Effect-directed fractionation and identification of cytochrome P4501A-inducing halogenated aromatic hydrocarbons in a contaminated sediment
Abstract
On the basis of a new fractionation method combined with in vitro ethoxyresorufin-O-deethylase (EROD) induction in a rainbow trout liver cell line (RTL-W1) and chemical analysis, halogenated aromatic hydrocarbons with dioxin-like activity were identified in a sediment extract from Bitterfeld, Germany. The fractionation method allowed a separation of different nonplanar and coplanar polychlorinated biphenyls (PCBs), polychlorinated naphthalenes (PCNs), dibenzo-p-dioxins (PCDDs), and dibenzofurans (PCDFs) with different degrees of chlorination. The dioxin-like activity at the investigated site could be quantitatively assigned to PCDD/Fs. Both PCBs and PCNs could be excluded as the cause of the measured effects on the basis of the fractionation procedure and bioanalytical results. Thus, the method allowed the chemical analysis to focus on PCDD/Fs, with significant reduction of the analytical expense. The EROD-induction potency of sediment-extract fractions was quantified, and toxicants were confirmed by the application of induction equivalent quantities on the basis of fixed-effect-level concentrations that exhibit 15% of the maximum induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin. This approach was designed to minimize methodological limitations due to superimposing inhibitory effects.
INTRODUCTION
Since the 1960s, halogenated aromatic hydrocarbons (HAHs) as anthropogenic hazardous contaminants in the environment have been of high public concern and subject to restrictions in their uses and emissions. They are typical persistent organic pollutants with a high potential for bioaccumulation and long-range transport to remote areas due to their generally high lipophilicity and their persistence under environmental conditions. Some of these HAHs, such as coplanar polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs), and polychlorinated naphthalenes (PCNs), are highly toxic and exhibit dermal toxicity, hepatotoxicity, teratogenicity, immunotoxicity, tumor promotion, hormonal and neurobehavioral changes, and lethality [1]. Because all these responses have been ascribed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) as a prototype inducer, compounds eliciting similar responses are collectively referred to as dioxin-like compounds. Dioxin-like toxicity is thought to be mediated through the arylhydrocarbon receptor (AhR). By binding to the AhR, dioxin-like compounds set off a cascade of cellular events leading to increased expression of the cytochrome P4501A (CYP1A) gene. One catalytic activity associated with the expression of CYP1A is ethoxyresorufin-O-deethylase (EROD) activity [2].
The ability of HAHs and other compounds to induce CYP1A is used as a basis for bioanalytical assessment of compounds exhibiting AhR-mediated effects. Several in vitro assays have been established within the last decade that measure EROD induction in fish liver cell lines (RTL-W1 [3] and PLHC-1 [4]), avian embryo hepatocytes [1], H4IIE rat hepatoma cells [5], and HEPA-1 mouse hepatoma cells [6]. Because of their speed, sensitivity, and reproducibility, in vitro EROD-induction assays are powerful tools for the specific detection of AhR-binding toxicants in complex environmental samples [5-8].
Environmental samples, such as sediments, mostly contain complex mixtures of toxicants. In addition to dioxin-like compounds, which are the focus of the present study, environmental samples may include other compounds influencing EROD activity. These include readily metabolized EROD inducers, such as polycyclic aromatic hydrocarbons (PAHs) [9], as well as EROD inhibitors and cytotoxic compounds. The separation of these compounds, the quantification of groupand compound-specific potentials of CYP1A induction in environmental mixtures, and the identification of unknown AhRmediated toxicants require the application of effect-directed fractionation procedures in combination with chemical analysis and biotesting.
Several efforts have been made to apply effect-directed fractionation to assign CYP1A induction in contaminated sediments to its substantial causes [8, 10-13]. Previous fractionation procedures were mostly limited to a few basic fractions, such as an aliphatic, a diaromatic, one or several PAH fractions, and sometimes, an additional polar fraction, but without further fractionation of dioxin-like compounds [8, 10-12]. On the basis of these fractionation procedures, it was rarely possible to assign EROD-inducing potency to the compounds causing the effect, because in general, only a minor portion of measurable EROD induction could be explained. A more sophisticated fractionation procedure for EROD-inducing compounds in sediment extracts was presented by Gale et al. [13], separating the fraction containing dioxin-like compounds into four subfractions that included three PCB fractions and one containing PCDD/Fs. We developed a fractionation procedure for the group-specific separation of nonplanar and coplanar PCBs, PCDDs, PCDFs, and PCNs with different numbers of ortho-chlorine substituents.
