A comparison of stream biological responses to discharge from wastewater treatment plants in high and low population density areas
Abstract
The ultimate purpose of this study was to identify broad relationships that may have relevance for the risk assessment of chemicals and materials that are discharged to receiving streams via municipal wastewater treatment plant (WWTP) effluents (e.g., consumer product ingredients). The effects of municipal wastewaters occurring in high population density (>500 persons per square mile, urban) and low population density (<500 persons per square mile, rural) environments were determined via analysis of biological, habitat, and water chemistry data collected both immediately upstream and downstream of 221 WWTPs in Ohio, USA. Several biological and chemistry indicators demonstrated poorer water quality in urban areas compared to rural areas. After considering the effect of river size, adverse effects downstream of WWTPs for both fish and macroinvertebrate communities were clearly identified for only urban areas. These data indicate that WWTP potency may be greater in urban areas compared to rural areas.
INTRODUCTION
Dyer et al. [1] compared biological responses of fish communities to local habitat metrics [2], water chemistry, and percentage cumulative municipal effluent in receiving waters at the watershed (emphasis on the Great Miami River) and state levels for Ohio, USA. Both watershed and state-level analyses indicated that cumulative municipal effluent (mean flow) was consistently associated with adverse impacts to fish community metrics, including the index of biotic integrity (IBI). Further, clusters of correlation coefficients for IBI versus habitat correlations indicated that the metroplexes of greater Dayton-Cincinnati (Great Miami River) and Cleveland (Chagrin and Cuyahoga Rivers) were different from other areas in the state. Within this cluster, percent effluent was the dominant factor addressing IBI scores. These findings lead us to this question: “Are effluents in urban areas more potent to receiving water biota than effluents in rural, or nonurban, areas?”
Impacts in urban watersheds may due to a plethora of factors, including urban nonpoint runoff of contaminants [3, 4], hydrologic alteration [5], in-stream habitat degradation [2], and increased nutrients [6]. The presence of these factors may be colocated and/or may covary with municipal wastewater discharge location and flows. In order to deduce the potential potency issues regarding the discharge of municipal waste-water in urban versus rural environments, we compared upstream fish and benthic macroinvertebrate communities to downstream communities at 221 wastewater treatment plants (WWTPs) in Ohio where in-stream habitat, chemistry, and biological measurements were made. Two null hypotheses were tested: Stream water quality in urban areas is the same as in rural areas, and WWTP discharges affect stream water quality in urban areas in the same way as it does in rural areas. These hypotheses are coarse in nature. Even so, testing these hypotheses could have major implications for establishing expectations, or biocriteria, for urban/rural areas as well as understanding the relative potential for impact from chemicals that are discharged in municipal effluents, such as pharmaceuticals and consumer product chemicals.
MATERIALS AND METHODS
Data sources and data preparation
River network. The baseline data for Ohio rivers was the U.S. Environmental Protection Agency's (U.S. EPA) Reach File, version 1, RF1. It represents rivers as a series of connected line sections at a scale of 1:500,000. However, the data do not have network features, which are essential for establishing upstream and downstream relationships. Therefore, ARC/INFO, version 7.0.4, commercial geographic information system software developed by the Environmental Systems Research Institute (Redlands, CA, USA), were used to create a river network from the RF1 line file [1].
Population data. United States Census data are considered the most reliable source for population information by geographic area. Census population data used in this study include total population by census tract. Census tracts are geographic areas within a county with relatively homogeneous demographic characteristics and a population size between 2,500 and 8,000 people. The census tract boundary and 1990 population data were extracted for the 2,861 census tracts in Ohio from the Environmental Systems Research Institute Data and Maps data set. The data were provided by Geographic Data Technology (Lebanon, NH, USA) and derived from the Bureau of the Census TIGER/Line files. The scale range of the data was between 1:35,000 and 1:1,200,000 [7]. Population density by census tract in Ohio then was calculated. The density varied from 0 to 540,000 people per square mile.
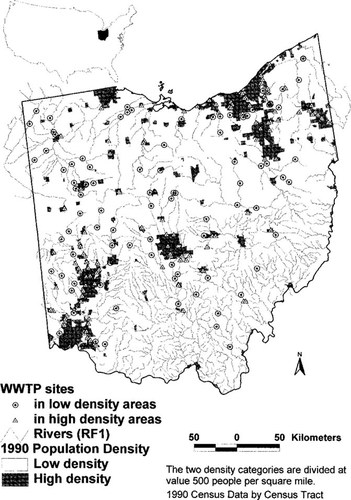
Location of wastewater treatment plants (urban and rural sites) in the state of Ohio, USA, having habitat, chemistry, and biological data for both upstream and downstream river segments.
