Interclonal variation of heavy metal interactions in Salix viminalis
Abstract
In the complex chemistry of soil, interactions between metals can be expected and these affect the uptake of the metals by the plants. The role of the metal–metal interaction may vary between different plants. This study was performed to investigate if variations exist in the interactions between Cd, Cu, and Zn on toxicity and accumulation of these metals in different clones of Salix viminalis. Two studies were performed. First, to study interaction at uptake, 10 clones with high or low accumulation capacity of Cd, Cu, and Zn, respectively, were treated with 0.3 μM Cd, 0.1 μM Cu, and 3 μM Zn (all three metals at the same time or separately). Second, to study the effect of one of the metals on the sensitivity of the plant to the other metals, three clones with high or low sensitivity to each of the three metals were used in a modified Weibull analysis. Examination of the results shows that interclonal variation exists in effects of metal interaction on metal accumulation and sensitivity exists. The uptake experiment showed that accumulation of Cu was decreased by the other metals, but only in clones with high Cu-accumulating properties because of decreased net uptake of Cu. The accumulation of Zn in roots was increased two- to threefold in all clones in the presence of the other metals because of a decreased translocation of Zn to the shoot. The accumulation of Cd was not changed by the presence of the other metals in any of the clones. The second experiment showed that the effect of interactions between the different metals on metal toxicity was present in all clones but appeared most frequently in the clone with high Zn resistance. Synergistic effects between Cu and Zn in the Zn-resistant clone suggested that this clone had evolved an additional site of toxic action that was absent in the other clones.
INTRODUCTION
In the complex chemistry in soils, interactions between heavy metals may have an important role in plant uptake. Therefore, the toxicity of a metal in the soil may depend on the presence of other heavy metals. Interactions between heavy metals in plants are known to alter uptake and accumulation of heavy metals as well as alter the sensitivity. Thus, one metal may change the uptake, translocation, accumulation, or toxicity of other metals [1-8]. Metals may interact antagonistically or synergistically, with no clear patterns between different metals. However, many of the reported interactions are contradictory, for example, Zn may decrease the uptake of Cd [9, 10] or increase the uptake of Cd [11], depending on species. This might be an effect of physiological differences between plant species; for example, McKenna et al. [12] reported that Cd stimulates the uptake of Zn in spinach but no interactions were found in lettuce. Furthermore, differences in interaction of metal accumulation in different tissues was reported by McKenna et al. [12], who showed interaction between Cd and Zn in young but not in old leaves of spinach.
Variation between species may be a result of differences in involved mechanisms; however, the mechanisms of interaction in accumulation and toxicity are poorly understood. One mechanism might be competition at the uptake site. Bowen [9] suggested that the interaction between Cu and Zn was such a competition. Competition at a certain site for toxic action by two metals also is possible, resulting in antagonistic interaction on toxicity [11]. Taylor [13] and Taylor et al. [14] showed that aluminum could interact antagonistically or synergistically, depending on the secondary heavy metal, by an-alyzing dose-response curves by using Weibull analysis. Sensitivity may be affected by indirect interactions; for example, a metal may stimulate tolerance mechanisms that affect the sensitivity of the plant to a second metal [11].
Greger et al. [15] reported that different clones of Salix viminalis accumulated Cd, Cu, and Zn to different degrees. Furthermore, the relation between clones in the accumulation capacity of Cu and Zn differs when comparing cultivation in soil versus hydroponic cultivation. However, in the case of Cd, the relationships between clones were the same in both media. A possible explanation could be that the uptake of Cu and Zn may be interfered with in some but not in other clones in the more complex soil solution. This leads to the question if interactions between metals may vary within the same species and, furthermore, if differences in metal interactions may be related to properties of the genotype such as high or low uptake or high or low metal sensitivity.
