Degradation and persistence of metolachlor in soil: Effects of concentration, soil moisture, soil depth, and sterilization†
Mention of specific products or supplies is for identification and does not imply endorsement to the exclusion of other suitable products or supplies.
Abstract
The present study evaluated the influence of soil depth, soil moisture, and concentration on the persistence and degradation of metolachlor in soil. Greater percentages of metolachlor persisted in subsurface soils than in surface soil regardless of the soil moisture or initial herbicide concentration. Larger quantities of bound residues and extractable degradation products were found in the surface soils as a result of the increased soil sorption and biodegradation of metolachlor associated with the surface soil, which had more organic matter. Saturated soil favored the dissipation of metolachlor and the formation of soil-bound residues. Significantly greater quantities of a dechlorinated metabolite were measured in the saturated surface soil compared to the unsaturated soil. Mineralization of metolachlor to CO2 and volatilization of metolachlor or metolachlor degradates was minimal in surface and subsurface soils at both soil moistures and herbicide concentrations. Increased metolachlor concentrations did not inhibit microbial activity; however, the greater rate of application did result in the reduced percentage of applied [14C]metolachlor that was bound to surface or subsurface soil. A significant reduction in the quantity of extractable metolachlor degradates and unextractable soilbound residues in sterile soil revealed the significance of biodegradation to the dissipation of metolachlor in soil.
INTRODUCTION
Dissipation of pesticides in soil is governed by chemical and physical properties of the pesticide and the soil as well as by climatic conditions [1, 2]. Conditions that favor rapid abiotic or biotic degradation of a compound may result in inadequate control of the targeted pest [3], whereas pesticides that are recalcitrant to degradation will remain in the environment. Increased persistence of pesticide residues in soil will increase the probability of pesticide translocation to surface water and groundwater. The presence of pesticides in the soil, their off-site movement to surface water and groundwater, and the detection of pesticide residues in food has heightened public interest regarding the persistence and toxicological significance of these substances [4-8].
Metolachlor (2-chloro-N-(2-ethyl-6-methylphenyl)-N-(methoxyprop-2-yl)acetamide) is a selective chloroacetamide herbicide used to control broadleaf and annual grass weeds in corn (Zea mays L.), soybean (Glycine max L. Merr.), peanut (Arachis hypogaea L.), and potato (Solanum tuberosum L.) [9]. Large quantities of metolachlor (22 million kg of active ingredient) are applied to agricultural fields in the United States, particularly in the Midwest, where most of the nation's corn and soybeans are grown [6, 10]. Biodegradation is the primary means of metolachlor dissipation in soil [11-14]. Factors that influence microbial activity, especially soil temperature and moisture content, in turn influence the persistence of metolachlor [13, 15]. Pesticides may also affect soil microbial activity depending on the rate of application, persistence, availability, and toxicity of the compound. Large concentrations of pesticides resulting from uneven pesticide application, improper disposal of empty containers, and mishandling and spills of highly concentrated pesticides may adversely affect microbial populations and inhibit microbial activity [16-18].
Whereas dissipation of extractable pesticides from soils is the net result of volatilization, plant uptake, leaching, runoff, adsorption, and/or abiotic or biotic degradation [19], the present study only addressed the dissipation of metolachlor through degradation, adsorption, and volatilization. Soil incubation experiments were conducted to determine the persistence of metolachlor at two concentrations in surface and subsurface soils under saturated and unsaturated conditions to gain a better understanding of the factors that influence the dissipation of metolachlor in soils.
MATERIALS AND METHODS
Chemicals
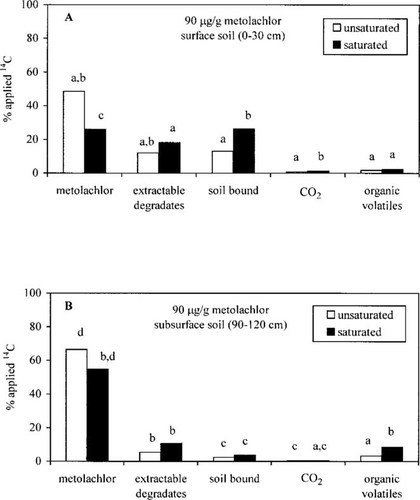
Metolachlor (2-chloro-N-(2-ethyl-6-methylphenyl)-N-(methoxyprop-2-yl)acetamide; CGA 24705, 97.3% pure); [U−14C] metolachlor (98.9% radiochemically pure), and the metolachlor degradates 4-(2-ethyl-6-methylphenyl)-5-methyl-3-morpholinone (CGA 40919, 99.8% pure) and N-(2-ethyl-6-methylphenyl)-2-hydroxy-N-(2-methylethyl)-acetamide (CGA 40172, 98.4% pure) were gifts from Ciba-Geigy (Greensboro, NC, USA) (Fig. 1).
Soil sampling
Soil was collected from a field with no previous pesticide history at the Iowa State University Agronomy and Agricultural Engineering Research Farm, near Ames, Story County, Iowa, using a Back Saverr̀ model N3 soil probe (Clements Associates, Newton, IA, USA). The soil was classified as Nicollet (fine-loamy, mixed, mesic Aquic Hapludoll)-Webster (fine-loamy, mixed, mesic Typic Haplaquoll) complex. Soil samples from the 0- to 30- and the 90- to 120-cm depths were air-dried, passed through a 2.4-mm sieve, and stored at 4°C. Replicate composite samples from each soil depth were analyzed according to standard protocol to characterize the soil physical and chemical properties (A&L Mid West Laboratories, Omaha, NE, USA) (Table 1). A porous plate/pressure membrane technique determined the moisture content of each soil at field capacity (0.33 bar, −33 kPa).
Metolachlor and two metolachlor degradates.
