A comparison of chelator-facilitated metal uptake by a halophyte and a glycophyte
Abstract
Phytoextraction is the use of plants to remove contaminants, in particular metals, from soil via root uptake and translocation to the shoots. Efficient phytoextraction requires high-biomass plants with efficient translocating properties. Halophytes characteristically accumulate large quantities of salts in aboveground tissue material and can have high biomass production. It has been speculated that salt-tolerant plants may also be heavy metal tolerant and, further, may be able to accumulate metals. This study compared growth and metal uptake by a halophyte, Atriplex nummularia, and a common glycophyte, Zea mays, in a minetailing contaminated soil:mulch mixture. Two chelators, ethylenediaminetetraacetic acid (EDTA) and rhamnolipid, were used to facilitate plant metal uptake. Despite a lower growth rate (2% growth/d) in the contaminated soil, the halophyte accumulated roughly the same amount of metals as the glycophyte on a mass basis (30–40 mg/kg dry wt). Neither plant, however, hyperaccumulated any of the metals tested. When treated with EDTA, specific differences in patterns of metal uptake between the two plants emerged. The halophyte accumulated significantly more Cu (2X) and Pb (1X) in the shoots than the glycophyte, but root metal concentrations were generally higher for the glycophyte, indicating that the halophyte translocated more metal from the root to the shoot than the glycophyte. For example, Zn shoot-to-root ratios ranged from 1.4 to 2.1 for Atriplex and from 0.5 to 0.6 for Z. mays. The biodegradable chelator rhamnolipid was not effective at enhancing shoot metal concentrations, even though radiolabeled chelator was found in the shoot material of both plants. Our results suggest that halophytes, despite their slower growth rates, may have greater potential to selectively phytoextract metals from contaminated soils than glycophytes.
INTRODUCTION
One approach to phytoremediation of metal-contaminated sites is removal of metal by phytoextraction. Phytoextraction requires efficient transfer of metals from the roots into the shoots to maximize metal removal by cropping of plant materials. It also requires high biomass yields. For example, the Indian mustard, Brassica juncea, has been reported to moderately accumulate a variety of metals (Pb, 3,600 mg/kg; Cu, 970 mg/kg; and Zn, 410 mg/kg) from contaminated soils with reasonable biomass yields of 7.2 t/ha/month [1]. Most plants utilized for metal accumulation are well-characterized crop plants, including sunflower (Heliantus annus), corn (Zea mays), pea (Pisum sitivum), and mustard (B. juncea) [2-5]. Interestingly, these plants are all classified as glycophytes or plants lacking salt-tolerance mechanisms.
Halophytes, such as the four-wing saltbush, Atriplex nummularia, are plants that tolerate saline soils and accumulate large quantities of salts in the aboveground tissue [6]. These plants can achieve biomass yields of 20 to 30 t/ha and have been shown to accumulate up to 40% NaCl in the biomass [6]. A few recent studies have provided evidence suggesting that halophytes may be useful for phytoremediation, particularly for salty soils [6-12]. For example, Thomas et al. [7] demonstrated that the halophyte Mesembryanthemum crystallinum regulates Cu uptake more effectively than Arabidopsis thaliana. In another study, Broadley et al. [10] identified the family Caryophyllidea, containing a number of halophyte taxa, as having characteristically high shoot Cs concentrations. Similarly, Williams et al. [12] found Zn concentrations in the roots and shoots of some halophytes to reflect sedimentary levels found in a polluted salt marsh. However, relatively little is known about metal tolerance and accumulation in halophytes.
Another requirement for efficient phytoextraction is that the metal contaminants be in a form available for plant uptake. This is generally not the case for weathered metal-contaminated soils, where the majority of the metal has become precipitated or tightly bound. Chelator applications significantly increase plant-available metal in most metal-contaminated soils [1-5, 13]. Blaylock et al. [1] report that the addition of 1 g EDTA/kg soil resulted in a 10-fold increase in metal uptake by B. juncea. Ethylenediaminetetraacetic acid and other synthetic chelators, however, can be toxic and nonbiodegradable, limiting their usefulness for continued soil applications [14-18].