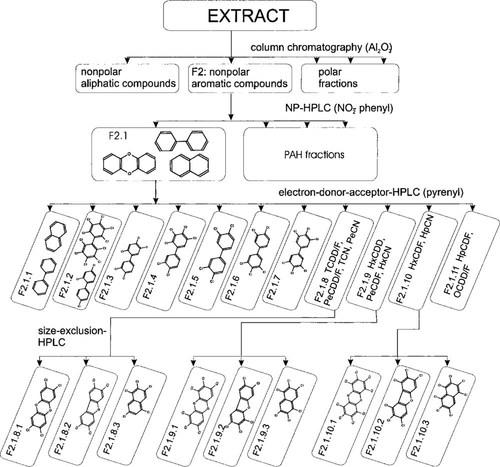
Fractionation procedure for the separation of dioxin-like halogenated aromatic hydrocarbons. Typical chemical structures are shown to characterize the various fractions. F = fraction; HpCDF = heptachlorodibenzofurans; HpCN = heptachloronaphthalenes; HPLC = high-performance liquid chromatography; HxCDD = hexachlorodibenzo-p-dioxins; HxCDF = hexachlorodibenzofurans; HxCN = hexachloronaphthalenes; NP = normal phase; OCDD/F = octachlorodibenzo-p-dioxin and octachlorodibenzofuran; PAH = polycyclic aromatic hydrocarbon; PeCDD/F = pentachlorodibenzo-p-dioxins and furans; PeCDF = pentachlorodibenzofurans; PeCN = pentachloronaphthalenes; TCDD/F = tetrachlorodibenzo-p-dioxins and furans.
The overall objective of the present study was to evaluate whether a combination of this fractionation procedure with biological analysis, using the RTL-W1 cell line, allows the identification of EROD-inducing HAHs in a complex environmental sample. Specifically, it was our aim to quantitatively ascribe EROD induction to the identified EROD inducers on the basis of chemical analysis and the concept of additive induction equivalent quantities (IEQs).
For the calculation of IEQs, fixed-effect-level concentrations rather than median effective concentrations (EC50s) were determined from EROD concentration-response curves to account for bell-shaped concentration-response curves with varying maxima [8]. Advantages and uncertainties of the applied method are discussed.
MATERIALS AND METHODS
Sediment sampling and sample extraction
The sediment was sampled with a corer at the creek Spittelwasser in the industrial region of Bitterfeld, Germany, which was a major site of chemical industry for more than 100 years. Industrial processes included chlor-alkali-electrolysis, pesticide and dye production, as well as photochemical and metallurgical processes. Spittelwasser sediments are highly contaminated with a wide range of pollutants, including pesticides, PAHs, organotin compounds [14-16], PCDD/Fs [17], and PCNs [18].
Approximately 20 sediment samples of the upper 20 cm were drawn, mixed, and homogenized. After freeze-drying the sediment, sand and coarser material were removed by sieving through a mesh of 63 μm. The sediment was Soxhlet extracted with dichloromethane for 24 h. Elemental sulfur was removed by shaking the extract with activated copper overnight.
Fractionation
Sediment extracts were fractionated in four steps according to the fractionation scheme in Figure 1. These steps included cleanup, normal-phase, electron-donor-acceptor, and size-exclusion.
Cleanup. Nonpolar aliphatic compounds and polar compounds were removed by applying a cleanup step on alumina (activity I; ICN Biomedicals, Eschwege, Germany), which was deactivated with 4.5% (w/w) distilled water. An aliquot of 10 ml of desulfurized sediment extract corresponding to 100 g of sediment was sorbed on 18 g of deactivated alumina by evaporation of the solvent in a rotation evaporator. The loaded alumina was transferred onto 60 g of neat deactivated alumina in n-hexane in a column with a diameter of 3 cm. Nonpolar aliphatic compounds were removed by eluting the column with 72 ml of n-hexane. Nonpolar aromatic compounds, including PAHs and HAHs, were subsequently eluted with 270 ml of n-hexane/dichloromethane (95%/5%, v/v).
Normal-phase high-performance liquid chromatography. Aliquots of 2 ml of the extracts cleaned up on alumina (Fig. 1, fraction F2) were fractionated at 10°C on a preparative stainless-steel column (21 × 250 mm) packed with nitrophenylpropyl silica (5 μmm of Nucleosil 100–5 NO2; Machery&Nagel, Düren, Germany) with a pore diameter of 100 Å. The n-hexane/dichloromethane (95%/5%, v/v) as mobile phase was delivered isocratically by a high-pressure pump (Kontron high-performance liquid chromatography [HPLC]-pump 422; Biotek Instruments, Neufahrn, Germany) at a flow rate of 19 ml/min. Solutes were detected on a dual mode ultravioletvisible light detector (Kontron HPLC detector 430; Biotek Instruments) at 250 and 280 nm. The HAHs were hardly retained and eluted as the first fraction (Fig. 1, fraction F2.1) followed by PAHs.
Electron-donor-acceptor HPLC. The diaromatic fraction F2.1 produced by normal-phase HPLC was further fractionated at 10°C injecting aliquots of 2 ml on a stainless-steel column (10 × 250 mm) equipped with a stainless-steel precolumn (10 × 20 mm), both packed with 2-(1-pyrenyl)ethyldimethylsilylated silica (5 μm of Cosmosil Pye; Nacalai Tesque, Kyoto, Japan) with an average pore diameter of 120 Å. Two high-pressure Kontron HPLC pumps delivered a flow of the mobile phase of 8 ml/min. The use of two additional high-pressure valves (Valco, Schenkon, Switzerland) allowed a separate elution of the precolumn and the main column according to the backflush method presented by Krahn et al. [19]. The elution program started isocratically with n-hexane eluting both precolumn and main column for 8 min. Subsequently, the precolumn was isolated, and the main column was eluted further for 2 min with n-hexane followed by a gradient from 100% n-hexane to 100% dichloromethane within 3 min. These conditions were held for 10 min. Subsequently, the precolumn was backflushed with dichloromethane for 5 min. Ultraviolet detection was performed at 250 and 280 nm. Eleven fractions were collected using the automated fraction collector and named F2.1.1 to F2.1.11 (Fig. 1).