Sterilization of soil
Before the application of metolachlor, surface and subsurface soils were autoclaved for 30 min at 120 ± 2°C SD on three consecutive days. The sterilization of each autoclaved soil was evaluated with microbial plate counts of autoclaved soil slurries applied to trypticase soy agar plates and plates containing nutrient agar with rose bengal and streptomycin. Sterile, ultrapure water was added to each soil to adjust and maintain soil moisture levels. In addition, samples were handled in a laminar flow hood, using sterile techniques, to minimize microbial contamination, survival, and growth during the study.
Soil incubation study
Herbicide application. The degradation of metolachlor in field soil was determined in surface (0–30 cm) and subsurface (90–120 cm) soil (nonsterile or autoclaved sterile soil) at two concentrations (9 and 90 μg/g) under unsaturated (0.33 bar, −33 kPa) and saturated (30% above the water content at −33 kPa) conditions following soil incubation procedures modified from those described by Kruger et al. [20]. A summary of the study design is given in Table 2. Ten randomly selected surface- or subsurface-soil cores were composited for each replication for a total of three replicate soils for both the surface and subsurface depths. A mixture of unlabeled and radiolabeled metolachlor, in acetone, was uniformly applied to each soil at concentrations of 9 and 90 μg/g to represent a field-application rate and a 10-fold field-application rate, respectively. Treated soils were thoroughly mixed to allow even distribution of the treating solution. After evaporation of the acetone, soils were divided into 50-g (dry wt) subsamples. Replicate subsamples (n = 12) were immediately extracted three times with 150 ml of methanol/water (9:1, v/v) to determine actual application rates and extraction efficiencies (99.9 ± 0.13% for 9 μg/g; 99.6 ± 0.10% for 90 μg/g SD).
Adjusting soil moisture. Test systems consisted of 50-g subsamples of treated soil in French square bottles (Fisher Scientific, Fair Lawn, NJ, USA) with the soil moisture adjusted to the gravimetric water content corresponding to −33 kPa or 30% above the gravimetric water content at −33 kPa using ultrapure water. The actual water content for each soil was 0.21 ± 0.01 g/g and 0.22 ± 0.01 g/g SD for the unsaturated surface and subsurface soil, respectively, and 0.81 ± 0.01 g/g and 0.83 ± 0.02 g/g SD for the saturated surface and subsurface soil, respectively. The quantity of water added to the soil in the saturated incubation chambers was sufficient to provide a layer of water (depth, ∼5 mm) above the soil, which ensured saturation in the saturated test systems. Incubation chambers were weighed each week, and ultrapure water was added as needed to the soil to maintain the soil moisture throughout the duration of the study.
Measuring volatilization and mineralization. Polyurethane foam and a vial containing 10 ml of 0.1 N NaOH were suspended above the treated soil in each incubation chamber to trap volatile [14C]-organic chemicals and 14CO2 produced from the degradation and mineralization, respectively, of [14C]metolachlor. Each test system was capped with a polytetrafluoroethylene covered neoprene stopper, sealed with paraffin film, and incubated in the dark at 24 ± 5°C SD. Both NaOH and polyurethane foam traps were changed periodically (NaOH, weekly; polyurethane foam, biweekly), which also allowed for frequent aeration of the incubation chambers to maintain aerobic conditions. A vial containing an aqueous solution of resazurin was placed inside test systems to monitor aerobicity.
| Soil depth (cm) | Soil texture | Sand (%) | Silt (%) | Clay (%) | OCa (%) | CECb (mEq/100 g) | pHc |
|---|---|---|---|---|---|---|---|
| 0–30 | Sandy clay loam | 56 | 24 | 20 | 1.5 | 14.6 | 5.5 |
| 90–120 | Sandy clay loam | 52 | 24 | 24 | 0.2 | 15.3 | 7.9 |
- a OC = organic carbon.
- b CEC = cation-exchange capacity.
- c Soil pH was measured in distilled water (1:1 w/w soil:distilled water).
| Test system identificationa | Application rate of metolachlor (μg/g) | Soil depth (cm) | Soil moisture (kPa)b | Incubation time (d) | Nonsterile or sterile (d) | Extracted soil and OV trapsc | Analysesd |
|---|---|---|---|---|---|---|---|
| Soil incubation study | |||||||
| A | 9 | 0–30 | −33 | 60 | Nonsterile | Yes | LSS, TLC, SC |
| B | 9 | 90–120 | −33 | 60 | Nonsterile | Yes | LSS, TLC, SC |
| C | 9 | 0–30 | −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| D | 9 | 90–120 | −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| E | 9 | 0–30 | 30% > −33 | 60 | Nonsterile | Yes | LSS, TLC, SC |
| F | 9 | 90–120 | 30% > −33 | 60 | Nonsterile | Yes | LSS, TLC, SC |
| G | 9 | 0–30 | 30% > −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| H | 9 | 90–120 | 30% > −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| I | 9 | 0–30 | −33 | 120 | Sterile | Yes | LSS, TLC, SC |
| J | 9 | 90–120 | −33 | 120 | Sterile | Yes | LSS, TLC, SC |
| K | 9 | 0–30 | 30% > −33 | 120 | Sterile | Yes | LSS, TLC, SC |
| L | 9 | 90–120 | 30% > −33 | 120 | Sterile | Yes | LSS, TLC, SC |
| M | 90 | 0–30 | −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| N | 90 | 90–120 | −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| O | 90 | 0–30 | 30% > −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| P | 90 | 90–120 | 30% > −33 | 120 | Nonsterile | Yes | LSS, TLC, SC |
| Microbial respiration study | |||||||
| Q | 9 | 0–30 | −33 | 0–8e | Nonsterile | No | IRGA |
| R | 90 | 0–30 | −33 | 0–8 | Nonsterile | No | IRGA |
| S | 900 | 0–30 | −33 | 0–8 | Nonsterile | No | IRGA |
| T | 9 | 90–120 | −33 | 0–8 | Nonsterile | No | IRGA |
| U | 90 | 90–120 | −33 | 0–8 | Nonsterile | No | IRGA |
| V | 900 | 90–120 | −33 | 0–8 | Nonsterile | No | IRGA |
| Observation | Data compared | Location of reported data |
|---|---|---|
| Effect of soil depth | A vs B, C vs D |
Table 3 |
| Effect of soil moisture | A vs E, B vs F, C vs G, D vs H |
Table 3 |
| Microbial degradation | C vs I, D vs J, G vs K, H vs L |
Figure 2 |
| Effect of metolachlor concentration | ||
| Microbial respiration | Q - V | Data not shown |
| 10 × application rate (effect of soil depth) | M vs N |
Figure 3 |
| 10 × application rate (effect of soil moisture) | M vs O, N vs P |
Figure 4 |
- a Control samples and test systems extracted 0, 18, and 30 d after application (DAA) for nonsterile soils and 0, 18, 30, and 60 DAA for sterile soil are not represented in this table. Each test system was replicated three times.