Wastewater treatment plants
Wastewater treatment facility location data were extracted from U.S. EPA's Needs Survey [8] and Permit Compliance System (PCS) [9] databases. A total of 567 Ohio WWTP facilities discharging to RF1 river reaches were included in the original data set. Of the 567 WWTPs, 221 were on river confluences and had both upstream and downstream river segments (Fig. 1). These WWTPs were overlaid with the Ohio human population density data layer. A threshold of 500 individuals per square mile was empirically determined to be the best value that divided the state into high-density (>500 persons per square mile, urban) and low-density (<500 persons per square mile, rural) areas. Since a quantitative definition of an urban area does not exist, the authors used this density classification scheme to distinguish the different human activity corresponding to each of the 221 WWTPs.
Calculation of cumulative effluent. Mean and critical low flow (7Q10) data for all receiving waters was sourced from U.S. EPA's RF1 river file [10]. These values were combined with flow data from municipal WWTPs to estimate dilution factor and cumulative percentage WWTP effluent. Cumulative percentage WWTP effluent serves as a surrogate for persistent wastewater contributions to receiving water quality. Percentage cumulative effluent was calculated as the ratio of WWTP flow to stream flow for headwater segments, for both mean and critical low (7Q10) stream flow. For all segments, WWTP flow included not only contributions from facilities on that river segment but also contributions from facilities upstream (e.g., main stem, tributaries) segments.
Water chemistry and toxic units. Ambient water chemistry data for the entire state of Ohio were extracted from U.S. EPA's STORET database [11]. Parameters extracted for the study include biological oxygen demand (5-d), total metal concentrations (Cd, Cu, Pb, Ni, and Zn, respectively), dissolved oxygen, hardness, total ammonia, total phosphorus, pH, and total suspended solids. Too few data for organic contaminants were available for use in this study. Water chemistry data were retrieved for the years 1990 to 1996 to overlap the time frame in which the entire state was monitored for its biotic integrity. The median and 90th-percentile concentrations for each water chemistry parameter per station were determined. The derivation of the total toxic load of metals and ammonia at each sampling site was based on additivity principles. Effect benchmarks for each chemical considered were based on established U.S. EPA water quality criteria. Algorithms used to derive toxic units are summarized in Dyer et al. [1, 12].
Habitat, fish, and macroinvertebrate data. Habitat, fish, and macroinvertebrate data for 1990 to 1996 were provided by the Ohio Environmental Protection Agency (Columbus, OH, USA). During this period, each of the state's major watersheds were sampled. Typically, the Ohio EPA samples each watershed once every five years. A description of the habitat metrics and their use in deriving the qualitative habitat evaluation index (QHEI) for Ohio are provided by Rankin [2]. Briefly, the QHEI is derived from six metrics: substrate, in-stream cover (Cover), channel quality (Channel), riparian/erosion (Riparian), Pool/Riffle, and Gradient. Scores for the first six metrics and raw data for Gradient (ft/mi) were used in this study. The sum of the six metrics is 100, with 100 indicating the highest-quality habitat. Waters with QHEI scores of less than 45 can be considered limiting to aquatic life, whereas waters with scores greater than 60 are considered good habitats [13]. Information on the status of fish communities was obtained using the 12 metrics corresponding to the IBI [13]. The number and total weight of fish per sample were also used in this study. Invertebrate community information corresponded to invertebrate community index (ICI) metrics [14]. Raw metric data were used instead of metric scores for both IBI and ICI metrics.