Size-exclusion chromatography. After a solvent exchange to tetrahydrofuran, the fractions F2.1.8 to F2.1.10 containing tetra- to heptachlorinated naphthalenes and PCDD/Fs were further fractionated by high-performance gel permeation chromatography at 10°C coupling two stainless-steel columns (25 × 600 mm) packed with a porous polystyrene-divinylbenzene copolymer with a pore size of 50 Å and a particle size of 10 μm (PLgel; Polymer Laboratories, Waltrop, Germany). Tetrahydrofuran was selected as mobile phase applying a flow rate of 10 ml/min. Ultraviolet detection was performed at 225 and 310 nm. Fractions F2.1.8, F2.1.9, and F2.1.10 were each separated into three subfractions named F2.1.8.1 to F2.1.8.3, F2.1.9.1 to F2.1.9.3, and F2.1.10.1 to F2.1.10.3 (Fig. 1), respectively, aiming at the separation of PCN, PCDD, and PCDF.
EROD induction in the RTL-W1 cell line
Induction of EROD was measured in the CYP1A-expressing permanent fish liver cell line RTL-W1 (rainbow trout, Oncorhynchus mykiss) [20] using confluent monolayers in 48-well tissue-culture plates. The method of dosing the cells and measuring EROD activity has been described in detail else-where [21, 22]. Cells were exposed to the extracts dissolved in dimethyl sulfoxide at a final concentration of 0.5% (v/v). Dilutions spanned six orders of magnitude, and each dilution was applied to the cells in triplicate wells with a duration of exposure of 24 h. In addition to extract dilution, a dilution series of 2,3,7,8-TCDD (1.21, 2.42, 4.48, 9.68, 38.8, and 77.5 pmol/L) was included on every plate as a positive control.
Analysis of concentration-response relationships and calculation of biologically derived IEQs
Concentration-response relationships. The assessment of EROD-induction potency was based on the establishment of concentration-response relationships applying geometrical dilution series with 24 dilution steps. Sample concentrations (CS) are given as sediment equivalents (SEq) per liter test medium (mg SEq/L).
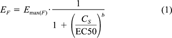 (1)
(1)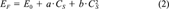 (2)
(2)Fixed-effect-level concentrations and biologically derived IEQs. The calculation of biologically derived IEQs (IEQB) was based on fixed-effect-level concentrations (EC15TCDD) [8]. The EC15TCDD values of the samples were calculated, based on concentration-response relationships, as the sample concentration CS exhibiting an EROD induction equivalent to 15% of the maximum EROD induction achieved by the 2,3,7,8-TCDD control on the same tissue culture (EF = 15; see Eqns. 2 and 3).
The EC15TCDD has been continuously found to yield statistically significant effects in any EROD-inducing experiment over years in our laboratory, and it was selected as a level low enough to identify EROD-inducing samples. The statistical uncertainty of EC15TCDD values was estimated graphically on the basis of the 95% confidence range, as shown in Figure 2, considering the dose range at which the outer limits of the 95% confidence interval crossed the 15% effect level (dashed line in Fig. 2).
 (3)
(3)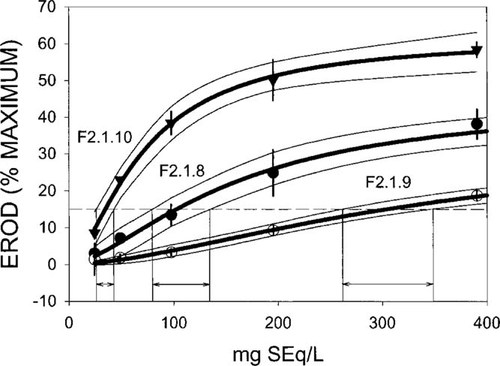
Graphical representation of the deduction of uncertainty ranges for fixed-effect-level concentration of 15% of maximum 2,3,7,8-tetrachlorodibenzo-p-dioxin induction (EC15TCDD) values from 95% confidence intervals of ethoxyresorufin-O-deethylase (EROD) induction data. The bold lines represent the dose-response curves for the respective fraction paralleled by hairlines indicating the 95% confidence intervals, which were used to obtain EC15TCDD values along with estimated deviations for effect concentrations (EC). The EC15TCDD values and the deviations were subsequently used to calculate mean induction equivalent quantities and deviations (see Fig. 4). The dashed line represents the 15% EROD induction level for TCDD. Error bars indicate the respective standard deviation. F = fraction; SEq = sediment equivalents.
Chemical analysis of PCDD/Fs
Identification and quantification of individual compounds were accomplished with a gas chromatograph (HP 5890 II, Agilent Technologies, Waldbronn, Germany) coupled with a high-resolution mass spectrometer (Finnigan MAT 95, Bremen, Germany). Compounds were separated on a fused-silica capillary column (DB-5MS, 60-m × 0.25-mm inner diameter, 0.25-μm film thickness; J&W Scientific, Folsom, CA, USA) using a constant flow of helium of 1.53 ml/min with an initial pressure of 27 psi (1.86 × 105 Pa) at 105°C. The column oven temperature was programmed from 105 to 180°C at a rate of 30°C/min and then to 260°C at 1.4°C/min followed by a rate of 30°C/min to the final temperature of 305°C with a final holding time of 10 min. Injection was done on column. The transfer-line temperature was held at 290°C. The mass spectrometer was operated at an electron impact energy of 70 eV with a mass resolution of 8,000 to 10,000. All compounds were determined in the multiple-ion detection mode at the two most intensive ions of the molecular ion cluster. The PCDD/Fs were quantified using 13C-labeled internal PCDD/F standards (EDF 8999 and EDF 957; Promochem, Wesel, Germany). Detection limits were approximately 0.1 ng/ml, corresponding to 0.01 ng/g sediment.
