rarely been simultaneously assessed. In the present study, the responses of C. riparius larvae to fenitrothion exposure were assessed at various levels of biological organization to better understand the impact of this compound on adult emergence. Fenitrothion is an organophosphate insecti- cide, which acts by irreversibly inhibiting the activity of acetylcholinesterase (AChE). The effects of this neurotoxicant on C. riparius were investigated at the biochemical (AChE and cytosolic superoxide dismutase [Cu, Zn-SOD] activities) and physiological levels (water content and dry wt of the larvae) as well as in terms of adult emergence. Residue analysis was also performed to verify the exposure level of the larvae to fenitrothion.
MATERIALS AND METHODS
Organisms
The C. riparius strain was provided by INERIS (Institut National de l'Environnement Industriel et des Risques, Verneuil- en-Halatte, France). Larvae were reared in the laboratory in aerated 25-L glass aquaria filled with dechlorinated tap water and were maintained under a constant temperature (20 ± 1°C SD) and a 14:10-h light:dark cycle. A 5-cm thick layer of washed siliceous sand and cellulose (Sigma-Aldrich, St Quentin Fallavier, France) mixture was used as the substrate. Adult chironomids were retained in wooden cages covered with steel wire mesh (mesh size, 1 mm), thus ensuring oviposition in the aquaria.
Exposure conditions
Glass tanks (20 × 15 × 20 cm) containing 2 L of dechlorinated tap water and 1 cm of substrate layer were used. Fourthinstar larvae were collected in the rearing aquaria using a hand net (mesh size, 0.25 mm). Larval instar was determined using head capsule size [26, 27]. At the beginning of the experiment, 2 ml of an acetonic solution of fenitrothion (Cluzeau Info Labo, Sainte Foy la Grande, France) were added to the experimental tanks. One-hundred larvae were then randomly introduced into each test aquarium. Exposure was carried out under constant temperature (20 ± 1°C SD), and a photoperiod of 14:10-h light:dark was used for all the experiments.
Fenitrothion fate
Two fate studies of different duration (24 and 72 h) were conducted. Water samples were collected at various times after the beginning of the experiment for fenitrothion residue analysis (1, 2, 4, and 24 h and 1, 24, 48, and 72 h after the beginning of the experiment for the 24-h and the 72-h studies, respectively). The C. riparius larvae were collected at each sampling time for AChE activity and fenitrothion residue measurements in the 24-h and 72-h study to investigate whether fenitrothion had penetrated into the animals and reached its target. The nominal concentration of fenitrothion in water at the beginning of the study was 10 μg/L, and three replicates were used. Exposure was renewed 48 h after the initial contamination in the 72-h experiment.
Exposure levels and duration
The results of previously performed acute toxicity tests [11] were used to determine sublethal exposure levels. Exposure levels ranged from one-thousandth to one-hundredth of the 24- h lethal concentration values of 50% (LC50; i.e., 2, 5, 10, and 20 μg/L) for all toxicity experiments. Lower exposure levels (0.0002, 0.002, 0.02, 0.2, and 2 μg/L) were used to evaluate the effects of fenitrothion on Cu,Zn-SOD and AChE activities in additional experiments. Exposure was not renewed during the experiment. Exposure was for 24 h, except for an additional study on Cu,Zn-SOD (96-h exposure) and for the assessment of the effects on adult emergence rate, in which the exposure lasted until the emergence was completed. Five replicate aquaria were used for each exposure level and control.
Fenitrothion analyses
At each sampling time, 15 ml of water and 10 larvae were collected in experimental tanks. Water was filtered through paper filters and introduced into a glass vial. Five milliliters of Pestipur hexane (SDS, Peypin, France) were added, and fenitrothion was extracted by mechanical agitation (3 × 5 min). Larvae were pooled, weighed, and homogenized for 2 min in 5 ml of Pestipur petroleum ether (SDS) containing 5 g of anhydrous sodium sulfate. Extraction was performed for 8 h by continuous petroleum ether distillation in a Soxhlet apparatus. Samples were concentrated using a rotary evaporator and cleaned-up on a Florisil column (Touzart&Matignon, Courtaboeuf, France). Following the extraction and purification steps, water and animal samples were evaporated, and residue was redissolved in hexane for quantification. Quantification was performed by gas chromatography (Gridel series 3000, Gridel Instrument, Suresnes, France) with an electroncapture detector (63Ni source) using 5 μl of extract. The extraction efficiencies for water and animal samples were 93.3 ± 7.1% (n = 3) and 85.6 ± 5.6% (n = 3), respectively.