Data integration
Biological, chemical, and habitat monitoring sites rarely occur at the same location. In order to compare data from each of these data sets in a spatially relevant manner, an aggregation scheme was applied. Based on the longitude and latitude values stored with the WWTPs, water chemistry, biology, and habitat sampling point data files, ARC/INFO® (Environmental Systems Research Institute) point coverages were created using the ARC/INFO geocoding function. The point coverages were then projected to the Albers conic equal-area projection, the same projection used for the RF1 river data. Then the RF1 river network was further divided into segments at significant hydrological features, such as the confluence of WWTP discharges and rivers. In addition, segments less than 30 m long were combined with the next downstream segment. Implementation of this segmentation scheme across the state resulted in 1,367 river segments, with an average segment length of approximately 12 km. For example, Figure 2 illustrates that the Kokosing River was divided into four segments. The first segment is from headwater to WWTP 648. The second segment is between WWTP 648 and WWTP 647. The third segment is between WWTP 647 and WWTP 646. The fourth (last) segment is from WWTP 646 to the confluence of the Kokosing and Mohican Rivers. The river segments were then labeled as upstream and downstream in relation to WWTPs. For example, segment 1 of the Kokosing River is the upstream segment for WWTP 648, and segment 2 of the Kokosing River is the downstream segment for WWTP 648. Meanwhile, segment 2 is also the upstream segment for WWTP 647. The ARC/INFO spatial overlay functions then were used to assign river segment number to each point file (water chemistry, habitat, and biological sampling points). If more than one monitoring site for a variable was found on the same segment, the value from the monitoring sites closest to the WWTP was used in further analysis. Figure 2 shows eight IBI sampling sites on the first section of the Kokosing River. The value from the last site (ID = 4406) was assigned to the upstream segment for WWTP 648. Although three IBI sites were found on the same segment (segment 2 of the Kokosing River), the value from IBI site 4405 was used to represent the downstream segment for WWTP 648, and IBI site 4403 was used to represent the upstream segment for WWTP 647. This method was adopted to reduce the impact of distance on the validity of the analysis.
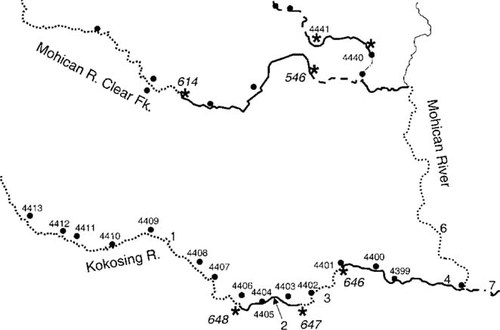
River segmentation scheme used to determine upstream and downstream effects of wastewater treatment plant (WWTP) discharge. River segments were broken on confluences from WWTPs (number of plant in italics, location = *) and other RFI streams. Biological sampling sites correspond to four-digit numbers. Segments are denoted by various dashed- or solid-line patterns, and large font values pertain to the segment number. When multiple biological sampling sites were present in a segment, only the nearest biological sampling sites to WWTPs were used in this study.
Statistical analysis
All sampling sites were split into four groups based on upstream or downstream relationships to WWTPs and whether they occurred in high or low population density areas. A two-tailed independent t test was employed for comparing the sample means between high and low population density groups upstream and downstream from WWTPs, respectively. River flow and dilution factors were also compared for high- and low-density areas using the independent t test. The test statistic is calculated from the sample mean of sites in urban area minus the sample mean of sites in rural area. The null hypothesis was that no difference exists between the two sample means at the 0.05 significance level.
A two-tailed paired t test was used to test for the change from upstream to downstream of WWTPs in high- and low-density areas, respectively. The test statistic was calculated from the variable value of downstream sites minus the value of upstream sites. The second null hypothesis tested was that the mean sample values did not change from upstream sites to downstream sites at the 0.05 significance level. All analyses were conducted with the statistical software SPSS® (Chicago, IL, USA).
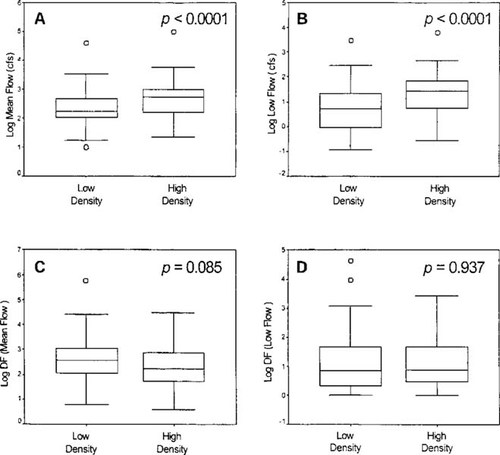
Comparison of the distributions of mean flow (A), low flow (B), dilution factor at mean flow (C), and dilution factor at low flow (D). Significance values correspond to results from two-tailed independent t tests. The boxes correspond to the mean and 1 standard deviation (SD), and the whiskers denote distances of 2 SD. The symbol (○) pertains to values outside 2 SD.