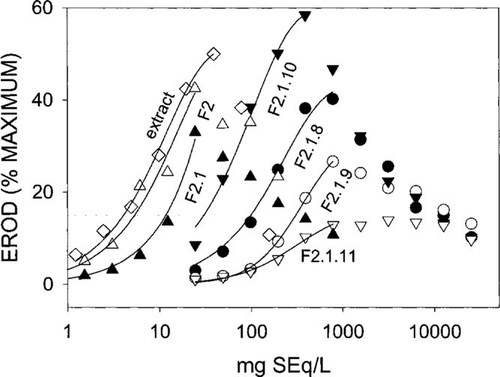
Dose-response curves for the ethoxyresorufin-O-deethylase (EROD) induction of the sediment extract and some fractions (F). The dashed line indicates the 15% effect level for 2,3,7,8 tetrachlorodibenzo-p-dioxin. Error bars were omitted for clarity. For typical standard deviations, see Figure 2. SEq = sediment equivalents.
Determination of chemically derived IEQs
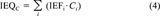 (4)
(4)The IEFs, according to Clemons et al. [23], are based on molar concentrations and have been transformed to mass-based IEFs.
RESULTS
Concentration-response relationships
The sediment extract as well as several HAH-containing fractions exhibited dose-dependent EROD induction. Concentration-response plots typically were asymmetrically peakshaped, with a steeper slope of the ascending branch compared to the descending one (Fig. 3). In most cases, the ascending branch obeyed the classical sigmoid concentration-response model, converging to a maximum induction level. These concentration-response relationships could be closely fitted to the logistic model with r2 values of more than 0.99, except for F2 and F2.1.8.1, with a somewhat poorer fit (Table 1), and F2.1 and F2.1.8.2, for which no reliable fit was achieved. Maximum levels depended on the respective sample and ranged from less than 15% (F2.1.11) up to 68% (extract) of the maximum induction achieved by 2,3,7,8-TCDD (Table 1). Typical standard errors of maximum level determination were 10 to 15%, but in the case of F2, it was more than 100%. The EC50 values were determined with typical standard error of 10 to 40%. Because of the high uncertainty of the maximum level for F2, an appropriate assessment of the EC50 was not possible (standard error > 200%).
In the cases of F2.1 and F2.1.8.2 (not shown), the ascending branch of the concentration-response curve represented only a part of a curve well below the point of inflection of the plot (Fig. 3). Thus, no reliable logistic concentration-response relationship, including maximum levels and EC50 values, could be modeled. Therefore, these dose relationships were fitted to a polynomial model (Table 2).
| Fraction | E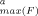 (% Emax(TCDD)) (% Emax(TCDD)) |
bb | r2 | EC50 (mg SEq/L)c | EC15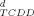 (mg SEq/L) (mg SEq/L) |
|---|---|---|---|---|---|
| Extract | 67.7 (±9.4) | −1.01 (±0.15) | 0.9945 | 13 (±4) | 3.8 (2.8–4.8) |
| MFx | 64.8 (±3.6) | −1.35 (±0.08) | 0.99967 | 18 (±2) | 7.3 (4.7–9.5) |
| F2 | 111 (±116) | −0.878 (±0.266) | 0.978 | 43 (±88) | 5.2 (3.6–7.5) |
| MF2.x | 31.3 (±1.4) | −1.81 (±0.19) | 0.9956 | 11 (±1) | 10.1 (9.3–11.3) |
| F2.1.8 | 44.9 (±3.4) | −1.56 (±0.246) | 0.993 | 159 (±24) | 100 (80–130) |
| F2.1.9 | 34.0 (±3.4) | −1.63 (±0.19) | 0.997 | 350 (±55) | 300 (260–350) |
| F2.1.10 | 61.9 (±2.4) | −1.56 (±0.15) | 0.998 | 72 (±6) | 35 (26–44) |
| MF2.1.x | 44.9 (±2.9) | −1.30 (±0.17) | 0.992 | 28 (±4) | 17 (14–21) |
| F2.1.8.1 | 57.9 (±7.5) | −0.96 (±0.23) | 0.967 | 580 (±230) | 200 (110–310) |
| F2.1.9.1 | 58.3 (±3.3) | −1.41 (±0.15) | 0.996 | 1,290 (±160) | 610 (520–720) |
| F2.1.10.2 | 33.4 (±0.16) | −1.59 (±0.02) | 0.99995 | 36 (±0.4) | 32 (30–33) |
- a Emax(F) = estimated maximum EROD induction of the fractions in percentage of maximum induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD). The values in parentheses represent the standard errors.
- b b = exponent with standard errors in parentheses.
- c EC50 = estimated effect concentrations inducing 50% of the individual maximum level including standard errors in parentheses; mg SEq/L = mg sediment equivalents per liter test medium.