Enzyme assays
A total of 10 larvae were collected 24 h after treatment from control and experimental tanks and pooled for AChE activity measurements. For Cu,Zn-SOD activity measurement, samples were collected after 24, 48, 72, and 96 h of exposure. Larvae were homogenized in 2.5 ml of tris-ethylenediaminetetraacetic acid buffer (40 mM, pH 7.8; Sigma-Aldrich) using a Potter-Elvehjem homogenizer (Biollock-Scientific, Illkvich, France). Crude homogenate was centrifuged for 15 min at 500 g (4°C), and supernatant was centrifuged for 30 min at 12,000 g (4°C). The resulting supernatant (postmitochondrial fraction) was used to measure the activity of Cu,Zn-SOD and AChE. The Cu,Zn-SOD activity was determined after hemoglobin precipitation and extraction in ethanol:dichloromethane (2:1, v/v) by the chemiluminescence method of Bensiger and Johnson [28] using a LKB Wallac type 1250 luminometer (EG2G Wallac, Turku, Finland). Arbitrary enzyme units were used, with one unit corresponding to the amount of enzyme that produced a 50% inhibition of chemiluminescence when compared to the control. The AChE activity was measured using the method of Ellman et al. [29]. Enzymatic activities were calculated relative to the protein content of the extracts, as measured by the method of Bradford [30].
Water content and body dry-weight measurement
Water content and body dry weight were measured on 10 larvae collected 24 h after the beginning of the exposure. Fresh weight was immediately measured. Larval dry weight was evaluated after placing the larvae at 105°C for 24 h, and water content was calculated from the respective dry and fresh weights. Weighing was performed to the nearest 0.1 mg.
Adult emergence rate
For the measurement of adult emergence rate, 100 fourthinstar larvae were introduced at the beginning of the experiment. Emerging adults were retained using wood cages covered with steel wire mesh and counted daily until the emergence had been completed in the control and experimental aquaria. Time to the completion of emergence varied from 10 to 15 d, according to the treatment. Every 2 d, 100 mg of Tetramin fish food flakes (Tetra, Courbevoie, France) were supplied to each aquaria. Test solutions were not renewed.
Statistics
Statistical differences were examined using the parametric t test. The no-observed-effect concentration (NOEC) and the lowest-observed-effect concentration (LOEC) for each parameter were determined using analysis of variance followed by Dunnett's test. Correlations were examined using Pearson's coefficient. All analyses were performed using StatViewr̀ 4.02 for a MacIntosh computer (SAS, Cary, NC, USA).
RESULTS
Fenitrothion residue analysis
Results of the 24-h study show that fenitrothion concentration decreased rapidly during the first 4 h and that less than 50% of the initial amount remained after 24 h (Fig. 1A). The same pattern was observed for the changes in fenitrothion concentration in water and larvae during the 72-h experiment (Fig. 1B). The AChE inhibition was significant as soon as 1 h after the beginning of exposure (70% inhibition when compared to control after 1-h exposure), and no recovery was observed during the experiment.
Biochemical and physiological effects
The Cu,Zn-SOD activity significantly increased (24–86% when compared to the control) and AChE activity significantly decreased (28–90% when compared to the control) at all the tested fenitrothion concentrations (Fig. 2A). The decrease in AChE activity occurred in a concentration-dependent manner (Pearson's r = - 0.931, p = 0.022). Exposure to fenitrothion caused an increase in Cu,Zn-SOD activity values (Fig. 2A), but not according to a concentration-dependent pattern.