The aim of this work was to investigate whether interactions between metals in terms of accumulation and toxicity of the metal differ between genotypes of a species with different sensitivity and accumulation capacity of the metal. The hypothesis was that the degree of interactions varies within the species and depends on the heavy metal sensitivity and accumulation capacity of the genotypes. Different clones of S. viminalis were used. This species has been shown to have large variation in heavy metal sensitivity and accumulation among closely related clones [16, 17]. The metals investigated were Cd, Cu, and Zn, which have been investigated by the authors regarding other effects on Salix. The work was divided into two experiments. First, the effect of interactions between metals was investigated on uptake-accumulation-translocation in one experiment in which plants had different accumulation capacities of the metals tested. Second, the effects of interactions between metals on toxicity were investigated where plants had different sensitivities to the metals.
| Root-accumulating properties | Metal concentration in root (μg/g dry wt) | |||||
|---|---|---|---|---|---|---|
| Clone | Cd | Cu | Zn | Cd | Cu | Zn |
| 1 | Low | Low | Low | 5 | 26 | 106 |
| 2 | Low | Low | Low | 7 | 30 | 119 |
| 3 | Low | Low | High | 10 | 19 | 1,812 |
| 4 | Low | Low | High | 7 | 34 | 1,697 |
| 5 | Low | High | Low | 9 | 260 | 85 |
| 6 | Low | High | Low | 12 | 187 | 129 |
| 7 | High | Low | Low | 103 | 24 | 94 |
| 8 | High | Low | Low | 134 | 33 | 118 |
| 9 | High | High | High | 142 | 196 | 1,529 |
| 10 | High | High | High | 119 | 244 | 1,773 |
MATERIALS AND METHODS
Plant material
In the experiment on the effects of interactions on metal accumulation in roots and shoots, 10 clones of S. viminalis L. (chosen from a collection of about 150 different Salix clones) with different capacities to accumulate Cd, Cu, and Zn in roots were used (Table 1). In the experiment on the effects of interactions on metal toxicity on root growth, three clones with different sensitivity to Cd, Cu, and Zn were used (Table 2).
Cultivation and growth conditions
Woody cuttings, 50 mm in length, from one-year-old shoots were mounted in Styrofoam™ plates, with six cuttings of the same clone in each plate, and placed in 1-L pots with 900 ml of 100 μM Ca(NO3)2 solution [18]. No additional nutrients were added to make a simple cultivation solution in order to limit possible interactions. Three replicates (three pots) were conducted for each treatment. The cuttings were treated from the first day with or without Cd, Cu, or Zn, added as CdCl2, CuCl2, and ZnCl2, in different concentrations and combinations in the two experiments (see below).
The water loss was compensated when 1% of the water had evaporated. Salix does not change pH very much; pH decreased from 6.5 to 6.0 during the experiment both in controls and treated plants. Cuttings were grown in a controlled-climate chamber equipped with metal halogen lamps (Osram, Powerstar HQI-R, Hanf, Oldenburg, Germany) providing a photon flux density of 200 μmol/m2/s for 16 h at 25°C. During the 8-h dark period, the temperature was 21°C. The relative humidity was 70 to 80%. Plants were harvested after 20 d and rinsed with 2 × H2O–20 mM Na2-ethylenedinitrilic tetraacetic acid-2 × H2O and the plant material was then treated as described below.
| Treatment metal (% of control) | |||
|---|---|---|---|
| Clone | Cd | Cu | Zn |
| High Zn resistance | 9 | 14 | 69 |
| High Cu resistance | 11 | 78 | 12 |
| High Cd resistance | 89 | 18 | 15 |
Experimental setup to evaluate accumulation and toxicity
In the experiment on the effects of interaction on accumulation, the clones were treated with one metal or all three metals at a time. The concentrations of the added metals were 0.3 μM Cd, 0.1 μM Cu, and 3 μM Zn and the concentrations were chosen based on a previous investigation by Landberg and Greger [17]. No metals were added in the controls. Metal accumulation was measured in roots and shoots. To estimate formation of complexes in the cultivation media, the free ionic strength (i.e., Cd2+, Cu2+, Zn2+, Ca2+, Cl−, and NO3-) was calculated by using the program MEDUSA (a chemical equilibrium program by Department of Inorganic Chemistry at Royal Institute of Technology, Stockholm, Sweden). The program showed that complexes with Cl− make up about 0.1% of total metal, other anion complexes (hydroxides, both in one-metal and three-metal treatments) make up to 1%. Thus, at least 99% of the metals exist as Cd2+, Cu2+, or Zn2+ in the cultivation solution.