- b Gravimetric water content at −33 kPa = unsaturated, 30% above gravimetric water content at −33 kPa = saturated.
- c Organic volatile (OV) traps = polyurethane foam traps used to capture [14C]-organic volatiles.
- d LSS = liquid scintillation spectroscopy of soil extracts, organic volatile trap extracts, and NaOH traps to determine mineralization; TLC = thin-layer chromatography of soil extracts; SC = combustion of extracted soil; IRGA = infrared gas analysis to measure CO2 evolution.
- e CO2 evolution was measured daily for 8 d.
Once removed from the soil incubation chambers, polyurethane foam traps were placed in vials containing 20 ml of hexane for a minimum of 4 h to extract organic volatiles. Scintillation cocktail (Ultima Gold™; Packard Instruments, Downers Grove, IL, USA) was added to each of two aliquots of the hexane extract and sodium hydroxide trapping solution, and the percentages of applied 14C associated with volatile [14C]-organic chemicals and 14CO2 were quantified by liquid scintillation counting (LSC) using a Rack-Beta™ model 1217 liquid scintillation counter (Pharmacia LKB Biotechnology, Gaithersburg, MD, USA).
Soil extraction. At the completion of each incubation period of 18, 30, 60, or 120 d after application (DAA), metolachlor and metolachlor degradates were extracted from the soil of three randomly selected test systems from each of the eight treatments with three 150-ml aliquots of methanol:ultrapure water (9:1, v/v). The soil and soil extract were separated by vacuum filtration, and soil on the filter paper was allowed to air-dry. Methanol in the soil extract was removed by rotary evaporation, and the remaining extract was brought to a final volume of 100 ml with ultrapure water. Aliquots of the extract were added to 5 ml of liquid scintillation cocktail and radioassayed to determine the activity of the soil extract.
Analyses of extractable metolachlor and degradates. Metolachlor and metolachlor degradates were removed from the aqueous extract using preconditioned Supelclean Envi-18™ 6-cc solid-phase extraction (SPE) cartridges (Supelco, Bellefonte, PA, USA) with an applied vacuum (50 kPa). Aliquots of the aqueous solution that passed through the cartridge were added to scintillation cocktail and radioassayed to determine the percentage of applied 14C that is characterized as unknown polar degradates. The SPE cartridges were air-dried, and metolachlor and metolachlor degradates were eluted using 10 ml of methanol, followed by 5 ml of ethyl acetate and then 5 ml of hexane. The level of 14C in the methanol, ethyl acetate, and hexane eluates was determined by LSC. Less than 0.5% of the applied 14C was detected in the ethyl acetate and hexane eluates; therefore, only the methanol eluates were characterized by thin-layer chromatography.
A portion of the methanol eluates, representing 0.03 μCi, was concentrated under N2 in a warm-water bath and spotted on 20- × 20-cm, 250-μm silica gel 60 F-254 plates along with radiolabeled metolachlor and the nonradiolabeled standards carbinol (N-(2-ethyl-6-methylphenyl)-2-hydroxy-N-(2-methylethyl) acetamide) and morpholinone (4-(2-ethyl-6-methylphenyl)-5-methyl-3-morpholinone). Silica gel plates were developed in a hexane:methylene chloride:ethyl acetate (6:1:3, v/v/v) solvent system [21]. The locations of the nonradiolabeled standards were identified with an ultraviolet lamp (254 nm). The X-Omat™ Kodak diagnostic film (Eastman Kodak, Rochester, NY, USA) was placed in contact with each plate and developed after four weeks to produce an autoradiogram, which located the extracted 14C-labeled compounds. The distance between the initial sample application area and the center of each developed sample spot was measured, and the silica gel was divided into zones according to identified sample locations. The silica gel from each zone was removed from the plate, added to 5 ml of scintillation cocktail, and radioassayed in a liquid scintillation counter. The SPE-bound 14C was calculated as the difference between the percentage of 14C measured in the soil extract before SPE extraction and the sum of the percentage of radioactivity in the SPE aqueous sample waste and SPE methanol, ethyl acetate, and hexane eluates.
Quantifying soil-bound residues. The quantity of unextractable soil-bound residues was determined by combusting 0.5 g of solvent-extracted soil, which had been mixed with 0.1 g of hydrolyzed starch, in a Packard sample oxidizer (Packard Instruments). The resulting 14CO2 was trapped in Carbo-Sorb™ E mixed with Permafluor™ V (Packard Instruments), and the 14C was quantified by LSC. Six pellets were combusted for each sample. Combustion efficiency was 99.4 ± 4.93%.