A concentration-dependent decrease in body water content was observed (Pearson's r = 20.978, p = 0.004) (Fig. 2B). A significant effect of fenitrothion on body dry weight was only observed for the highest exposure concentration (decrease of 21% when compared to the control).
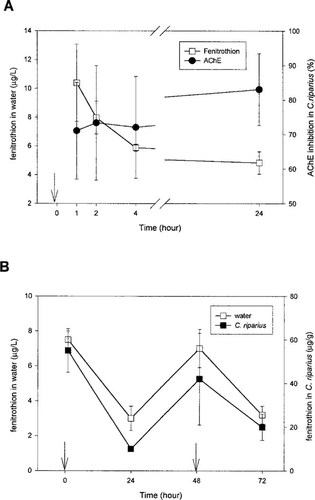
Changes with time of fenitrothion concentration in water (mean 6 SE (n = 3. Acetylcholinesterase (AChE) inhibition in Chironomus riparius larvae (A; mean ± SE, n = 3) and fenitrothion concentration in C. riparius larvae (B; mean ± SE, n = 3) are shown. The AChE inhibition is expressed as a percentage of enzyme activity measured before exposure (time 0).
A significant increase in Cu,Zn-SOD activity was observed in larvae exposed to very low concentrations of fenitrothion (0.002, 0.02, and 0.2 μg/L) (Fig. 3). The AChE activity was only significantly decreased (35% inhibition when compared to the control) in larvae exposed to 0.2 μg/L of fenitrothion (Fig. 3).
An additional 96-h study was done regarding the activity of Cu,Zn-SOD in larvae exposed to 2 and 5 μg/L of fenitrothion (Fig. 4). Activation of Cu,Zn-SOD after exposure to 2 μg/L of fenitrothion was statistically significant for 48 h. Afterward, a decrease in enzyme activity was observed, but values remained higher (although not significantly so) than those in the control larvae. A significant effect was observed in the larvae exposed to 5 μg/L of fenitrothion only 24 h after the beginning of exposure.
Adult emergence
A complete inhibition of emergence was observed at the two highest concentrations (10 and 20 μg/L) (Fig. 5), whereas the lower concentrations did not have any significant effect. A negative relationship was observed between the fenitrothion concentration and the adult emergence (Pearson's r = 20.883, p = 0.047).
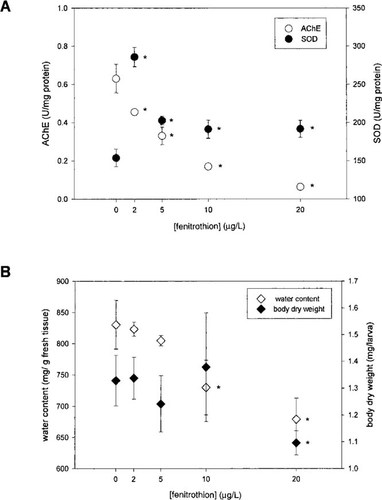
Effects of fenitrothion exposure on superoxide dismutase (SOD) and acetylcholinesterase (AChE) activities (A; mean ± SE, n = 5) and water content and body dry weight (B; mean ± SE, n = 5) in fourth-instar larvae of Chironomus riparius exposed for 24 h to sublethal concentrations of fenitrothion. The asterisk denotes a significant difference from control (p < 0.05).
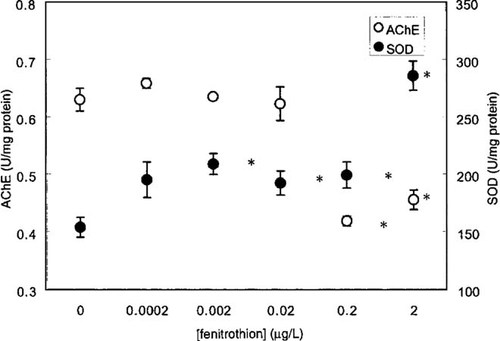
Effects of fenitrothion exposure on superoxide dismutase (SOD) and acetylcholinesterase (AChE) activities (mean ± SE, n = 5) in fourth-instar larvae of Chironomus riparius exposed for 24 h to sublethal concentrations of fenitrothion. The asterisk denotes a significant difference from control (p < 0.05).