- d EC15TCDD = effect concentrations that induces 15% of the maximum level induced by 2,3,7,8-TCDD. The 95% confidence intervals are shown in parentheses.
The EC15TCDD values could be calculated for both models with typical confidence intervals of 10 to 40%. The ratio of the commonly used EC50 value to the EC15TCDD suggested no invariable value, but this depends on the analyzed fraction. For the fractions analyzed in the present study, typical ratios from 1 to 3.5, with a mean value of two, are observed. This suggests that deviations of EC50-based EROD-induction activities from fixed effect level-based values by a factor of two or three can be expected. The fraction F2, with an EC50: EC15TCDD ratio of 8.3, was not considered in these estimations because of the high uncertainty of the EC50 value.
IEQB values in sediment extract fractions
The IEQB values for the sediment extract fractions are given in Figure 4. An estimation of uncertainty of IEQB values was done on the basis of the intervals estimated for the EC15TCDD values and are shown as error bars. In addition to the IEQB values of the fractions shown are the following: The IEQB values of the initial sample before fractionation, the IEQB values of the reconstituted mixture after fractionation (signified by the letter M), and the calculated sum of the IEQB values of the respective fractions (signified by the letter S). The comparison of the IEQB values of the reconstituted mixtures (M) with those of the initial samples gives a measure for the overall recovery for every fractionation step. The comparison of the calculated sums of the fraction IEQB values (S), which assumes additivity of effect concentration, with the IEQB values of the reconstituted mixture (M) provides an indication of the deviation of the true mixture from IEQ additivity.
Primary fractions. Approximately 72% of the IEQB values of the sediment extract could be recovered in the primary fraction F2 (Fig. 4A), which is characterized by nonpolar aromatic compounds. Polar fractions contributed approximately 10% of the IEQB values, whereas the nonpolar aliphatic fraction F1 did not induce EROD significantly. In the reconstituted mixture (MFx), approximately 52% of the IEQB values of the extract were recovered.
Secondary fractions. The diaromatic fraction F2.1, which contained PCBs, PCNs, PCDDs, and PCDFs, contributed IEQs of approximately 1.8 μg SEq/L, representing approximately half the total induction potency of F2 (Fig. 4B). In addition, 1.3 μg SEq/L were recovered in PAH fractions. Their further investigation is in progress. Approximately 52% of the IEQB values were recovered in the reconstituted mixture MF2.x. Similar to the primary fractions, the calculated sum of the fraction IEQB values (SF2.x) was slightly higher than that of the fraction MF2.x values (68%).
Tertiary fractions. The fraction F2.1 was subsequently separated by electron-donor-acceptor chromatography (Fig. 1) into the following subfractions: Nonhalogenated bulk diaromatic compounds, PCB fractions with increasing chlorination and decreasing numbers of ortho-chlorine substituents, and PCDD/Fs and PCNs with more than four chlorine substituents and an increasing degree of chlorination. Bulk diaromatic and PCB fractions (F2.1.1 to F2.1.7) failed to significantly induce EROD (Fig. 4C). In contrast, the PCDD/F- and PCN-containing fractions F2.1.8 to F2.1.10 were potent EROD inducers, with F2.1.10 as the most active fraction. Also, the fraction F2.1.11, which was characterized by hepta- and octachlorodibenzo-p-dioxins and dibenzofurans, induced EROD. However, the 15% level was not achieved. Thus, no IEQ value could be calculated.
| Fraction | E0a | aa | ba | r2 | EC15TCDD (mg SEq/L)b |
|---|---|---|---|---|---|
| F2.1 | 0.629 (±0.066) | 0.800 (±0.015) | 0.0217 (±0.001) | 0.999988 | 13.2 (13.0–13.5) |
| F2.1.8.2 | 1.44 (±0.67) | 0.123 (±0.009) | 0 | 0.965 | 110 (97–128) |
- a Estimated parameters of polynomial regression with standard errors in parentheses.
- b EC15TCDD = concentration that induce 15% of the maximum level induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD); mg SEq/L = mg sediment equivalents per liter test medium. The 95% confidence intervals are shown in parentheses.
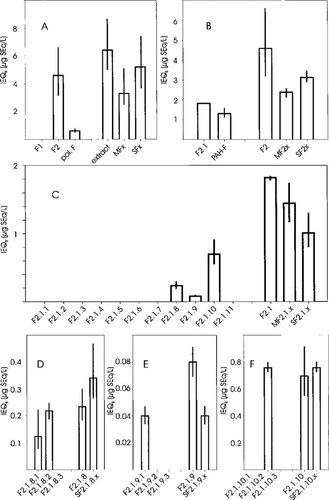
Biologically derived induction equivalent quantities (IEQB) for the sediment extract fractions, including estimated uncertainties derived from effect concentration uncertainty (see Fig. 2). Panels illustrate IEQB values for the various fractions according to Figure 1. A. Primary fractions. B. Secondary fractions. C. Tertiary fractions. D-F. Quaternary fractions. In each panel, bars along the vertical axis indicate the IEQB value for the individual fractions, which are indicated by the letter F and a number. Pol. F indicates the sum of IEQB values of two polar fractions and PAH (polycyclic aromatic hydrocarbon)-F the sum of IEQB values of PAH fractions. The bars along the horizontal axis indicate the IEQB values in the following order: The initial sample before fractionation, the reconstituted mixtures of the individual fractions (indicated by the letter M), and the sums calculated based on individual IEQB values of the fractions (indicated by the letter S). The IEQB values are given as mg sediment equivalents per liter test medium (mg SEq/L).