The objective of this study was to compare root and shoot metal accumulation from an aged contaminated soil by a halophyte (A. nummularia) and a glycophyte (Z. mays) in the presence and absence of metal chelators. Two chelators were used, a synthetic chelator (EDTA) and a microbially produced chelator (rhamnolipid). Rhamnolipid is produced by Pseudomonas aeruginosa and has been shown to chelate metals such as Pb and Cd in both soil and solution [14-20].
MATERIALS AND METHODS
Soils
The contaminated and uncontaminated soils used in this study were collected from an industrial site in Tucson (AZ, USA). The contaminated soil is a mine-tailing waste disposed of approximately 30 years ago. The uncontaminated soil was collected from a nearby site containing no tailings and supporting plant growth. Composite samples of contaminated and uncontaminated soil were collected and passed through a 20- mm sieve, and a 100-g subsample of composite was sent to an U.S. Environmental Protection Agency (U.S. EPA)-approved laboratory for total (hard and soft) metals, pH, conductivity, and salinity (Table 1). These data were certified with the appropriate quality assurance/quality control protocols (CSAL Analytical Laboratory, Tucson, and Laboratory Consultants, Phoenix, AZ, USA). The toxicity characteristic leaching procedure (TCLP) metal concentrations of both the contaminated and the uncontaminated soils as well as the contaminated soil:mulch mixture were determined and are presented in Table 1. The TCLP metal concentrations were determined by the following modified procedure (U.S. EPA Method 1311). Ten-gram soil samples were incubated in a rotary shaker (200 rpm) overnight (18 ± 2 h) with 200 ml of solution 1 and centrifuged at 6,000 g for 10 min. The supernatant was filtered through TCLP-certified glass filters (VWR Scientific, Westchester, PA, USA) and analyzed for metals by flame atomic absorption (AA) spectrophotometry (Model IL, Video 12, Thermo Jarrell Ash, Franklin, MS, USA). One liter of extracting solution 1 contained 5.7 ml glacial acetic acid and 64.3 ml of 1-N NaOH. The solution pH was adjusted with NaOH to 4.93 6 0.05. All supplies were of reagent grade containing trace metal concentrations. All glassware were acid washed in 10% nitric acid and rinsed thoroughly with double-distilled water prior to experimentation. The TCLP standards and sample blanks were verified regularly by AA spectrophotometry.
| Soil | Contaminated | Uncontaminated | Mixturea |
|---|---|---|---|
| Analytes | Total (mg/kg soil) | ||
| Lead | 2,000 | 50 | 1,000 |
| Zinc | 2,500 | 60 | 1,300 |
| Copper | 21,000 | 280 | 10,000 |
| Iron | 150,000 | 17,000 | 75,000 |
| Nitrate | 170 | 170 | − |
| Sulfate | 6,500 | 890 | − |
| Sodium | 680 | 170 | − |
| General characteristics | |||
| pH | 7.9 | 8.5 | − |
| Salinity (dS/m) | 1.4 | 0.9 | − |
| Conductivity (mmhos/cm) | 3.2 | − | − |
| TCLP (mg/L extract solution) | |||
| Lead | 12.5 (±0.3) | NDb | ND |
| Zinc | 26.7 (±7) | ND | 10.0 (±0) |
| Copper | 33.3 (±7) | 0 (±0) | 17.3 (±4) |
- a The mixture soil is a 1:1 (mass basis) mixture of contaminated soil and El Toro mulch. Metal values reported for the mixture soil are estimates based on the contaminated soil. The mulch used to supplement the soil had minimal TCLP levels of Pb, Zn, or Cu (data not shown).
- b ND = not determined.
It was determined in a preliminary plant screening (data not shown) that in order to facilitate plant growth, organic amendments were required in the contaminated soil. In this study, both soils were mixed 1:1 (w/w) with forest mulch and soil conditioner (El Toro Forest Mulch, Triple A Fertilizer, Tucson, AZ, USA). The soil mixtures were used for all experiments unless noted otherwise.
Chelators
Both EDTA and 14C-EDTA (specific activity 5 0.69 μCi/mmol) were purchased from Sigma Scientific (St. Louis, MO, USA). Rhamnolipid, a mixture of mono- and dirhamnolipid forms, was generously donated by Jeneil Biosurfactant (Saukville, WI, USA). The 14C-monorhamnolipid (specific activity = 0.435 μCi/mmol) was produced and purified as described by Maslin and Maier [20].