Microbial respiration study
Technical-grade metolachlor (97.3% pure) in acetone was applied to each surface and subsurface soil at concentrations of 9, 90, and 900 μg/g. The treated soils were thoroughly mixed and divided into 25-g subsamples, which were placed into jars, and the soil moisture was adjusted to field capacity (−30 kPa). The jars were capped with polytetrafluoroethylenecovered neoprene stoppers containing two glass tubes sealed with rubber septa and incubated at 25 ± 2°C. Untreated soil and acetone-treated soil were the nonsterile control samples and autoclaved untreated soil was the sterile control samples. Each treatment or control was replicated three times. The CO2 evolution was measured daily with an infrared gas analyzer (Model 300; Mine Safety Appliances, Pittsburgh, PA, USA) following the methods of Edwards [22] and Walton et al. [23].
Calculations and statistical analyses
Analysis of variance and least-square means determined significant difference between treatments. Rate constants were calculated based on the assumption that dissipation followed first-order kinetics. Linear regressions of the natural log of concentration versus time determined first-order rate constants (k) and coefficients of determination (r2). Time for 50% dissipation (DT50) were calculated by the formula DT50 = 0.693/k [24]. Statistically significant differences were determined with analysis of variance and general linear models. In the analysis of the microbial activity study, the standard errors that did not overlap were considered to be significantly different.
RESULTS AND DISCUSSION
Recovery of applied 14C
To characterize the dissipation of metolachlor, a mass-balance analysis of the applied chemical is necessary (i.e., attempting to account for all 14C applied as the sum of remaining extractable metolachlor, extractable degradates, unextractable soil-bound residues, CO2 resulting from mineralization, volatile organic chemicals, and residues bound to SPE cartridge packing). Overall, the average mass balance for all treatments was 92.8 ± 7.8%, with the average mass balance for all treatments on days 18, 30, 60, and 120 at 95.5 ± 7.3%, 93.6 ± 6.7%, 89.6 ± 8.9%, and 88.7 ± 7.4%, respectively.
Effect of soil depth
Loss of parent molecule. A greater (p < 0.0007) percentage of the applied [14C]metolachlor was recovered from the subsurface soils (90–120 cm) than from the surface soils (0–30 cm) at all sampling times when soil moisture was maintained at field capacity (−33 kPa) (Table 3). The rate constant for the dissipation of metolachlor was threefold greater in the unsaturated surface soil than in the subsurface soil, which resulted in a shorter extrapolated DT50 for the surface soil (DT50 = 81 d) as compared to subsurface soil (DT50 = 289 d) (Table 4). The DT50 of metolachlor in our surface soil was similar to previously reported DT50s of metolachlor in sandy loam soil at 6% (w/w) soil moisture (DT50 = 80.6 d) [3] and in clay loam soil in flask studies and intact soil cores (DT50 = 56.8 and 71.0 d, respectively) [25]. Bouchard et al. [26] reported a DT50 value of 65.1 d for metolachlor in silt loam at a 40- to 50-cm depth. To our knowledge, no DT50 values have been reported for metolachlor in subsurface soils at depths below 90 cm.
Extractable degradates. The mass-balance data presented in Table 3 reveals that degradation was important to the dissipation of metolachlor in both the unsaturated surface and subsurface soil. Almost twice as much (p < 0.0487) total extractable degradates were recovered from unsaturated surface soils than from subsurface soil (Table 3). Rates of extractable degradate accumulation in the unsaturated surface and subsurface soils were comparable (p < 0.7534) (Table 4).
Extractable degradates consisted of a carbinol (N-(2-ethyl-6-methylphenyl)-2-hydroxy-N-(2-methylethyl)-acetamide), morpholinone (4-(2-ethyl-6-methylphenyl)-5-methyl-3-morpholinone), and unknown polar compounds (degradation products of which we did not have known standards). The percentage of applied 14C that was characterized as carbinol was similar in the unsaturated surface and subsurface soil at 60 and 120 DAA (Table 3). Less than 3% of the applied [14C]metolachlor was degraded to extractable [14C]morpholinone metabolite in both unsaturated surface and subsurface soils at 120 DAA. Unsaturated surface soils contained a greater percentage of polar degradates than did unsaturated subsurface soil at 120 DAA. The quantity of polar degradates measured in the unsaturated surface soil doubled from 60 to 120 DAA. Unknown, less polar degradates measured in the SPE solvent eluates were less than 9% of the applied 14C for unsaturated surface soil and less than 5% for unsaturated subsurface soil. LeBaron et al. [27] reported that the carboxylic acid degradate of metolachlor, the oxidized version of the carbinol degradate, was the major degradation product of metolachlor in soil. In addition, Aga et al. [28] identified an ethanesulfonic acid metabolite of metolachlor, which is formed via a glutathione conjugation pathway in soil. The increase in unknown polar degradates measured at 120 DAA possibly resulted from the formation of the carboxylic acid and sulfonic acid degradates, which were not identified with the methods of extraction and analysis utilized in the present study.