For the toxicity experiments, root growth based on the length of the longest root was used as the end-point. The plants were treated with or without 7 μM Cd, 3 μM Cu, or 70 μM Zn in the absence and the presence of a second toxic metal. The second metal was added in a series of different concentrations (0, 1, 3, 5, 7, or 10 μM Cd or 0, 0.3, 1, 3, 5, or 7 μM Cu or 0, 10, 30, 50, 70, or 100 μM Zn). The concentrations were chosen based on a previous investigation by Landberg and Greger [17].
Analysis of metals
Roots and shoots were dried (105°C for 48 h) and wetdigested in HNO3:HClO4 (7:3, v/v) according to the method of Frank [19]. The Cd, Cu, and Zn concentrations were analyzed by atomic absorption spectrophotometry with flame atomizer (AA-100A, Varian, Springvale, Australia) with analytical blanks and standard addition. The metal recovery was 98 to 107%.
Calculations and statistical treatments
 (1)
(1)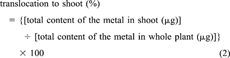 (2)
(2) (3)
(3) (4)
(4)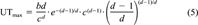 (5)
(5)In all experiments, data for plants harvested from the same pot (n = 4) were pooled into one replicate. Three replicates were conducted for each treatment. The data from each clone were shown to have normal distribution (p = 0.21; Shapiro-Wilk W test, n = 18 [22]). The least significant difference in correlation tests, when comparing two parameters, was calculated at the ρ = 0.05 level. The least significant difference in t tests was calculated at the ρ = 0.05 level.
RESULTS
Accumulation of Cu, Cd, or Zn in roots was studied in the absence or presence of the other two metals (Fig. 1). The Cd net root uptake, translocation to shoot, accumulation in shoot (not shown), and accumulation in root (Fig. 1) were not affected. Zinc accumulation in roots, on the other hand, increased two- to threefold in all clones in the presence of the other metals. In contrast, the accumulation of Cu in roots decreased significantly in the presence of Cd and Zn; however, this occurred only in clones with high Cu-accumulating properties in the roots.
Further analyses were performed on clones that were affected by the presence of additional metals (Fig. 1) on interactions of net uptake, translocation to the shoot and shoot accumulation in the absence and the presence of the other metals (Fig. 2). The other metals increased significantly net uptake of Zn but decreased the translocation of Zn to the shoot in all clones (Fig. 2A), resulting in that the accumulation of Zn in the shoot was not affected. In the presence of Cd and Zn (Fig. 2B), the net uptake of Cu decreased significantly in the high-Cu-accumulating clones, which had about 10 times higher accumulation capacity than the low-accumulating ones (Table 1). The translocation of Cu to the shoot was unaffected and less Cu was accumulated in the shoot in the presence of Cd and Zn.
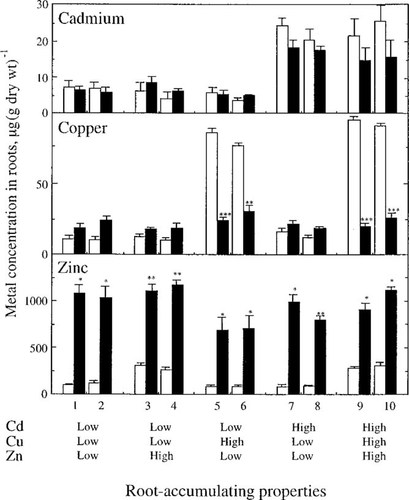
Metal concentration of Cd, Cu, and Zn in roots (μg Me/g dry wt) of 10 clones of Salix viminalis. The properties of metal accumulation in roots of the clones are indicated, where high and low indicate high and low accumulation of the metal in roots, respectively, in relation to the other clones. Plants were treated for 20 d with 0.3 μM Cd, 0.1 μM Cu, and 3 μM Zn separately (empty bars) or with all three metals together (filled bars). n = 3 ± standard error. Significant differences in parameters between treatments with one and all metals at the time: * ρ ≤ 0.050, ** ρ ≤ 0.010, *** ρ ≤ 0.001.