Plant tissue metal analysis
Plant samples analyzed for metal content were oven dried (65°C) for 2 d, ground in a stainless-steel Wiley Mill (A.H. Thomas, Philadelphia, PA, USA), and passed through a 20- mesh screen. Five-hundred-milligram samples were ashed in a furnace at 500°C for 5 h. The ashed samples were digested at 150°C in 3-M hydrochloric acid (HCl) for 3 min and brought to 10-ml volume with ddH2O. Samples were filtered through Whatman 42 paper (Clifton, NJ, USA) prior to analysis by AA. All glassware was acid washed in 10% nitric acid and rinsed thoroughly with double-distilled water prior to experimentation. Standards and sample blanks subjected to the same procedure were verified regularly.
Transplant preparation
The two plants chosen for this study were Z. mays and A. nummularia. Zea maysl was chosen as the model glycophyte because it is commonly used in phytoremediation studies [2-5]. Atriplex nummularia was chosen as the model halophyte because it is a native desert plant and has frequently been observed growing on disturbed sites. In addition, both plant varieties chosen were drought tolerant. More important, the plants chosen here grew better in the contaminated soil than other varieties/species screened directly in the contaminated soil (no mulch amendment; data not shown).
Seeds of Z. mays cv. Mayo Tuxpeno (Native Seed Search, Tucson, AZ, USA) were sown into a commercial potting soil (El Toro Potting Soil). The seedlings were watered daily for two weeks. Uniform seedlings were selected and transplanted into 300-ml pots containing approximately 250 g of the contaminated soil:mulch mixture). For A. nummularia, uniform established plants obtained from clippings were selected and transplanted as described for Z. mays. In most cases, the transplants were allowed to establish roots under a daily watering regime for a week prior to receiving chelator treatment.
Relative growth rates
Relative growth rates (RGRs) were determined for A. nummularia and Z. mays in the absence of chelator. Five plants were sown into the contaminated soil:mulch mixture and an uncontaminated soil:mulch mixture and watered every other day to field capacity with tap water. The plants were harvested after 40 d. Initial and final dry-weight measurements were obtained for the shoot material. These samples were further processed and analyzed for Pb content. The RGRs were calculated by the compound interest formula RGR (grams new growth per gram existing growth per day, g/g/d) = (lnFinal wt 2 lninitial wt)/number of days. In Table 2, the data are presented as percentage growth per day by multiplying RGR by 100.
| Percentage growth/day (g/g/d) | Shoot Pb concentrations (mg/kg) | |||
|---|---|---|---|---|
| Contam. | Uncontam. | Contam. | Uncontam. | |
| Plants | ||||
| Atriplex | 2.0 (±0.1) | 5.0 (±0.7) | 31 (±3) | 20 (±2) |
| Zea mays | 6.0 (±0.4) | 11 (±0.1) | 41 (±28) | 35 (±38) |
| ANOVAa | ||||
| Plant type | ||||
| F ratio | 80 | 1.0 | ||
| p value | <0.001 | 0.30 | ||
| Soil | ||||
| F ratio | 170 | 0.5, 7.0b | ||
| p value | <0.001 | 0.5, 0.02b | ||
| Plant and soil | ||||
| F ratio | 2.7 | 0.1, 0.4b | ||
| p value | 0.1 | 0.5, 1.0b | ||
- a ANOVA = analysis of variance.
- b Test statistics subtracting an outlier value from the Z. mays shoot Pb concentration data.
Chelator-facilitated metal-uptake experiments
Metal uptake by both plants was measured in the presence and absence of chelator. Both plants were treated every other day with distilled water or a 5-mM chelator solution (either EDTA or rhamnolipid) for a total application rate of 5 mmol chelator/kg dry soil over a 7-d period. The plants were harvested 24 h after the final watering event. On harvest, plants were cut 1 cm above the soil, and the shoots were washed with deionized water. The roots were washed first in distilled water to remove excess soil and then washed in deionized water. After washing, root and shoot samples were dried for metal analysis. All treatments were conducted in triplicate. The experiment was duplicated for a total of six plants per treatment.