Quaternary fractions. The PCNs (F2.1.x.3) were separated from PCDDs and PCDFs in fractions F2.1.x.1 and F2.1.x.2 with size-exclusion chromatography. For all three fractions (F2.1.8, F2.1.9, and F2.1.10), EROD-induction potency was recovered in the PCDD- and PCDF-containing subfractions, with a recovery of 50 to 100% (Fig. 4D-F). None of the PCN subfractions exhibited EROD induction.
Confirmation of EROD inducers
Because the size-exclusion chromatography with subsequent biotesting (Fig. 4D-F) indicated no significant contribution of PCNs to EROD-induction potency, the confirmation of EROD inducers in fractions F2.1.8, F2.1.9, and F2.1.10 using the IEQ concept was based on PCDD/Fs only. The fractionation procedure excluded PCBs from these fractions. The PCDD/F concentrations and IEQ values calculated by multiplication of the IEFs by the concentrations of the respective compounds are presented in Table 3. When no IEFs for our test system were available, IEQC values were calculated on the basis of international TEFs [24]. These values are indicated in Table 3, are highly uncertain, and should be taken only as a rough estimate.
The highest IEQC value of a single compound was contributed by 1,2,3,4,7,8-hexachlorodibenzofuran, with 25 μg/kg in fraction F2.1.10, accounting for approximately one-fifth of the IEQC values of the entire extract. High IEQC values were also found for 2,3,4,7,8-pentachlorodibenzofuran, which was distributed between F2.1.8 and F2.1.9 with 7.2 μg/kg, 1,2,3,7,8-pentachlorodibenzofuran with 2.1 μg/kg and 2,3,7,8-tetrachlorodibenzofuran with 1.6 μg/kg. The polychlorinated dibenzo-p-dioxin with the highest IEQC value was 1,2,3,7,8-pentachlorodibenzo-p-dioxin, with 0.87 μg/kg. The total IEQC value of PCDDs attained only approximately 4% of the respective value for the PCDFs.
Comparison of IEQC and IEQB values
The sums of IEQC values derived from analytical data and IEFs from the literature exceed the IEQB values in all three of the fractions F2.1.8, F2.1.9, and F2.1.10 by factors of 1.6, 3, and 2, respectively (Fig. 5). This indicates that the analyzed PCDD/Fs are the cause of the measured effects in the ERODinducing fractions despite a slight overestimation of the activity by the IEQC approach.
DISCUSSION
Concentration-response relationships and fixed-effect-level concentrations
Concentration-response relationships for EROD induction by sediment extracts and their fractions, which represent complex environmental mixtures, are typically characterized by a sigmoid increase up to a maximum level of EROD activity followed by a less-steep decline. Thus, concentration-response curves usually have an a asymmetric peak shape (Fig. 3). The decline of EROD activity at higher concentrations can be explained by a superposition of EROD-induction and -inhibiting effects [4, 8, 25]. Data presented by Hahn et al. [4] and Jung et al. [26] indicated that maximum levels of CYP1A, induced in a fish cell line by different compounds, were similar despite different maximum levels of EROD activity. At low effect levels EROD induction and CYP1A induction proceed in parallel, but with increasing inducer concentrations the increase of EROD activity is diminished likely due to enzyme inhibition. The EC50 values of CYP1A induction for some azaarenes and nitro-PAHs, which are inducers with diminished EROD maximum levels compared to 2,3,7,8-TCDD, have been shown to be approximately one order of magnitude higher than the values derived from enzyme activity [26]. This phenomenon is expected to be fortified in complex mixtures because of the presence of many compounds that may add to the inhibiting effects. As a result, individual maximum values of complex mixtures and derived parameters such as EC50 values are of questionable significance in representing an inextricable superposition of inducing and inhibiting effects. Therefore, evaluation methods providing data that are less affected by inhibition should be applied [26].
Besides the principal shortcomings of EC50-based evaluations, this method also has practical limitations. A maximal response is defined as the magnitude of response where the S-shaped concentration-response curve reaches the upper plateau [27]. Because the concentration-response curves of complex environmental fractions do not always include the part converging to this upper plateau (e.g., F2.1 and F2.1.8.2), maximum values and, therefore, EC50 values are not determinable in every case. Nevertheless, the ascending part of the concentration- response relationships can be closely fitted, such as with a polynomial model providing proper estimates for effect concentrations at distinct effect levels (Table 2).