Interactions between metals on metal sensitivity, measured as effects on root-length growth, were analyzed by using the Weibull model (Table 3). The lower the toxicity threshold (TT95b) and the higher the maximum unit toxicity (UTmax), the more sensitive is the plant (in this case, the root growth). The highest TT95b and the lowest UTmax values in this study were found in those clones with the highest resistance to the metal (except the UTmax value for Zn in the Zn-resistant clone; Table 3). In the case of the Cu-resistant clone, the Cu sensitivity was the same in the presence of the other two metals as when they were absent. However, this was not the case with the sensitivity to Zn and Cd in the Zn- and Cd-sensitive clones, respectively.
For further analysis of metal interactions on metal sensitivity (the root-length growth), the different Weibull parameters (i.e., a, b, c, and d; see the Materials and Method section) were analyzed (Table 3). The parameter a was always zero because the treatment started before the formation of roots and the strongest toxic effect in this experiment is completely inhibited growth of the root. In each treatment, addition of the secondary metal resulted in a significant reduction in the value of the growth-response parameter b. Thus, the secondary metal reduced root growth more than the primary metal, indicating a multiplicative effect. This effect appears irrespective of clone and metal-sensitivity properties. In the case where parameters c and d were unaffected by addition of the secondary metal, the effect on root-length growth was interpreted as no interactions. When parameters c or d increased in the presence of a secondary metal, the interaction was interpreted as antagonistic. Such an antagonistic interaction was found in two cases, when Zn was added as secondary metal to the Cu-treated Cdresistant clone, and when Zn was added to the Cd-treated Znresistant clone. Finally, when parameters c and d decreased in the presence of a secondary metal, this was interpreted as a synergistic effect. Synergistic interactions were found in the Zn-resistant clone treated with Cu with Zn and Zn with Cu, whereas no interactions were found in the Cu-resistant clone.
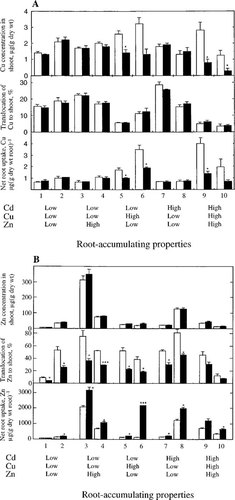
Metal concentration in the shoot, translocation to the shoot, and net uptake by 10 clones of Salix viminalis. (A) Zinc. (B) Copper. The properties of metal accumulation in roots of the clones are indicated, where high and low indicate high and low accumulation of the metal in roots, respectively, in relation to the other clones. Plants were treated for 20 d with 0.3 μM Cd, 0.1 μM Cu, and 3 μM Zn separately (empty bars) or with all three metals together (filled bars). n = 3 ± standard error. Significant differences in parameters between treatments with one and all metals at the time: * ρ ≤ 0.050, ** ρ ≤ 0.010, *** ρ ≤ 0.001.
Effects of clone-independent interactions between metals on sensitivity could be analyzed by comparing the parameters c and d as well as the toxicity threshold and maximum unit toxicity for the different metal combinations (Table 4). A decrease in parameter d was found in the Cd with Cu and Cu with Cd treatments, indicating a synergistic effect when these two metals were combined. Increased toxicity threshold values were found in Cu treatment in the presence of Cd, suggesting a decrease in Cu sensitivity caused by Cd addition on rootlength growth. Decreased Cu and Zn sensitivity was found in the presence of Zn and Cu, respectively, as indicated by decreased maximum unit toxicity values.
DISCUSSION
In this work, intraspecies differences in S. viminalis in effects of interaction between Cd, Cu, and Zn could be observed in the degree of sensitivity, measured as root-length growth, and accumulation of the metals (Fig. 2A and B and Tables 3 and 4). In some of the cases, the interactions were similar in all of the S. viminalis clones studied, whereas other interactions varied among the clones. For example, in all clones, Zn accumulation in roots was stimulated two- to threefold in the presence of the other metals, whereas Cu accumulation was decreased by the other metals only in highaccumulating clones. Metal interactions on toxicity also were found, mainly in the Zn-resistant clone (Table 3). Possible effects of Cl ions are unlikely in this study. Prestudies by the authors, with NaCl as treatment, showed effects only at concentration higher than 5 μM (by Cl or Na; data not shown).