| 60-d incubationa | 120-d incubationa | |||||||
|---|---|---|---|---|---|---|---|---|
| Soil moisture: | Unsaturatedb | Saturatedc | Unsaturatedb | Saturatedc | ||||
| Soil depth (cm): | 0–30 | 90–120 | 0–30 | 90–120 | 0–30 | 90–120 | 0–30 | 90–120 |
| Metolachlor | 51.6A | 79.8B | 33.6CE | 72.8BD | 25.2CF | 63.9D | 18.0F | 40.9AE |
| Extractable degrad.d | 20.7AC | 10.1B | 23.9AC | 8.7B | 31.2CD | 17.9AB | 33.4CD | 28.3C |
| Carbinolc | 1.0A | 0.5A | 14.8B | 0.9A | 0.5A | 1.1A | 24.3C | 1.8A |
| Morpholinonef | 1.7AB | 1.1A | 0.8A | 1.2A | 2.9B | 0.7A | 0.7A | 2.5A |
| Polar degradatesg | 9.2AB | 4.8ABD | 1.9AD | 1.5D | 21.2C | 11.4B | 2.6A | 7.4ABD |
| Unknown degradesh | 8.7A | 3.7B | 6.5AB | 5.3AB | 6.7AB | 4.8AB | 5.9AD | 16.6C |
| Soil-bound residues | 17.2A | 3.8B | 30.8C | 3.7B | 27.6C | 8.3D | 36.9AB | 15.2A |
| CO2 | 0.6A | 0.3AB | 0.6A | 0.1B | 1.9C | 0.6A | 1.2D | 1.5E |
| Organic volatiles | 0.5A | 1.8B | 0.6A | 2.2B | 0.6A | 4.0C | 1.2D | 5.3D |
| SPE lossi | 0.7A | 0.0A | 6.1A | 12.1A | 4.0A | 3.1A | 2.0A | 6.4A |
| Total | 91.2A | 95.7AB | 95.6AB | 99.6B | 90.6A | 97.8AB | 92.7A | 97.7AB |
- a Incubation at 25 ± 2°C. Means in each row followed by the same letter are not statistically different (p = 0.05).
- b Unsaturated = 0.3 bar or −33 kPa (field capacity).
- c Saturated = 30% above the water content, −33 kPa.
- d Extractable degrad. = sum of extractable degradates, including the carbinol, morpholinone, polar degradates, and unknown degradates.
- e Carbinol = N-(2-ethyl-6-methylphenyl)-2-hydroxy-N-(2-methylethyl)-acetamide.
- f Morpholinone = 4-(2-ethyl-6-methylphenyl)-5-methyl-3-morpholinone.
- g Polar degradates = unknown metolachlor degradates in the solid-phase extraction (SPE) aqueous extract.
- h Unknown degradates in the SPE solvent eluate.
- i SPE loss = % of applied 14C measured in the soil extract before SPE - sum of % applied 14C measured in the SPE aqueous extract and solvent eluates.
Soil-bound residues. Sorption of metolachlor and/or metolachlor degradates contributed to the dissipation of extractable metolachlor in both the unsaturated surface and the subsurface soil. Unsaturated surface soils contained three- to fourfold more soil-bound residues than unsaturated subsurface soils (p = 0.0001) (Table 3). Soil-bound residues increased from 17.2 to 27.6% in unsaturated surface soil and from 3.8 to 8.3% in unsaturated subsurface soil at 60 and 120 d. Greater quantities of soil-bound residues formed in the surface soil relative to the subsurface soil, presumably as a result of the greater quantity of organic carbon in the surface soil. Surface soil often contains more organic matter than subsurface soil [29, 30]. The organic carbon content of the soil has been found to be the most important soil property influencing the adsorption of metolachlor [30-35]. The main adsorption mechanisms of acetanilide herbicides result from multifunctional H-bonds and charge-transfer bonds between humic acids and the herbicides[35] and from hydrogen bonds between carboxyl or hydroxyl groups of organic substances and the carbonyl oxygen of metolachlor [36]. Sorption of metolachlor to the soil has also been correlated with cation-exchange capacity (r = 0.85) and the clay content (r > 0.70) of the soil [19, 30, 31, 37]. Analysis of soil in the present study revealed that the cationexchange capacity and the clay content of the subsurface soil were slightly greater than those of the surface soil, whereas surface soil contained more organic carbon (1.5% vs 0.2%) (Table 1). The rate of formation of unextractable soil-bound residues was similar in unsaturated surface and subsurface soils (Table 4).
| K (r2)a | |||||
|---|---|---|---|---|---|
| Soil depth (cm) | Soil moisture | DT50b (r2) (d) | Metolachlor dissipationc | Unextractable residue formationd | Degradate accumulatione |
| 0–30 | Uf | 81 (0.95) | −0.0086 (0.96)Ag | 0.0102 (0.82)A | 0.0066 (0.96)A |
| 90–120 | U | 289 (0.88) | −0.0024 (0.88)B | 0.0120 (0.86)A | 0.0020 (0.86)A |
| 0–30 | Sf | 50 (0.88) | −0.0130 (0.84)C | 0.0127 (0.75)A | 0.0156 (0.77)B |
| 90–120 | S | 94 (0.88) | −0.0076 (0.95)A | 0.0252 (0.99)B | 0.0166 (0.98)B |
- a Rate constants (coefficient of determination).
- b DT50 values were calculated based on first-order reaction kinetics.
- c Calculation based on first-order reaction kinetics of the percentage of applied [14C]metolachlor remaining in the soil extract.
- d Calculation based on first-order reaction kinetics of the percentage of applied 14C remaining in the extracted soil.
- e Calculation based on the first-order reaction kinetics of the percentage of applied 14C characterized as [14C]degradation products in the soil extract. This calculation assumes the degradation of the transformation products was minimal.
- f U = unsaturated soil = −33 kPa; S = saturated soil = 30% above the water content at −33 kPa.
- g Means followed by different letters are significantly different at p ≤ 0.05 (least-significant-difference test) within each column.
Volatilization/mineralization. Significantly larger quantities of metolachlor and/or metolachlor degradates volatilized from the unsaturated subsurface soils than from the surface soils, but the quantity of applied 14C that volatilized was less than 5% for both unsaturated soils (Table 3). The mineralization of [14C]metolachlor to 14CO2 was minimal (<2%) in both the unsaturated surface and subsurface soils.