Chelator complexation of soil bound metals
The ability of the two chelators to complex Pb, Cu, and Zn from the contaminated soil was determined. Triplicate soil: mulch samples were incubated (1:4 solid-liquid) with 5 mM NaNO3 (control), EDTA, or rhamnolipid (all pH = 7.5, ionic strength 5 5 mM) at 200 rpm for 20 h. Each sample was centrifuged at 10,000 g to pellet the soil. The supernatant was filtered through a Whatman 42 filter and analyzed for Pb, Cu, and Zn by AA.
Chelator uptake by plants
An experiment was designed to quantify chelator uptake by the halophyte and the glycophyte; 1 mmol 14C-labeled rhamnolipid or EDTA was added in 50 ml water to each established transplant (specific activity of 0.1 μCi/plant). Triplicate sets of plants were incubated in sealed, transparent, Plexiglas chambers and harvested after 24 h. Plants were separated into root and shoot material, allowed to dry at room temperature for 2 d, and then macerated into fine particles. Samples (0.25 g) were digested with 2 ml of Na-hypochlorite at 60°C according to the instructions provided by Packard Biosciences (http://ww2.packardinst.com/packard/ecom/), now Perkin-Elmer Life Sciences (Norwalk, CT, USA). Fourteen milliliters of Hionic- Fluor (Perkin-Elmer Life Sciences) scintillation fluid was added to the digested suspension and assayed for radioactivity in a Packard scintillation counter (TriCarb 1600, Packard, Meriden, CT, USA).
Statistical analysis
Two-way analysis of variance (ANOVA) was conducted to compare mean Pb, Cu, and Zn uptake by the two plants across treatments using the SYSTAT 10 statistical software (CPSS, Chicago, IL, USA). ANOVA was also undertaken to compare mean relative growth rates of the two plants in the uncontaminated and contaminated soil as well as to compare the mean chelator uptake by the two plants. Both F ratios and p values are reported in the tables at alpha = 0.05.
RESULTS
Soil characteristics and plant growth
Both soils used in this study were collected from the same site. Other than the extensive heavy metal contamination found for the contaminated soil, both soils had similar general chemical characteristics (Table 1). The glycophyte (Z. mays) had a two- to threefold higher relative growth rate than the halophyte (A. nummularia) in both the contaminated and the uncontaminated soil:mulch mixtures (p < 0.001; Table 2). Growth of both plants was inhibited in the contaminated soil:mulch mixture when compared to the uncontaminated soil:mulch mixture (p < 0.001). The halophyte showed a few minor signs of stress when grown in the contaminated soil:mulch mixture. In contrast, and despite its higher relative growth rate, the glycophyte showed profound signs of metal stress, including severe chlorosis and mortality when grown in the contaminated soil:mulch mixture.
Plant metal uptake
Metal uptake by both the halophyte (A. nummularia) and the glycophyte (Z. mays) was measured in the presence and absence of chelator (either 5 mmol EDTA or rhamnolipid/kg dry soil). Plants were allowed to establish roots in the contaminated soil:mulch mixture one week prior to the application of treatment solutions. In the absence of chelator, total metal (Pb + Cu + Zn) accumulation by the two plants was similar, ranging from 920 mg/kg for the halophyte to 950 mg/kg for the glycophyte. Metal accumulation generally followed the order Cu » Zn > Pb, reflecting the fact that the metals were present in the soil at 11,000, 1,250, and 1,000 mg/kg, respectively (Table 1). In general, both plants accumulated 2- to 11- fold more metal in the roots than the shoots with one exception: The halophyte accumulated more Zn in the shoot than the root (Figs. Fig. 1., Fig. 2.) regardless of treatment. Table 3 shows detailed test statistics on the mean metal uptake data presented for the shoots (Fig. 1) and the roots (Fig. 2). With respect to Pb uptake into the shoots, both plant type (p < 0.05) and treatment effects were significant (p < 0.005), but the interaction term (plant type × treatment) was not. Atriplex nummularia accumulated slightly more Pb in the shoot material than Z. mays but only when treated with EDTA (p < 0.05). A different pattern emerged with respect to Pb uptake into the roots. Both treatment effects and the interaction term were significant, but no difference was observed in Pb uptake between the two plant types. With respect to Cu uptake into the roots, plant type, treatment, and the interaction term were all significant. In this case, Z. mays accumulated significantly more Cu than A. nummularia across all treatment types (p < 0.005). With respect to Cu uptake into the shoots, only treatment effects were significant. Lastly, with respect to Zn metal uptake into the root, all test statistics were significant. Zea mays accumulated more Zn in the roots than A. nummularia over all treatments. Only plant type and treatment were significant with respect to Zn uptake into the shoots. In contrast to root Zn uptake, A. nummularia accumulated significantly more Zn in the shoots than Z. mays regardless of treatment.