RESULTS
High density versus low density
The ranges of mean and low river flows for both rural and urban areas were similar (Fig. 3a and b). However, the mean values for both mean and low flows in high-density areas were significantly greater than mean values in low-density areas. The ranges of dilution factors from WWTPs were similar for both low- and high-density areas (Fig. 3c and d). In contrast to the flow analysis, no significant differences were observed for mean dilution factors in rural versus urban areas. The distances of fish and macroinvertebrate sampling sites to WWTPs were similar for both urban and rural settings, corresponding to approximately 1 km for upstream and downstream (Fig. 4).
Fish metrics that serve as measures of good water quality were generally greater in low-density areas versus high-density areas (Table 1). Significantly greater mean values were observed for both upstream and downstream sites in rural areas for metrics such as the total number of fish per sample, percentage tolerant species, and numbers of darter, sculpin, and minnow species. A significantly lower proportion of fish with deformities, fin erosions, lesions and tumors (DELTs), percent top carnivores, and the total weight of fish sampled were observed in the low-density areas. Significantly greater species richness was noted downstream of WWTPs in low-density areas. The number of sunfish species and percentage of round-bodied suckers were greater in upstream urban settings. No significant differences were observed for the IBI.
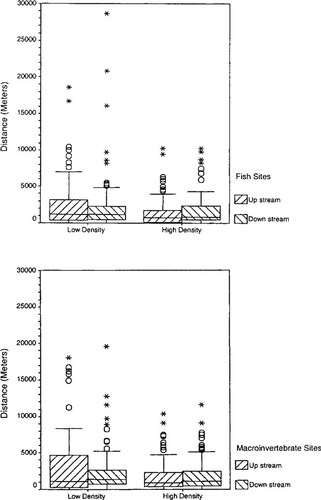
A comparison of the distances of macroinvertebrate and fish sampling sites to wastewater treatment plant locations for both urban (high-density) and rural (low-density) areas. The boxes correspond to the mean and 1 standard deviation (SD), and the whiskers denote distances of 2 SD. The symbol (○) pertains to values outside 2 SD.
Significantly greater numbers of total macroinvertebrates and caddisfly taxa were observed in upstream urban sites as compared to upstream rural areas (Table 2). Conversely, the number of diptera taxa was significantly less in urban areas. Several metrics that pertain to the ICI showed significant reductions in downstream urban areas, including the number of diptera taxa, number of invertebrate taxa collected via Hester-Dendy samplers and dip net (qualitative taxa), and number of mayfly taxa. No significant differences were observed for ICI. In-stream habitat quality (Table 3) did not show significant differences between high- and low-density sites for either upstream or downstream conditions, with the exception of significantly greater Pool scores in urban areas.
Significant differences between urban and rural settings for water chemistry parameters were mostly evident for downstream comparisons (Table 4). Significantly greater concentrations of cadmium (90th percentile), lead (50th percentile), total phosphorus (90th percentile), total zinc (50th percentile), and total toxic units from metals and ammonia (50th percentile) were all elevated in downstream urban segments as compared to downstream rural segments. The only parameter that showed significant differences for both upstream and downstream segments was the percentage WWTP effluent at mean river flow, both larger in urban than rural areas. Upstream urban areas were also significantly greater than rural areas for the percent cumulative effluent at low flow.
Upstream versus downstream
Paired t tests of upstream-to-downstream change in fish metrics for high- and low-density areas receiving discharge of municipal wastewater yielded two very different sets of responses (Table 5). The first set of responses reflected an adverse effect of effluent exposure to fish in urban settings, while an enrichment or little effect of effluent was observed in rural situations. In addition to the IBI, sensitive metrics such as the number of darter and sculpin species, percentage of darter species, numbers of sunfish species, and total species richness were all significantly greater in upstream segments in highdensity areas. None of these same metrics showed the same response in rural settings. For instance, significant increases in the numbers of sensitive and sucker species were observed downstream of WWTP discharges in low-density areas. No other significant differences were determined for rural areas.