Effect of soil moisture
Loss of parent molecule. Under saturation, greater (p < 0.0042) quantities of metolachlor persisted in the unsaturated soils than in the saturated soils, with the exception of the day 60 subsurface soil and the day 120 surface soil, which were not statistically different (p < 0.21) (Table 3). The rate of dissipation of metolachlor was significantly faster (p < 0.05) in the saturated soils than in the corresponding unsaturated soils, which resulted in substantially shorter extrapolated DT50 values for metolachlor in the saturated surface and subsurface soils (Table 4).
Extractable degradates. The percentages of the applied radioactivity associated with total extractable degradation products were comparable in three of the four saturated-versusunsaturated soil pairs for days 60 and 120 DAA (p < 0.7797) (Table 3). However, calculated rates of total extractable degradate accumulation were twofold greater in the saturated than in the unsaturated surface soil and eightfold greater in the saturated than in the unsaturated subsurface soil (p < 0.0196) (Table 4). In addition, 47-fold more carbinol metabolite was measured in the extract of the saturated surface soil than in the unsaturated surface soil at 120 DAA (Table 3). In saturated soil, conditions for the oxidation of the carbinol degradate to the carboxylic acid degradate [27] are less favorable than those in unsaturated soil; therefore, one would expect to see a buildup of the carbinol degradate in the saturated soil. This would explain the greater carbinol degradate to polar degradate ratio measured in the saturated surface soil (24.3% carbinol vs 2.6% polar degradates) relative to the unsaturated soil (0.5% carbinol vs 21.2% polar degradates) at 120 DAA.
Soil-bound residues. At 120 DAA, a significantly larger (p < 0.0041) percentage of the applied 14C was bound to the saturated soil than to the unsaturated soil for both surface and subsurface soils (Table 3). The rate of soil-bound residue formation was greater in the saturated subsurface soil than in the unsaturated subsurface soil, whereas rates were similar for the saturated and unsaturated surface soil (Table 4).
Examination of our data shows metolachlor dissipation was more rapid in the saturated soils than in the unsaturated soils, with first-order DT50 values of metolachlor that were significantly shorter in the saturated soils. These results are in agreement with the findings of Walker and Brown [3], who observed that the first-order half-life of metolachlor decreased from 80.6 d in soil with a 6% w/w soil moisture to 20.9 d in the same soil with a 15% w/w soil moisture. Combustion of the extracted soils revealed that saturated soils contained a significantly greater percentage of applied 14C as soil-bound residues than the amount bound to the unsaturated soils. We were unable to identify the bound residues. The greater quantities of bound residues in the saturated soils possibly resulted from increased degradation of metolachlor in the saturated soil and the adsorption of metolachlor degradates and metolachlor to the soil. This would explain our observation in the comparison of saturated with unsaturated soil of an increase in metolachlor dissipation (Tables 3 and 4), an increase in the rate of degradate accumulation (Table 4), and an increase in the percentage of soil-bound residues (Table 3) in the saturated soil, whereas the percentages of extractable degradates were similar for the saturated and unsaturated soils (Table 3). Zheng et al. [38] showed that the Freundlich adsorption coefficient (Kfa) of metolachlor in soil increased with a decreasing soil to water ratio. This suggests that a greater quantity of the applied metolachlor was associated with the soil of the unsaturated system relative to the saturated system. Therefore, a greater quantity of the applied metolachlor was dissolved in the soil water of the saturated soil and was more bioavailable to microbial degradation.
Volatilization/mineralization. Greater quantities of metolachlor and/or metolachlor degradates volatilized from the saturated surface and subsurface soils compared to the unsaturated soils at 120 DAA, but the quantity of [14C]volatiles was less than 6% of the applied 14C (Table 3). The mineralization of [14C]metolachlor to 14CO2 was minimal (<2%) in the saturated and unsaturated surface and subsurface soils.
Microbial degradation
Surface soils that are rich in organic matter favor the growth of heterotrophic microorganisms [29]. Konopka and Turco [39] as well as Ghiorse and Wilson [40] observed that microbial biomass declined by several orders of magnitude with soil depth. Biodegradation has been shown to be the primary mechanism of metolachlor dissipation in soil [11-14]. In fact, repeated soil applications of metolachlor have been found to result in adapted microbial populations that have an enhanced ability to degrade metolachlor [41]. Therefore, factors that affect microbial activity, such as the difference in microbial abundance between surface and subsurface soils, may influence the persistence of metolachlor.
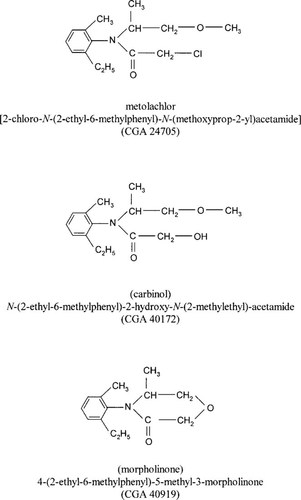
Loss of parent molecule. Soil extracts from unsaturated sterile surface soil contained significantly greater (p = 0.0001) quantities of metolachlor than the unsaturated nonsterile surface soil (Fig. 2A), indicating a microbial contribution to dissipation. In contrast, the percentage of applied [14C]metolachlor remaining in the sterile and nonsterile unsaturated subsurface soil was not statistically different (p = 0.6534) (Fig. 2B). This is reflected in the calculated DT50 values, extrapolated from metolachlor dissipation rate constants, in which the DT50 of metolachlor in unsaturated nonsterile surface soil (81 d) was less than half the DT50 in unsaturated sterile surface soil (210 d) and unsaturated nonsterile and sterile subsurface soil (289 and 217 d, respectively).