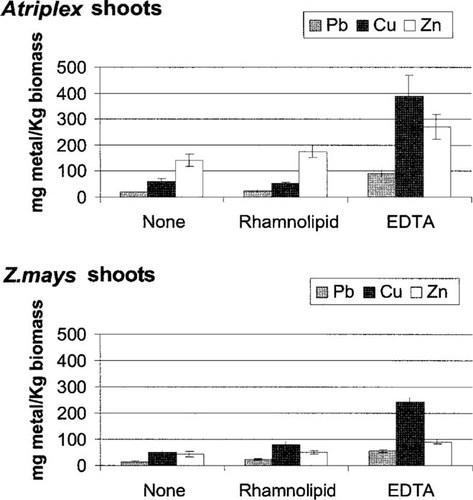
Shoot metal (Pb, Cu, and Zn) concentrations per kilogram plant biomass (dry wt) receiving a total of 5 mM ethylenediaminetetraacetic acid (EDTA), 5 mM rhamnolipid, or no chelator treatment. Both plant types received (in total) 1 mmol of chelator/kg dry soil over a one-week period prior to harvesting. Transplants were allowed to establish new roots in the contaminated soil:mulch mixture for one week prior to a one-week chelator application period. Error bars represent standard error (n = 6).
| Pb | Cu | Zn | |
|---|---|---|---|
| Shoot metal concentrations | |||
| Plant type | |||
| F ratio | 5 | 1 | 35 |
| p value | 0.03 | 0.3 | <0.001 |
| Treatment | |||
| F ratio | 21 | 24 | 3 |
| p value | <0.001 | <0.001 | 0.03 |
| Plant type and treatment | |||
| F ratio | 2 | 2 | 1 |
| p value | 0.2 | 0.1 | 0.3 |
| Root metal concentrations | |||
| Plant type | |||
| F ratio | 2 | 15 | 4 |
| p value | 0.2 | <0.001 | 0.05 |
| Treatment | |||
| F ratio | 7 | 37 | 17 |
| p value | 0.001 | <0.001 | <0.001 |
| Plant type and treatment | |||
| F ratio | 4 | 12 | 4 |
| p value | 0.02 | <0.001 | 0.02 |
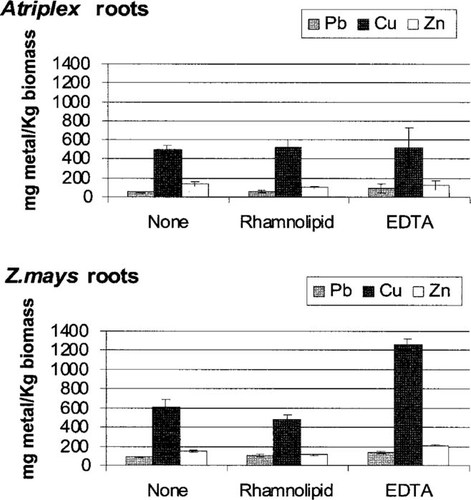
Root metal (Pb, Cu, and Zn) concentrations per kilogram plant biomass (dry wt) receiving a total of 5 mMethylenediaminetetraacetic acid (EDTA), 5mM rhamnolipid, or no chelator treatment. Both plants received (in total) 1 mmol of chelator/kg dry soil over a one-week period prior to harvesting. Transplants were allowed to establish new roots in the contaminated soil:mulch mixture for one week prior to a one-week chelator application period. Error bars represent standard error (n = 6).