As found with the fish metrics and IBI, paired t tests for invertebrate metrics and ICI yielded similar trends, indicating adverse effects of effluent exposure (downstream) in urban areas and enrichment effects in rural areas (Table 6). Six out of the 13 metrics in urban areas indicated poorer invertebrate community integrity downstream of WWTPs in urban areas. The numbers of mayfly, ephemeroptera, plecoptera, and trichoptera and qualitative taxa in addition to the percentage of dipteran and Tanytarsini taxa were significantly less in downstream sections, whereas significant increases in the percentage of oligochaetes in downstream sites were observed. In contrast, rural areas showed significant increases in the number of caddisfly and mayfly taxa sampled via Hester-Dendy artificial substrates downstream of WWTP inputs.
None of the metrics corresponding to the QHEI or the QHEI itself was found to be significantly different between upstream and downstream areas for both urban and rural areas. Upstream-to-downstream trends for water chemistry in urban and rural sites were different (Table 7). Significantly greater concentrations of median and 90th-percentile Cd and total phosphorus were observed downstream of urban WWTPs. Median zinc and lead concentrations were also significantly greater at downstream of urban sites. In contrast, in the rural sites, median lead was significantly less in downstream river segments.
DISCUSSION
Rivers in urban areas were highly significantly larger than their rural counterparts. While dilution factors at the point of discharge did not show urban-versus-rural differences, a significantly greater portion of river flow could be attributed to the cumulative discharges from municipal WWTPs. Importantly, this meant that the testing of hypothesis 1 (stream water quality in urban areas is the same as in rural areas) required a recognition of the effect of river size (flows), whereas the second hypothesis (WWTP discharges affect stream water quality in urban areas in the same way as they do in rural areas, i.e., upstream-to-downstream comparisons mediated by WWTP effluents) was not confounded by factors such as dilution and distances from discharges.
| Mean | Sample size | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Signa | Positionb | Mean difference | t | P | High density | Low density | High density | Low density |
| Index of biotic integrity | + | Up | −0.761 | −0.499 | 0.62 | 36.06 | 36.83 | 111 | 71 |
| Down | −2.416 | −1.649 | 0.10 | 34.35 | 36.77 | 111 | 71 | ||
| No. darter and sculpin species | + | Up | −0.839 | −2.945 | 0.00* | 1.99 | 2.83 | 111 | 71 |
| Down | −1.252 | −5.127 | 0.00* | 1.65 | 2.90 | 111 | 71 | ||
| No. darter species | + | Up | −0.801 | −2.967 | 0.00* | 1.91 | 2.71 | 111 | 71 |
| No. fish per sample | Down | −1.142 | −4.911 | 0.00* | 1.59 | 2.73 | 111 | 71 | |
| + | Up | −218.385 | −2.369 | 0.02* | 710.25 | 928.64 | 111 | 71 | |
| % Fish with deformities, eroded fins, lesions, or tumors | Down | −257.277 | −2.818 | 0.01* | 663.29 | 920.57 | 111 | 71 | |
| − | Up | 1.384 | 2.059 | 0.04* | 3.18 | 1.80 | 111 | 71 | |
| Down | 1.710 | 2.708 | 0.01* | 3.56 | 1.85 | 111 | 71 | ||
| No. intolerant fish species | + | Up | 0.018 | 0.078 | 0.94 | 1.28 | 1.26 | 111 | 71 |
| Down | −0.161 | −0.748 | 0.46 | 1.20 | 1.36 | 111 | 71 | ||
| No. minnow species | + | Up | −0.924 | −2.809 | 0.01* | 4.79 | 5.71 | 111 | 71 |
| Down | −1.392 | −4.084 | 0.00* | 4.57 | 5.96 | 111 | 71 | ||
| % Omnivores | − | Up | 4.084 | 1.692 | 0.09 | 27.06 | 22.97 | 111 | 71 |
| Down | 3.634 | 1.477 | 0.14* | 28.39 | 24.75 | 111 | 71 | ||
| % Round-bodied suckers | + | Up | 6.949 | 3.365 | 0.00* | 14.19 | 7.24 | 111 | 71 |
| Down | 3.777 | 1.882 | 0.06 | 12.85 | 9.07 | 111 | 71 | ||
| No. sensitive species | + | Up | 0.603 | 1.050 | 0.30 | 5.20 | 4.60 | 111 | 71 |
| Down | −0.669 | −1.232 | 0.22 | 4.80 | 5.47 | 111 | 71 | ||
| No. sunfish species | + | Up | 0.422 | 2.173 | 0.03* | 3.49 | 3.06 | 111 | 71 |
| Down | −0.043 | −0.223 | 0.82 | 3.12 | 3.17 | 111 | 71 | ||
| No. sucker species | + | Up | 0.503 | 1.863 | 0.06 | 3.24 | 2.74 | 111 | 71 |
| Down | 0.077 | 0.299 | 0.76 | 3.18 | 3.10 | 111 | 71 | ||
| % Tolerant fish species | − | Up | −9.641 | −2.861 | 0.00* | 30.52 | 40.16 | 111 | 71 |