In some of the clones, the net uptake of Cu in roots was reduced in the presence of Cd and Zn (Fig. 2). This may be a result of competition at the uptake site, probably by Zn (the concentration of Zn was 30 times higher than that of Cu in the cultivation solution), as earlier suggested for tomato and rice by Bowen [9]. The interactive effect was observed only in those clones that, in one-metal treatment, had a high ability to accumulate Cu in their roots. Because clones with low Cuaccumulating properties were not affected, we suggest that the uptake sites where the other metal interacts differs in highcompared to low-accumulating clones (Fig. 1). The difference could be due to several factors, for example, competition at the uptake site or changes in the efflux rate induced by the other metals.
In contrast to Cu, Zn accumulation in roots was shown to increase in the presence of Cu and Cd in all clones except for root accumulation properties (Fig. 1). Increase of Zn accumulation by other metals also has been reported in the literature for Silene vulgaris [23]. In the present study, this was due to an increased net uptake in the roots and a simultaneous decreased translocation of Zn to the shoot, also resulting in similar Zn concentration in the shoot as in the single-metal treatment (Fig. 2). This could be due to either an increased uptakeor a decreased efflux of Zn by the roots, in both cases involving cation competition at the root surface [24], in this case by Cu, Cd, or both. The effect by Cd and Cu on Zn distribution in Salix also might be due to ion competition at binding sites, that is, between Zn and Cu or Cd, thereby reducing the amount of Zn translocated to the shoot. However, this would have resulted in a concomitant reduced Cd or Cu concentration in roots or increased Cd or Cu translocated to the shoots, which was not observed in this study (Figs. Fig. 1., Fig. 2.). Instead, the increased net uptake of Zn itself may have caused the observed change in the Zn distribution, as also was found when comparing Zn distribution after cultivation in 10 and 70 μM Zn, respectively (data not shown). Net uptake of Zn was significantly higher, whereas the translocation of Zn to the shoot was lower in plants cultivated at a high concentration of Zn in the media (70 mM; data not shown) compared to 10 μM Zn treatment. Salix viminalis, like some other species (e.g., Holcus lanatus [25]), is likely to have mechanisms to keep heavy metal concentrations in the shoot low to protect them from damage by too high metal concentration. A low translocation of Cd, Zn, and Cu to the shoot has been found in clones of different Salix species (S. daphnioides, S. purpurea, S. triandra and S. viminalis) originating from areas contaminated with these three metals compared to Salix from uncontaminated areas [17].
| Weibull parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clone Treatment | Secondary metal | b | c | d | TT95b | UTmax | r2 | Interaction |
| High Cd resistance | ||||||||
| Cd × Me | - | 212 | 8.10 | 4.95 | 2.81 | 21.0 | 0.86 | |
| Cu | 34*** | 8.27 | 3.04 | 2.48 | 20.6 | 0.90 | Multiplicative | |
| Zn | 39*** | 4.83 | 2.51 | 2.67 | 15.6 | 0.92 | Multiplicative | |
| Cu × Me | - | 206 | 0.95 | 2.36 | 0.27 | 101.4 | 0.90 | |
| Cd | 110*** | 2.49 | 1.31 | 0.35 | 21.1 | 0.90 | Multiplicative | |
| Zn | 46*** | 2.56* | 1.37 | 0.29 | 28.8* | 0.91 | Antagonistic | |
| Zn × Me | - | 220 | 48.4 | 1.97 | 10.7 | 1.75 | 0.97 | |
| Cd | 112*** | 52.1 | 2.86 | 24.2 | 2.82 | 0.92 | Multiplicative | |
| Cu | 27*** | 69.0 | 2.39 | 19.9 | 1.41 | 0.95 | Multiplicative | |
| High Cu resistance | ||||||||
| Cd × Me | - | 183 | 6.04 | 4.76 | 2.23 | 29.7 | 0.84 | |
| Cu | 67*** | 5.02 | 3.40 | 2.09 | 26.1 | 0.93 | Multiplicative | |
| Zn | 29*** | 7.66 | 1.61 | 1.21* | 28. | 0.93 | Multiplicative | |
| Cu × Me | - | 163 | 4.82 | 1.62 | 0.77 | 15.9 | 0.99 | |