We compared the ability of EDTA to complex and solubilize metals from the soil with its ability to enhance metal uptake by plants. The EDTA increased metal solubilization by 62- fold (Pb) to 138-fold (Cu) (data not shown) over soils extracted with NaNO3. The EDTA significantly increased the amount of Pb, Cu, and Zn accumulated by both plants in both the shoot and the roots, with total metal accumulation ranging from 1,500 mg/kg for the halophyte to 2,000 mg/kg for the glycophyte. This reflects an approximate doubling of metal uptake by both plants in the presence of EDTA. Ethylenediaminetetraacetic acid particularly facilitated the translocation of metals from the roots to shoots in all cases. This is reflected in the significant increase in shoot-to-root metal ratios (Table 4). For example, shoot-to-root ratios increased twofold over those not receiving treatment for the halophyte and one-half-fold for the glycophyte. Considering how effective EDTA was at complexing metals from the soil, only a small fraction of the EDTA-complexed metal was actually taken up by the plants. In contrast to EDTA, rhamnolipid treatment had no effect on metal accumulation in comparison to those plants receiving water alone (Figs. Fig. 1., Fig. 2.). Rhamnolipid was also much less effective at complexing and removing metal from the contaminated soil during the soil washing experiment, removing only 4- to 33-fold more metal than the NaNO3 control (data not shown).
The halophyte and the glycophyte exhibited specific differences in metal translocation patterns. In fact, the halophyte had significantly higher shoot-to-root metal accumulation ratios than the corn across all treatments (Table 4). Specifically, the halophyte shoot-to-root ratios were 2.2-fold, 1.4-fold, and 2.8-fold greater than the glycophyte shoot-to-root ratios for Pb, Cu, and Zn, respectively. As was mentioned previously, the addition of EDTA significantly increased this difference between the two plants to 2.4-fold, 3.8-fold, and 4.1-fold for Pb, Cu, and Zn, respectively. These data indicate that the halophyte translocates all three metals, but in particular Zn, more effectively to shoots than the glycophyte, especially in the presence of EDTA.
| Pb | Cu | Zn | |
|---|---|---|---|
| Shoot-to-root metal ratio | |||
| Treatment/plant type | |||
| EDTAa | |||
| Atriplex | 1.00 ± 0.29 | 0.73 ± 0.37 | 2.14 ± 0.99 |
| Zea mays | 0.41 ± 0.10 | 0.19 ± 0.05 | 0.52 ± 0.14 |
| Rhamnolipid | |||
| Atriplex | 0.38 ± 0.14 | 0.50 ± 0.39 | 1.75 ± 0.56 |
| Zea mays | 0.23 ± 0.09 | 0.19 ± 0.12 | 0.60 ± 0.19 |
| Water | |||
| Atriplex | 0.37 ± 0.09 | 0.13 ± 0.05 | 1.35 ± 0.89 |
| Zea mays | 0.17 ± 0.05 | 0.09 ± 0.02 | 0.48 ± 0.17 |
| ANOVA | |||
| Plant type | |||
| F ratio | 90 | 40 | 110 |
| p value | <0.001 | <0.001 | <0.001 |
| Treatment | |||
| F ratio | 70 | 60 | 5 |
| p value | <0.001 | <0.001 | 0.007 |
| Plant type and treatment | |||
| F ratio | 20 | 40 | 4 |
| p value | <0.001 | <0.001 | 0.01 |
- a EDTA = ethylenediaminetetraacetic acid.
Plant chelator uptake
Chelator-facilitated plant metal uptake is believed to be due, in part, to increased metal solubility. It may also be due to preferential root-to-shoot translocation of organo-bound heavy metal complexes, that is, Pb-EDTA [4, 5]. Since both chelators employed in this study increased the soluble fraction of the soil-bound metals (data not shown), we hypothesized that rhamnolipid's failure to enhance metal uptake was due to either exclusion from the root or failure to pass the metal from the root to the shoot. To test this hypothesis, we exposed both plants to soil solutions containing 14C-EDTA or 14C-rhamnolipid. Surprisingly, both plants translocated considerably less EDTA (only 0.01–0.09% of the total applied to each plant) than rhamnolipid (Table 5; p = 0.003). A small fraction of the added rhamnolipid (0.06–0.24%) was found in the plant tissue with the amount found in the roots exceeding that found in the shoots by two- to fourfold. Mineralization of both chelators was found to be negligible over the 24-h incubation period (data not shown), signifying that the radiolabel found in the plant was representative of the intact chelator and not a by-product of rhizosphere metabolism.