| Down | −6.956 | −2.113 | 0.04* | 30.59 | 37.54 | 111 | 71 | ||
| % Top carnivores | + | Up | 3.428 | 3.344 | 0.00* | 8.36 | 4.94 | 111 | 71 |
| Down | 3.152 | 3.075 | 0.00* | 8.63 | 5.47 | 111 | 71 | ||
| Total species richness for the sampling pass | + | Up | −0.186 | −0.214 | 0.83 | 17.72 | 17.91 | 111 | 71 |
| Down | −2.152 | −2.482 | 0.01* | 16.72 | 18.88 | 111 | 71 | ||
| Weight (kg) of fish per distance | + | Up | 30.649 | 3.586 | 0.00* | 75.92 | 45.27 | 111 | 71 |
| Down | 29.490 | 3.312 | 0.00* | 79.56 | 50.07 | 111 | 71 | ||
- a + indicates that higher value represents higher quality; — indicates that higher value represents lower quality.
- b Up indicates that the monitoring site is upstream from a wastewater treatment plant (WWTP) discharge point; Down indicates that the monitoring site is downstream from a WWTP discharge point.
- * p < 0.05.
Metrics for fish and macroinvertebrate community biotic integrity showed that water quality may be significantly more degraded in urban areas than rural areas. Significantly fewer numbers of fish per sample as well as fewer numbers of darter, sculpin, and minnow species were observed in both upstream (of WWTP) and downstream segments in urban areas compared to rural areas. It can be successfully argued that these significant differences were simply a matter of river size. That is, darter, sculpin, and minnow species are dominant in smallorder streams [13]. Two other significant relationships support a conclusion that river size is a major determinant in the urbanversus-rural comparison: the significantly increased percentage of carnivores and total weight of fish sampled in both upstream and downstream urban segments and the significantly greater percentage of round-bodied suckers and number of sunfish species in upstream segments. Both relationship sets are indicative of larger streams. Even so, it appears that adverse effects may exist in urban environments via the significantly decreased numbers of fish taxa in downstream urban segments and the increased percentage of fish with deformities, fin erosions, lesions, and tumors in both upstream and downstream segments.
Biological effects of river size were also noted by the significantly greater numbers of caddisfly taxa and total number of individuals in the Hester-Dendy samplers in the high-density areas compared to the lower-density areas. Even so, impacts were clearly noted in urban areas, as the number of diptera taxa were less in both upstream and downstream sites compared to their rural counterparts. Significantly fewer numbers of mayfly taxa and total macroinvertebrate taxa from both qualitative (dip net) and Hester-Dendy samples in downstream urban areas indicated that these areas were adversely impacted. Upstream-to-downstream analysis within the urban category indicated that these WWTPs may be associated with these impacts. Significantly fewer numbers of ephemeroptera, plecoptera, and trichoptera, mayfly and qualitative taxa were observed downstream of WWTPs. Significant reductions in the percentage of diptera and Tanytarsini taxa, coupled with an increased percentage of oligochaete worms, further support the conclusion of impacts below urban WWTPs. Conversely, these trends were not observed in the rural areas. Significantly increased numbers of caddisfly and mayfly taxa were observed downstream of WWTPs in rural areas. Clearly, these data indicate that the WWTP discharge in rural areas contribute to an enrichment phenomena rather than one reminiscent of toxicity, as identified in the urban areas.
An examination of the water chemistry data in both upstream and downstream areas supported the previous conclusion. Metals (Cd, Pb, Zn) and total phosphorus concentrations were significantly greater in downstream urban segments as compared to both downstream rural segments and upstream urban segments. This was not the case for rural areas, where it was found that median lead concentrations were less in downstream segments as compared to upstream areas. Even so, caution is needed not to overinterpret the statistical significance of the water chemistry data with the biological trends so as to establish cause-and-effect relationships.