Extractable degradates. Metolachlor degradation was significantly (p < 0.0424) reduced in the sterile surface and subsurface soil. At 120 DAA, only 5 and 7% of the applied 14C was characterized as extractable metolachlor degradates in the unsaturated sterile surface and subsurface soil compared to 31 and 18% in the unsaturated nonsterile surface and subsurface soil, respectively. The carbinol and morpholinone metabolites accounted for less than 3% of the applied 14C in sterile and nonsterile unsaturated surface and subsurface soil. Formation of unidentified polar metabolites was reduced from 21.2 to 0.7% when nonsterile and sterile unsaturated surface soils were compared and from 11.4 to 0.4% of the applied 14C when nonsterile and sterile unsaturated subsurface soils were compared.
Influence of microbial degradation on the dissipation of metolachlor. Comparisons can be made for each category (e.g., metolachlor, extractable degradates, etc.) within each graph and among the graphs A to D. Bars in each category with the same letter are not statistically different (p = 0.05).
Soil-bound residues. The percentages of applied 14C characterized as unextractable soil-bound residues were compared between sterile and nonsterile soils to determine the importance of microbial degradation to the formation of unextractable bound residues. Soil-bound residues were significantly (p < 0.0289) reduced in unsaturated sterile soil compared to the nonsterile soils (Fig. 2A and B). At 120 DAA, soil-bound residues accounted for 27.6% of the applied 14C in nonsterile unsaturated surface soil and 16.7% in sterile unsaturated surface soil. Subsurface soil contained 8.3% soil-bound residues in unsaturated nonsterile soil, whereas soil-bound residues were not detected in the unsaturated sterile subsurface soil. The significant reduction in the quantity of soil-bound residues in the sterile soil relative to nonsterile soil suggests that soilbound metolachlor metabolites that are formed during microbial degradation contribute to the quantity of unextractable soil-bound residues.
Volatilization/mineralization. Volatile organic chemicals represented 0.6 and 4% of applied 14C in the nonsterile unsaturated surface and subsurface soils, respectively, whereas no volatile organic chemicals were measured in the sterile soils. Mineralization of [14C]metolachlor to 14CO2 was significantly reduced (p < 0.0446) in the sterile soils; however, 14CO2 represented less than 2% of the applied 14C for both sterile and nonsterile unsaturated surface and subsurface soil (Fig. 2A and B).
Sterile and nonsterile saturated soils. Metolachlor degradation was significantly reduced in the sterile saturated surface and subsurface soil (Fig. 2C and D). A comparison of extractable metolachlor degradates and soil-bound residues in sterile and nonsterile saturated surface and subsurface soils revealed that extractable degradates were reduced from 33.4% (nonsterile) to 7.9% (sterile) and from 28.3% (nonsterile) to 7.8% (sterile), respectively, whereas soil-bound residues were reduced from 36.9% (nonsterile) to 26.8% (sterile) and from 15.2% (nonsterile) to 2.7% (sterile) in saturated surface and subsurface soil, respectively. Mineralization and volatilization were reduced to less than 1% in the sterile saturated soil.
Our results comparing the sterile and nonsterile soils confirm the findings of Bouchard et al. [26] as well as Beestman and Deming [11], who have shown that the degradation of acetanilide herbicides is greatly reduced in autoclaved soils. Overall, the increased persistence of metolachlor and the reduced formation of extractable degradates and unextractable soil-bound residues in the autoclaved soils supports the observations that biodegradation is very important to the dissipation of metolachlor in soil.
Effect of metolachlor concentration
Microbial respiration study. Carbon dioxide evolution was not significantly depressed in unsaturated surface or subsurface soils treated with 9, 90, or 900 μg/g of metolachlor, indicating that metolachlor concentrations at 10- and 100-fold the field rate did not have an adverse effect on the soil microbial organisms (data not shown).
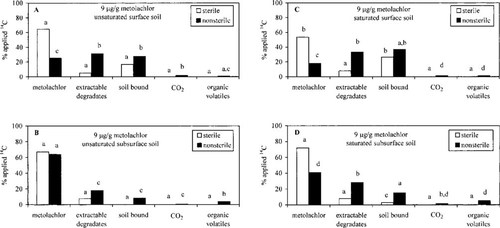
Effect of soil depth on metolachlor degradation when applied at 10-fold the field rate. Similar trends were observed in the comparison between the soils treated with metolachlor at 90 μg/g and those in soils treated at 9 μg/g. When applied at 90 μg/g, metolachlor was more persistent in the unsaturated subsurface soil than in the surface soil (p < 0.0232), and the surface soil contained a greater percentage (p = 0.0001) of unextractable soil-bound [14C]residues (Fig. 3). The unsaturated surface soil contained twofold more extractable degradates (11.9%) than the subsurface soil (5.4%); however, the difference was not statistically significant. Similar to the soil treated with metolachlor at 9 μg/g, surface and subsurface soils contained comparable quantities of the carbinol degradate (<1%) and a greater percentage of the morpholinone metabolite in the unsaturated surface soil (2.2%) than in the unsaturated subsurface soil (0.5%). Less than 1% of the applied [14C]metolachlor was mineralized to 14CO2. Volatile organic chemicals accounted for less than 4% of the applied 14C in both the unsaturated surface and subsurface soils.
Effect of a metolachlor concentration of 90 μg/g on the persistence and degradation of metolachlor in unsaturated (−33 kPa) surface and subsurface soil. Bars in each category with the same letter are not statistically different (p = 0.05).