DISCUSSION
Metals, when present in excess in the soil, interfere with proper enzymatic functions and can inhibit overall plant growth. Establishment of plants for phytoextraction in heavily contaminated soils may be quite difficult. The mine-tailings soil employed in this study is considered highly metalliferrous, consisting of 15% Fe, 2% Cu, 0.2% Pb, and 0.25% Zn, among other metals. Very few plants are actually found growing directly in the mine tailings on site. It is presumed that the high heavy metal loading found for this soil is solely responsible for the lack of natural plant establishment in the contaminated soil both in the greenhouse and on site. The contaminated soil is slightly more acidic and saline than the uncontaminated soil, but when mixed with mulch and soil conditioner, the contaminated soil supported adequate plant growth.
| mmol Chelator/kg plant | mg Metal/mg chelatora | |||
|---|---|---|---|---|
| EDTAb | Rhamnolipid | EDTA | Rhamnolipid | |
| Plant material | ||||
| Shoots | ||||
| Atriplex | 0.10 (±0.01) | 0.80 (±0.40) | 0.80 (±0.10) | 0.06 (±0.03) |
| Zea mays | 0.04 (±0.01) | 0.20 (±0.02) | 1.4 (±0.25) | 0.10 (±0.01) |
| Roots | ||||
| Atriplex | 0.10 (±0.03) | 1.10 (±0.40) | 2.8 (±1.0) | 0.20 (±0.02) |
| Zea mays | 0.20 (±0.10) | 0.70 (±0.30) | 3.2 (±2.4) | 0.30 (±0.20) |
| Shoots | Roots | Shoots | Roots | |
| ANOVA | ||||
| Plant type | ||||
| F ratio | 15 | 0.7 | 30 | 0.3 |
| p value | 0.003 | 0.4 | <0.001 | 0.6 |
| Treatment | ||||
| F ratio | 20 | 10 | 230 | 30 |
| p value | 0.002 | 0.002 | <0.001 | <0.001 |
| Plant type and treatment | ||||
| F ratio | 8 | 2 | 20 | 0.06 |
| p value | 0.02 | 0.2 | 0.002 | 0.8 |
- a Total mg metal (Pb + Zn + Cu)/mg chelator recovered from corresponding plant material (dry wt).
- b EDTA = ethylenediaminetetraacetic acid.
Effective phytoextraction requires that plants be both metal tolerant and accumulate metal in the aboveground tissue. It has been suggested that salt-tolerant plants may be better adapted to coping with environmental stresses, including heavy metals [7, 21], than salt-sensitive (glycophytic) crop plants (Z. mays, B. juncea, P. sitivum, and so on) commonly chosen for phytoextraction research. In this study, growth of both the halophyte and the glycophyte was stunted in the contaminated soil:mulch mixture compared to the uncontaminated soil:mulch mixture. When comparing the two plant types, the glycophyte had a faster growth rate than the halophyte regardless of the soil:mulch mixture. Despite their slower growth rate, halophytes such as A. nummularia are known to achieve biomass yields as high as 30 t/ha even under salt stress conditions [6]. Further, unlike Z. mays, which is an annual plant, A. nummularia is a perennial plant that continues to accumulate biomass at an exponential rate throughout the growing season (E.P. Glenn, personal communication). It should be noted that the slower growth rate of the halophyte did not correspond to a decrease in metal uptake. In fact, both plants accumulated similar levels of metals (on a mass basis), but the symptoms of metal toxicity (stunted growth and chlorosis) demonstrated by the halophyte were far less severe than those demonstrated by the glycophyte when sown in the contaminated soil:mulch mixture.
Both plants accumulated metals in the order Cu > Zn > Pb, reflecting the metal concentrations found in the contaminated soil and/or the bioavailability of the metal. Both Cu and Zn are essential for maintaining plant metabolic processes, but in excess concentrations both metals are phytotoxic. Lead, on the other hand, is not an essential metal. In addition, Cu and Zn are considerably more bioavailable than Pb in soils. Both Zn and Cu tend to form ionic instead of covalent chemical bonds, making them less stable and prone to resolubilization [22]. Conversely, Pb forms strong covalent complexes, such as inorganic oxides, carbonates, or sulfides, that are much more stable. Even though Pb (2,000 mg/kg dry soil) and Zn (2,500 mg/kg dry) are at similar concentrations in the soil, less Pb (<0.01% of the total) was accumulated in the aboveground plant material than Zn (0.1%). This was especially true for the halophyte. Williams et al. [12] observed similar metal loading of essential (Cu, Zn, Mn, and Fe) rather than nonessential metals (Pb and Cd) for a group of salt marsh plants in the United Kingdom. In our study, as in theirs, neither the halophyte nor the glycophyte hyperaccumulated any one metal (Cu, Pb, or Zn) from the contaminated soil:mulch mixture. In fact, both plants accumulated significantly less than 1% of their biomass in total (Pb + Cu + Zn) metal.