| Mean | Sample size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Unit | Signa | Positionb | Mean difference | t | P | High density | Low density | High density | Low density |
| Invertebrate community index | + | Up | 0.129 | 0.057 | 0.95 | 36.61 | 36.48 | 93 | 53 | |
| Down | −4.094 | −1.825 | 0.07 | 34.35 | 38.45 | 93 | 53 | |||
| Caddisfly | Count | + | Up | 1.504 | 3.512 | 0.00* | 4.14 | 2.64 | 101 | 63 |
| Down | 0.257 | 0.610 | 0.54 | 3.64 | 3.38 | 101 | 63 | |||
| % | Up | 2.921 | 1.332 | 0.18 | 13.68 | 10.75 | 93 | 53 | ||
| Down | 3.326 | 1.664 | 0.10 | 14.15 | 10.83 | 93 | 53 | |||
| Diptera taxa | Count | + | Up | −2.206 | −2.110 | 0.04* | 11.50 | 13.71 | 101 | 63 |
| Down | −3.505 | −3.725 | 0.00* | 11.02 | 14.52 | 101 | 63 | |||
| % | Up | 3.628 | 1.122 | 0.26 | 59.11 | 55.48 | 93 | 53 | ||
| Down | −3.872 | −1.225 | 0.22 | 54.11 | 57.98 | 93 | 53 | |||
| Ephemeroptera, plecoptera, and trichoptera | Count | + | Up | 0.069 | 0.077 | 0.94 | 10.56 | 10.49 | 101 | 63 |
| Down | −1.493 | −1.683 | 0.09 | 9.22 | 10.71 | 101 | 63 | |||
| Invertebrate taxa | Count | + | Up | 0.289 | 0.135 | 0.89 | 28.45 | 28.16 | 101 | 63 |
| Down | −4.018 | −2.019 | 0.05* | 26.98 | 30.99 | 101 | 63 | |||
| Mayfly taxa | Count | + | Up | 0.601 | 1.115 | 0.27 | 5.22 | 4.62 | 101 | 63 |
| Down | −1.031 | −1.950 | 0.05* | 4.50 | 5.53 | 101 | 63 | |||
| % | Up | −2.649 | −1.039 | 0.30 | 15.17 | 17.82 | 93 | 53 | ||
| Down | −4.441 | −1.748 | 0.08 | 13.93 | 18.37 | 93 | 53 | |||
| Oligochaete taxa | % | − | Up | −1.108 | −0.671 | 0.50 | 6.10 | 7.21 | 93 | 53 |
| Down | 3.569 | 1.775 | 0.08 | 9.16 | 5.59 | 93 | 53 | |||
| Other taxa | % | − | Up | −5.033 | −1.061 | 0.29 | 45.86 | 50.90 | 93 | 53 |
| Down | 2.148 | 0.454 | 0.65 | 50.54 | 48.39 | 93 | 53 | |||
| Qualitative taxa | Count | + | Up | −2.581 | −1.262 | 0.21 | 39.39 | 41.97 | 101 | 63 |
| Down | −5.620 | −2.714 | 0.01* | 36.16 | 41.78 | 101 | 63 | |||
| Tanytarsini taxa | % | + | Up | 5.363 | 1.661 | 0.10* | 23.91 | 18.55 | 93 | 53 |
| Down | −0.394 | −0.128 | 0.90 | 20.34 | 20.74 | 93 | 53 | |||
| Total macroinvertebrates | Count | + | Up | 2,938.311 | 2.624 | 0.01* | 6,524.61 | 3,586.27 | 101 | 63 |
| Down | −117.260 | −0.093 | 0.93 | 5,502.40 | 5,619.66 | 101 | 63 | |||
- a + indicates that higher value represents higher quality; — indicates that higher value represents lower quality.
- b Up indicates that the monitoring site is upstream from a wastewater treatment plant (WWTP) discharge point; Down indicates that the monitoring site is downstream from a WWTP discharge point.