| C (μg/kg sediment)/IEQc (μg/kg sediment) | ||||||
|---|---|---|---|---|---|---|
| Compounda | IEF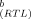 |
TEF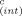 |
F2.1.8 | F2.1.9 | F2.1.10 | F2.1.11 |
| 2,3,7,8-TCDD | 1.0 | 1.0 | 0.32/0.32 | —d | − | − |
| ∑TCDD | NAe | NA | 19/NA | − | − | − |
| 1,2,3,7,8-PeCDD | 2.3 | 1 | 0.38/0.87 | − | − | − |
| ∑PeCDD | NA | NA | 90/NA | − | − | − |
| 1,2,3,4,7,8-HxCDD | 0.9 | 0.1 | − | 0.18/0.16 | − | − |
| 1,2,3,6,7,8-HxCDD | 0.16 | 0.1 | − | 0.56/0.09 | − | − |
| 1,2,3,7,8,9-HxCDD | NA | 0.1 | − | 0.50/0.05f | − | − |
| ∑HxCDD | NA | NA | − | 3.0/NA | − | − |
| 1,2,3,4,6,7,8-HpCDD | NA | 0.01 | − | − | − | 1.4 0.014f |
| ∑HpCDD | NA | NA | − | − | − | 1.9/NA |
| OCDD | NA | 0.0001 | − | − | 4.5/0.0005f | 12/0.0012f |
| 2,3,7,8-TCDF | 0.21 | 0.1 | 7.6/1.6 | − | − | − |
| ∑TCDF | NA | NA | 110/NA | − | − | − |
| 1,2,3,7,8-PeCDF | 0.19 | 0.05 | 11/2.1 | 0.06/0.01 | − | − |
| 2,3,4,7,8-PeCDF | 1.8 | 0.5 | 1.6/2.9 | 2.4/4.3 | − | − |
| ∑PeCDF | NA | NA | 74/NA | 10/NA | − | − |
| 1,2,3,4,7,8-HxCDF | 0.94 | 0.01 | − | − | 27/25 | − |
| 1,2,3,6,7,8-HxCDF | NA | 0.01 | − | − | 18/0.18f | − |
| 1,2,3,7,8,9-HxCDF | NA | 0.01 | − | − | 0.05/0.0005f | − |
| 2,3,4,6,7,8-HxCDF | NA | 0.01 | − | − | 5.1/0.051f | − |
| ∑HxCDF | NA | NA | − | 9.7/NA | 110/NA | − |
| 1,2,3,4,6,7,8-HpCDF | NA | 0.01 | − | − | − | 24/0.24f |
| 1,2,3,4,7,8,9-HpCDF | NA | 0.01 | − | − | − | 22/0.22f |
| ∑HpCDF | NA | NA | − | − | − | 59/NA |
| OCDF | NA | 0.001 | 8.5/0.009f | 38/0.038f | 1.7/0.002f | 160/0.16f |
- a HpCDD = heptachlorodibenzo-p-dioxin; HpCDF = heptachlorodibenzofuran; HxCDD = hexachlorodibenzo-p-dioxin; HxCDF = hexachlorodibenzofuran; OCDD = octachlorodibenzo-p-dioxin; OCDF = octachlorodibenzofuran; PeCDD = pentachlorodibenzo-p-dioxin; PeCDF = pentachlorodibenzofuran; TCDD = tetrachlorodibenzo-p-dioxin; TCDF = tetrachlorodibenzofuran.
- b [23].
- c [24].
- d— = Not detected.
- e NA = not available.
- f Derived from international TEF values.
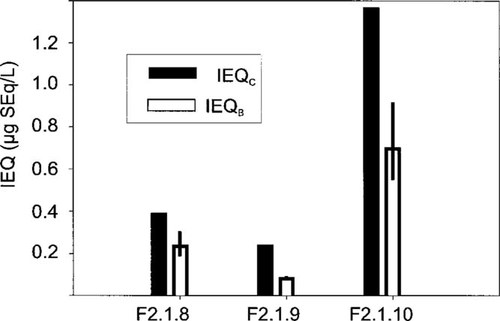
Comparison of the sum of chemically derived induction equivalent quantities (∑IEQC) and the biologically derived value (IEQB) for the fractions F2.1.8 to F2.1.10.
To allow a comparison of EROD-inducing potencies on a unique effect level and to overcome the principal and practical restrictions linked to EC50 values, fixed-effect-level EC values have been suggested [8]. Normalization of this level to the well-defined maximum level of a positive control enhances the reproducibility of results and the comparability of effect concentrations between experiments as well as laboratories. The prototype inducer of EROD, 2,3,7,8-TCDD, is recommended as a positive control [8, 11], because it induces EROD activity with the lowest effect concentration and the highest maximum level. The EROD induction of 2,3,7,8-TCDD fits well to the logistic model. Unlike other compounds, EROD activity induced by 2,3,7,8-TCDD in RTL-W1 cells reaches a well-defined plateau without superimposing inhibiting effects over a wide concentration range (data not shown). The maximum level can be assessed with a standard error of less than 5%. Therefore, a normalization of EROD induction of samples to maximum EROD induction of 2,3,7,8-TCDD as a positive control is recommended. To minimize the influence of inhibitory effect, the effect level is kept as low as possible, yet still high enough to represent a significant effect. These criteria are met with the EC15TCDD value applied in the present study.