Despite the fact that the halophyte did not hyperaccumulate metals while growing in the highly metalliferrous soil (as it does Na1 and Cl2 in salty soils), its metal accumulation pattern differed significantly from the glycophyte. These differences became more pronounced when EDTA was applied to the soil. The halophyte translocated more metal from the roots to the shoots than the glycophyte, especially Zn. Likewise, Williams et al. [23] report that a number of halophytes (Atriplex included) growing in a heavy metal-polluted salt marsh in the United Kingdom exhibited similar shoot-to-root ratios. The selective translocation of Zn (among a variety of other metals found in our soil) by Atriplex would suggest that salt tolerance confers an advantage in regulating ion uptake over salt sensitivity. Although we compared only one representative halophyte to one representative glycophyte in this study, a similar study comparing the response to Cu stress between Messembryathemum crystallinum (another halophyte) and Arabidopsis thaliana (a glycophyte) demonstrated enhanced Cu uptake and higher Cu tolerance by M. crystallinum [7]. Taking into consideration our data and those reported elsewhere [6-11], it can be suggested that halophytes may be superior plants for long-term establishment in co-metal-contaminated soils.
It could also be argued that since Z. mays is a monocot and A. nummularia a dicot, the comparison in metal uptake between these two plants is somehow complicated by the anatomical and physiological differences between these plants. Nevertheless, our results are consistent with current salt-tolerance research [6, 21, 24]. In general, halophytes such as Atriplex are more restrictive in regulating salt/mineral intake via the roots than glycophytes. Furthermore, some halophytes characteristically translocate and store salts/minerals in vacuoles found in the leaf tissue, providing for greater osmotic control/water movement [6, 24]. Therefore, they tend to translocate ions from roots to shoots more effectively than glycophytes. Thus, the basic differences between halophytes and glycophytes observed with respect to Na uptake and transport appear to apply to heavy metals as well, though the differences we observed were not nearly as large as those observed for Na.
Results from this study confirm that EDTA can solubilize metals from the soil and facilitate metal uptake by plants [1-5, 13]. One major drawback to the use of synthetic chelators such as EDTA is that they are highly recalcitrant to microbial degradation and may persist in the soil for decades [16, 17]. In fact, concerns have been raised regarding the use of synthetic chelators in the field [16-18]. In this study, we compared the ability of EDTA and a biodegradable chelator, rhamnolipid, to facilitate plant metal uptake. Rhamnolipid, like EDTA, complexed soil-bound metals. However, rhamnolipid was not effective at enhancing metal uptake by either the halophyte or the glycophyte, even though it was found in the shoot material of both plants. One reason for this may be that, compared to EDTA, rhamnolipid has a significantly lower binding affinity for metals. For example, the stability constant for EDTA-Pb is 17.88 compared to 8.58 for rhamnolipid-Pb, which represents a nine-order-of-magnitude difference in the relative strength of the two complexes [19]. A second possibility is that the metal-bound rhamnolipid complex is excluded from the root entirely, whereas the free rhamnolipid molecule is not. Continued research is required to identify environmentally compatible metal chelators that may be used to aid in phytoextraction scenarios.
To summarize, our results provide further evidence that salt tolerance may play a role in conferring metal tolerance in halophytes. The fact that Atriplex spp. and other halophytes selectively translocate Zn from contaminated soils and that an increase in stress response factors was observed for M. crystallinum when exposed to increasing Cu concentrations suggests that halophytes may have an advantage over the glycophytes typically employed in most phytoremediation strategies for some sites [1-5, 7, 23]. These data also clearly demonstrate the need to identify or design chelators that are environmentally compatible but that effectively complex metals from soil and facilitate their translocation to the aboveground plant material.
Acknowledgements
This research was funded in part by a research contract with the U.S. Postal Service and by Grant P42 ES04940 from the National Institute of Environmental Health Sciences.