| Cd | 31*** | 4.43 | 1.75 | 0.80 | 17.8 | 0.92 | Multiplicative | |
| Zn | 36*** | 5.12 | 1.51 | 0.99 | 13.4 | 0.96 | Multiplicative | |
| Zn × Me | - | 159 | 64.3 | 3.72 | 28.8 | 2.20 | 0.94 | |
| Cd | 39*** | 61.9 | 2.45 | 30.1 | 1.96 | 0.95 | Multiplicative | |
| Cu | 64*** | 72.3 | 2.21 | 18.7 | 1.27 | 0.95 | Multiplicative | |
| High Zn resistance | ||||||||
| Cd × Me | - | 263 | 5.32 | 3.15 | 2.06 | 23.1 | 0.93 | |
| Cu | 18*** | 7.57 | 2.33 | 2.12 | 12.6* | 0.98 | Multiplicative | |
| Zn | 51*** | 7.80* | 3.29 | 3.16* | 16.4 | 0.96 | Antagonistic | |
| Cu × Me | - | 229 | 1.97 | 2.17 | 0.49 | 35.8 | 0.99 | |
| Cd | 25*** | 2.00 | 1.59 | 0.66 | 31.7 | 0.92 | Multiplicative | |
| Zn | 60*** | 2.16 | 0.74** | 0.53 | 18.4** | 0.93 | Synergistic | |
| Zn × Me | - | 229 | 87.1 | 5.67 | 51.7 | 2.43 | 0.98 | |
| Cd | 26*** | 70.3 | 2.26 | 18.8* | 1.32 | 0.94 | Multiplicative | |
| Cu | 16*** | 59.3** | 3.10 | 22.9* | 2.03 | 0.89 | Synergistic | |
- a Significant differences in parameters between treatments with one and two metals at the time: * ρ ≤ 0.050, ** ρ ≤ 0.010, *** ρ ≤ 0.001; p = 0.05 at r2 = 0.81.
The Zn-resistant clone was most susceptible to interactions (Table 3). This clone showed synergistic interaction between Cu and Zn, but in other clones these metals showed either antagonistic or no interaction (Table 3). An explanation could be that different sites of toxic action for Zn and Cu occur in the Zn-resistant clone, which generates a synergistic effect, whereas only one site of toxic action exists for these metals in the other clones, resulting in an antagonistic effect between Zn and Cu. Moreover, this possible specific site of toxic action in the Zn-resistant clone also may be used by Cd, thus Cd has the same site of action as Zn, because when Cd was added as primary metal and Zn was added as the secondary metal, an antagonistic interaction was found (Table 3). However, this was not the case when the dose response to Zn in the presence of Cd was investigated (Table 3), because the concentration of the free Zn ion was much higher than that of Cd, which depressed the activity of Cd. This is probably also the reason for the antagonistic effect in the former case where Cd was added as the primary metal and Zn was added as the secondary metal (Table 3). Among the clones, the Cu-resistant clone was most stable in Cu sensitivity against interactions by other metals in comparison to the Cu-sensitive clones (Table 3).
| Weibull parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | |||||||||||||
| c | d | TT95b | UTmax | ||||||||||
| Primary metal | Secondary metal | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Cu | Cd | + | 0 | 0 | - | - | - | + | + | + | - | + | - |
| Cd | Zn | - | + | + | - | - | 0 | 0 | - | + | - | 0 | - |
| Zn | Cd | + | 0 | - | + | - | - | + | 0 | - | + | - | - |
| Cu | Zn | + | 0 | 0 | - | + | - | 0 | + | 0 | - | - | - |
| Zn | Cu | + | + | - | + | - | - | + | - | - | - | - | - |
In conclusion, we have shown that properties such as high or low accumulation of heavy metals and sensitivity to metals are important in interactions between heavy metals in clones of S. viminalis. Thus, genotypes with different properties, such as high or low resistance to a specific metal, may respond in totally different ways when grown on a soil contaminated with a mixture of heavy metals because of various metal interaction effects both on their accumulation capacity and their toxic response. Therefore, in situations with high levels of metals, for example, in physiological experiments and cultivation on contaminated areas, attention to the special properties of the genotypes will be needed.
Acknowledgements
We thank the Section of Short Rotation Forestry and Department of Genetics at the Swedish University of Agriculture, and Svalöf-Weibull AB. We also thank Lena Kautsky. This work was financed by the Carl-Fredrik von Horn, Carl Trygger, and Futura foundations.