The 90-μg/g concentration of metolachlor did not inhibit microbial activity; however, this rate of application did result in a larger percentage of the applied metolachlor being detected in the soil extract of the 90-μg/g treatment soil (surface, 48.6%; subsurface, 66.5%) than in that of the 9-μg/g treatment soil (surface, 25.2%; subsurface, 63.9%), but the values were not statistically (p < 0.72) different for the subsurface soil. Metolachlor DT50 values in the unsaturated surface soil and subsurface soil treated with 90 μg/g were 182 and 289 d, respectively, compared to 81 and 289 d, respectively, for the soils treated at 9 μg/g. The 9-μg/g treatment soils contained a significantly (p < 0.0188) greater percentage of the applied 14C as extractable degradation products in both the surface and the subsurface soil. A significantly (p < 0.0026) larger percentage of the applied 14C was bound to the surface and subsurface soil of the 9-μg/g treatment soil than for the 90-μg/g treatment soil. Obrigawitch et al. [31] observed similar trends in which the percentage adsorption of metolachlor to soil and the distribution coefficient (Kd) of metolachlor decreased as the concentration of metolachlor applied to the soil increased.
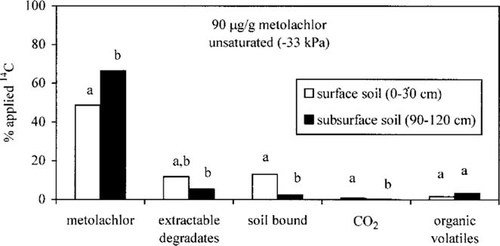
Effect of soil moisture on metolachlor degradation when applied at 10-fold the field rate. Similar trends to those observed at 9 μg/g were observed when saturated and unsaturated soil treated at 10-fold the field rate were compared. Greater quantities of metolachlor were measured in unsaturated surface (48.6%) and subsurface soil (66.5%) than in saturated surface (26.1%) and subsurface soil (54.8%), respectively (Fig. 4A and B). Calculated DT50 values of metolachlor in the saturated soil were 83 d in the surface soil and 173 d in the subsurface soil, compared to 182 and 289 d for unsaturated surface and subsurface soil, respectively. The percentage of total extractable degradates were similar for saturated and unsaturated soil; however, formation of the carbinol degradate was greater in the saturated surface soil (7.7%). Less than 1% of the carbinol degradate was measured in the unsaturated surface soil or in the saturated and unsaturated subsurface soil. Similar to the soil treated with 9 μg/g of metolachlor, saturated surface soil treated with 90 μg/g of metolachlor contained significantly more soil-bound residues (26.2%) than unsaturated surface soil (13.0%) at 120 DAA. Mineralization of [14C]metolachlor to 14CO2 was minimal (<1.5%) for surface and subsurface soils treated with 90 μg/g of metolachlor. Greater quantities of volatile organic chemicals were measured in the unsaturated subsurface soil (8.6%) than in the saturated subsurface soil (3.3%), whereas the levels measured in the unsaturated and saturated surface soil were comparable (<2.5%).
CONCLUSIONS
The dissipation of metolachlor in surface and subsurface soils, under saturated and unsaturated conditions, was determined. Significantly larger quantities of metolachlor were detected in the soil extracts of the subsurface soils regardless of the soil moisture or herbicide concentration. The greater quantity of organic matter in the surface soil resulted in both increased biodegradation and sorption of the applied [14C]metolachlor. Saturated soil favored the dissipation of metolachlor, primarily with the increased formation of soil-bound residues. Biodegradation was shown to be very important in the dissipation of metolachlor in soil, and a significantly lower quantity of unextractable soil-bound residues in sterile soil revealed the significance of biodegradation to the binding of applied 14C. Volatilization of metolachlor and metolachlor degradates and mineralization of metolachlor to CO2 were minimal in surface and subsurface soils at both soil moistures and herbicide concentrations. Increased metolachlor concentrations did not inhibit microbial activity; however, the greater rate of application did result in an increased percentage of extractable [14C]metolachlor and a reduced percentage of unextractable soil-bound residues.
Effect of a metolachlor concentration of 90 μg/g on the persistence and degradation of metolachlor in unsaturated (−33 kPa) and saturated soil (30% above the water content at −33 kPa). Comparisons can be made for each category (e.g., metolachlor, extractable degradates, etc.) within each graph and among graphs A and B. Bars in each category with the same letter are not statistically different (p = 0.05).
Greater persistence and greater availability of herbicide residues in soil increases the probability of herbicide translocation. Therefore, a reduction in the binding and the biodegradation of metolachlor in subsurface soils suggests metolachlor residues that leach to subsurface soil will have a greater potential to contaminate groundwater as a result of their increased persistence and availability. In addition, the greater percentage of extractable metolachlor and reduced percentage of soil-bound residues in the soil treated with 90 μg/g relative to the soil treated with 9 μg/g suggests that larger concentrations of metolachlor in soil are more available and have a greater potential to leach. This was observed by Zheng et al. [38], who reported that the mobility of metolachlor in soil columns increased with the applied metolachlor concentration. Clearly, the biodegradation of metolachlor in the surface soil is extremely important to its overall persistence and potential mobility. The degradation of metolachlor to more water-soluble degradates suggests that metolachlor metabolites are more mobile than the parent molecule and have a greater potential to be transported to surface water and groundwater, which may impact water quality depending on their toxicological significance.
Acknowledgements
Funding for this research was provided by Ciba Crop Protection/Novartis and the U.S. Department of Agriculture-Cooperative State Research, Education, and Extension Service Management Systems Evaluation Area Program. The authors thank Ciba Crop Protection/Novartis for providing analytical standards and radiotracers. We thank E. Arthur, Patricia Rice, J. Anhalt, K. Tollefson, C. McCullough, P. Khuon, J. Ramsey, K. Wedemeyer, and B. Nelson for their help in sample collection and technical support; M. Wallendorf for his assistance in the statistical analysis; and W. Koskinen for his helpful suggestions and comments during the writing of this manuscript. This paper is journal Article J-19706 of the Iowa Agriculture and Home Economics Experiment Station (Ames, IA, USA).