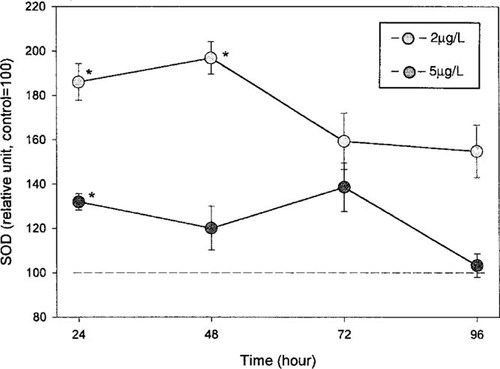
Effects of fenitrothion on superoxide dismutase (SOD) activity (mean ± SE, n = 5) in fourth-instar larvae of Chironomus riparius. Results are expressed as a percentage of the corresponding control value. Fenitrothion exposure levels were 2 and 5 μg/L, and measurements were performed 24, 48, 72, and 96 h after exposure. The asterisk denotes a significant difference from the control (p < 0.05).
Comparison of C. riparius responses to fenitrothion exposure
The NOEC and LOEC values for the different parameters are summarized in Figure 6. The LOEC values for Cu,Zn-SOD and AChE activities were 0.002 and 0.2 μg/L, respectively (i.e., 1 × 106 and 1 × 104 lower than the 24-h LC50, respectively). The LOEC values for water content of the larvae and adult emergence were identical (10 μg/L; i.e., 200-fold lower than the 24-h LC50). Effects on body dry weight were only observed at the highest tested concentration (20 μg/L; i.e., 100-fold lower than the 24-h LC50).
DISCUSSION
In this study, parameters were measured at various levels of biological organization to investigate the effects of fenitrothion exposure in fourth-instar C. riparius larvae. As expected, results show that the responses of biochemical parameters were much more sensitive than physiological or population-level descriptors. The most sensitive endpoint was Cu,Zn-SOD activity, whereas dry weight of the larvae was the least sensitive. Enzymes activities, physiological (water content and dry wt), and population-level parameters (adult emergence rate) and mortality were related to fenitrothion exposure in a concentration-dependent manner.
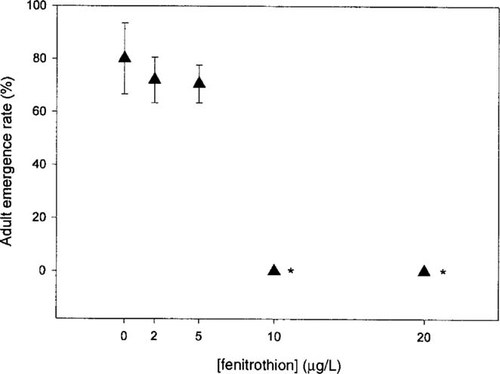
Effects of fenitrothion exposure on Chironomus riparius adult emergence rate (mean ± SE, n = 5). Results are expressed as a percentage of the total number of larvae introduced at the beginning of the experiment. The asterisk denotes a significant difference from the control (p < 0.05).
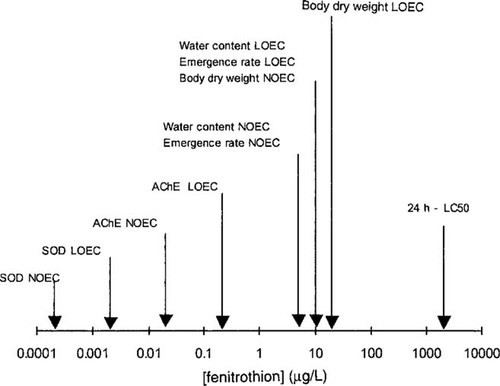
Response spectrum of various parameters measured in Chironomus riparius exposed to fenitrothion. (Data for the 24-h 50% lethal concentration (LC50) values are from [11].) AChE = acetylcholinesterase; LOEC = lowest-observed-effect concentration;NOEC = no-observed-effect concentration; SOD = superoxide dismutase.