Identification of effective groups of compounds
The combination of the applied fractionation procedure with a high-throughput screening test for EROD induction allows a proper assignment of EROD-induction potencies to specific groups of substances even without chemical analysis. The major EROD-inducing activity is due to nonpolar aromatic compounds (F2), whereas aliphatic compounds (F1) are not EROD inducers and polar compounds contribute a minor part of the activity (Fig. 4A). This is in agreement with the results of previous studies [11]. In the reconstituted mixture (MFx), approximately 52% of the IEQ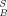 of the extract were recovered, suggesting losses during open-column chromatography by, for example, irreversible binding of polar compounds on alumina. The higher recovery in the fractions compared to the reconstituted mixture suggests the presence of compounds in noninducing fractions that diminish the bioavailability of EROD inducers or the biological response itself. However, with respect to the confidence intervals, the differences between the extract, MFx, and SFx, are not significant.
of the extract were recovered, suggesting losses during open-column chromatography by, for example, irreversible binding of polar compounds on alumina. The higher recovery in the fractions compared to the reconstituted mixture suggests the presence of compounds in noninducing fractions that diminish the bioavailability of EROD inducers or the biological response itself. However, with respect to the confidence intervals, the differences between the extract, MFx, and SFx, are not significant.
The activity of the nonpolar aromatic fraction (F2) can be attributed as approximately half to the diaromatic fraction containing known EROD-inducing HAHs (PCBs, PCNs, and PCDD/Fs) (Fig. 4B) and the other half to PAHs.
Further fractionation of the diaromatic fraction (F2.1) by electron-donor-acceptor chromatography (Fig. 4C) with subsequent biotesting clearly indicates that, in this sample, PCBs do not contribute to overall EROD-inducing potencies and need not be considered for chemical analysis to identify causative compounds. This is in agreement with the results of a previous study at the same creek, which indicated that PCBs did not significantly contribute to overall EROD-induction potency at this site [8]. Approximately 79% of the IEQ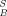 of F2.1 were recovered in the reconstituted mixture MF2.1.x. The calculated sum SF2.1.x is lower (56%), indicating that fractions other than F2.1.8 to F2.1.10 (e.g., F2.1.11) may contribute to the EROD-induction potency of MF2.1.x even if they do not achieve the 15% induction level required for IEQB calculation.
of F2.1 were recovered in the reconstituted mixture MF2.1.x. The calculated sum SF2.1.x is lower (56%), indicating that fractions other than F2.1.8 to F2.1.10 (e.g., F2.1.11) may contribute to the EROD-induction potency of MF2.1.x even if they do not achieve the 15% induction level required for IEQB calculation.
Biological analysis identified F2.1.8 to F2.1.10 as potent fractions. Subsequent size-exclusion chromatography (Fig. 4D–F) and biotesting of subfractions excluded PCNs as the cause of this effect and suggested tetra- to heptachlorinated dibenzo-p-dioxins and furans as major EROD inducers in the sediment extract, even if a contribution of similar compounds, such as polychlorinated dibenzothiophenes (i.e., the sulfur analogues of PCDFs), cannot be excluded [28].
Confirmation of EROD inducers
Published IEFs are based on EC50 values and, therefore, are in a strict sense only applicable if maximum induction levels of the respective compound and 2,3,7,8-TCDD are equivalent and if the concentration-response plots are parallel [27]. Previous studies indicate that this assumption rarely applies to PCDD/Fs or other EROD inducers [4]. However, because to our knowledge no fixed effect level-derived IEFs are yet available, EC50-derived IEFs were applied as a first approximation. Thus, for all compounds with maximum induction levels lower than that of 2,3,7,8-TCDD, these IEFs overestimate the relative EROD-induction potency of the respective compound.
In our investigation, the sums of the IEQC values, derived from analytical data and IEFs from literature, exceed the biologically derived IEQB values in all three of the fractions F2.1.8, F2.1.9, and F2.1.10 by factors of 1.6, 3, and 2, respectively (Fig. 5). This overestimation of the activity by the IEQC approach is thought to result from EC50-based IEFs, which do not consider the lower maximum induction levels by most inducers in comparison to 2,3,7,8-TCDD. The EROD-induction potencies of these compounds are systematically overestimated compared to 2,3,7,8-TCDD.
CONCLUSIONS
The combination of a tailor-made, multistep fractionation procedure, a bioanalytical identification and quantification of EROD-inducing fractions, and subsequent chemical analysis proved to be a powerful tool to allocate measurable EROD induction to distinct toxicants, providing an appropriate basis for targeted emission-reduction and remediation activities. The CYP1A activity in the sediment extract investigated in the present study could be predominantly allocated to tetra- to hexachlorinated dibenzo-p-dioxins and furans. We observed no indications that further, nonidentified diaromatic HAHs contribute significantly to CYP1A-activity.
Effect-directed identification and confirmation of AhRbinding toxicants in complex environmental mixtures using EROD induction and the concept of IEFs and IEQs require a proper quantification of the enzyme-inducing potency of mixtures and individual compounds. Because of varying maximum levels and slopes of concentration-response relationships of EROD activity due to superposing inhibiting effects, EC50 values for EROD appear to be a questionable measure of AhR-mediated activity. Instead, a common agreement on a certain fixed effect level is required as a basis for the determination of effect concentrations, IEFs, and IEQ values. We suggest a low but significant effect level of 15% relative to the maximum induction of 2,3,7,8-TCDD as a positive control. To date, all available IEFs are EC50 based. Such values of EROD inducers with an induction maximum lower than that of 2,3,7,8-TCDD systematically overestimate their individual AhR-mediated activity. Therefore, fixed effect level-based IEFs for individual compounds are required.
Acknowledgements
We thank A. Sperreuter, I. Ränker, and I. Christmann for technical assistance. The study was funded, in part, by the Deutsche Bundesstiftung Umwelt.