Chemical analysis revealed that the changes with time in the concentration of fenitrothion in water and larvae exhibited a similar pattern, with rapid absorption and excretion (or detoxification) of this compound by the larvae, thus suggesting a low probability of long-term bioaccumulation. Despite its associated difficulties and limitations, use of the internal body concentration of fenitrothion may have distinct advantages compared with use of environmental concentrations as a toxicological index. In terms of toxicity, bioaccumulation data can be explained more meaningfully and make a better connection between the accumulated dose and the toxicological effect, thus permitting better interpretation of the hazard associated with complex exposure routes [31].
The activity of the antioxidant enzyme Cu,Zn-SOD showed a surprisingly higher sensitivity than AChE, the direct target of fenitrothion. This response may result from particular characteristics of C. riparius larvae, which contain a relatively high amount of hemoglobin in the hemolymph. The ability of C. riparius larvae to live in hypoxic or polluted environments is related to this high hemoglobin content [32, 33]. However, the auto-oxidation of hemoglobin is an important source of superoxide radical [34]. Previous studies have shown that enzymatic radical scavengers, including Cu,Zn-SOD and Mn- SOD, are abundant in C. riparius larvae [35] and that early Cu,Zn-SOD and Mn-SOD response was nonstressor-specific [10]. Moreover, the time-course study revealed that recovery of Cu,Zn-SOD activity occurred 48 or 72 h after exposure, depending on the concentration tested. Thus, the early response of Cu,Zn-SOD to low concentrations of fenitrothion may be considered as a homeostasis-maintaining process rather than as an indicator of permanent adverse effects of fenitrothion. Its response at high fenitrothion concentrations probably reflects an adverse effect of the exposure, because perturbations of physiological parameters were observed at these concentrations. Homeostatic responses of Cu,Zn-SOD seem to have little impact at higher levels of biological organization. Therefore, Cu,Zn-SOD should perhaps be considered as an early warning biomarker of pollution rather than as a specific biomarker of an adverse effect. The level of AChE inhibition increased as soon as the larvae were exposed to fenitrothion; this rapid response reflects the mode of action of fenitrothion.
Because NOEC and LOEC values for water content of the larvae and for adult emergence were identical, it may be hypothesized that larval osmoregulation is directly related to population- level effects and, thus, that severe perturbation of this process may lead to a complete failure of emergence. However, more detailed studies should be performed to identify the mechanistic link between these two processes. Decrease of body dry weight, an indicator of animal growth, at the highest concentration of insecticide suggests that alteration of this parameter might be considered as the consequence of a serious progression of the toxic effect.
Chironomids are considered to be good biological models for the study of chronic effects of environmental pollutants at the population level because of their short life cycle, high fecundity, and ability to be reared under laboratory conditions [21-23]. The response of several biomarkers to toxicants has already been evaluated [4, 7, 10, 11]. Various biochemical parameters measured in C. riparius larvae, such as AChE, Cu,Zn-SOD, glutathione S-transferases, electron-transport system, and energy yielding-substrates, have already demonstrated a high sensitivity toward environmental pollutants [4, 7]. Results of the present study suggest that population-level changes are less sensitive than biochemical responses. However, few direct experimental demonstrations of the wider relationships between biochemical effects and their subsequent consequences at higher levels of biological organization have appeared. Characterization of the causal relationships between biomarker responses and effects at higher biological levels would help to define the sublethal hazards of chemicals in this animal.
In freshwater ecosystems, chemical pollution is frequently caused by a complex mixture of pollutants. This considerably increases the difficulty in predicting pollutant effects and emphasizes the need for studies involving multiple biological endpoints to identify pertinent biomarkers. Simultaneous measurement of various biological parameters gives the opportunity to obtain data at different levels of biological organization, and it may help to fully understand the effect of a toxicant on organisms. In addition, the determination of population-level parameters improves the interpretation of data collected at lower biological levels [36].