- * p < 0.05.
| Mean | Sample size | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Position* | Mean difference | t | P | High density | Low density | High density | Low density |
| Qualitative habitat evaluation index | Up | 2.438 | 1.288 | 0.20 | 65.98 | 63.55 | 106 | 81 |
| Down | 0.130 | 0.070 | 0.94 | 65.75 | 65.62 | 106 | 81 | |
| Channel quality | Up | 0.478 | 0.997 | 0.32 | 14.01 | 13.53 | 106 | 81 |
| Down | −0.080 | −0.180 | 0.86 | 13.99 | 14.07 | 106 | 81 | |
| Gradient | Up | 1.072 | 0.631 | 0.53 | 9.90 | 8.82 | 106 | 81 |
| Down | 1.488 | 0.977 | 0.33 | 9.07 | 7.58 | 106 | 81 | |
| Instream cover | Up | 0.282 | 0.653 | 0.51 | 12.32 | 12.04 | 106 | 81 |
| Down | −0.114 | −0.274 | 0.78 | 12.10 | 12.21 | 106 | 81 | |
| Pool | Up | 0.800 | 2.821 | 0.01* | 9.03 | 8.23 | 106 | 81 |
| Down | 0.743 | 2.777 | 0.01* | 9.29 | 8.55 | 106 | 81 | |
| Riffle | Up | 0.328 | 1.056 | 0.29 | 3.55 | 3.22 | 106 | 79 |
| Down | 0.293 | 0.948 | 0.34 | 3.55 | 3.26 | 106 | 79 | |
| Riparian/erosion | Up | −0.169 | −0.778 | 0.44 | 6.01 | 6.18 | 106 | 81 |
| Down | −0.209 | −1.063 | 0.29 | 6.06 | 6.27 | 106 | 81 | |
| Substrate | Up | 0.228 | 0.368 | 0.71 | 13.17 | 12.95 | 106 | 81 |
| Down | −0.694 | −1.167 | 0.24 | 12.95 | 13.64 | 106 | 81 | |
- a Up indicates that the monitoring sites is upstream from a wastewater treatment plant (WWTP) discharge point; Down indicates that the monitoring site is downstream from a WWTP discharge point.
- * p < 0.05.
| Mean | Sample size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Sign | Percentile | Positionb | Mean difference | t | P | High density | Low density | High density | Low density |
| Biochemical oxygen demand, 5 d, 20°C (mg/L) | ||||||||||
| — | 50 | Up | 2.279 | 1.064 | 0.29 | 4.83 | 2.55 | 54 | 43 | |
| Down | 1.661 | 0.768 | 0.44 | 4.54 | 2.88 | 54 | 43 | |||
| 90 | Up | 2.479 | 1.106 | 0.27 | 6.94 | 4.46 | 54 | 43 | ||
| Down | 1.683 | 0.745 | 0.46 | 6.65 | 4.97 | 54 | 43 | |||
| Cadmium, total (μg Cd/L) | — | 50 | Up | −0.067 | −1.046 | 0.30 | 0.21 | 0.28 | 95 | 61 |
| Down | 0.052 | 1.725 | 0.09 | 0.26 | 0.21 | 95 | 61 | |||
| 90 | Up | −0.018 | −0.258 | 0.80 | 0.28 | 0.30 | 95 | 61 | ||
| Down | 0.173 | 2.514 | 0.01* | 0.41 | 0.24 | 95 | 61 | |||
| Copper, total (μg Cu/L) | — | 50 | Up | −0.221 | −1.033 | 0.30 | 9.72 | 9.94 | 95 | 61 |
| Down | 0.174 | 0.598 | 0.55 | 10.05 | 9.88 | 95 | 61 | |||
| 90 | Up | −0.532 | −1.076 | 0.28 | 10.44 | 10.98 | 95 | 61 | ||
| Down | 0.167 | 0.233 | 0.82 | 11.24 | 11.08 | 95 | 61 | |||
| Dissolved oxygen (mg O2/L) | + | 50 | Up | 0.798 | 1.702 | 0.09 | 7.14 | 6.34 | 77 | 50 |
| Down | 0.042 | 0.095 | 0.92 | 6.77 | 6.72 | 77 | 50 | |||
| 90 | Up | 0.447 | 0.727 | 0.47 | 9.27 | 8.82 | 77 | 50 | ||
| Down | −0.260 | −0.425 | 0.67 | 8.72 | 8.98 | 77 | 50 | |||
| Hardness, total (mg CaCO3/L) | — | 50 | Up | −28.067 | −1.966 | 0.05* | 271.83 | 299.90 | 94 | 59 |
| Down | −25.967 | −1.923 | 0.06 | 268.78 | 294.75 | 94 | 59 | |||
| 90 | Up | −29.811 | −1.884 | 0.06 | 301.75 | 331.56 | 94 | 59 | ||
| Down | −22.580 | −1.469 | 0.14 | 305.09 | 327.67 | 94 | 59 | |||
| Lead, total (μg Pb/L) | — | 50 | Up | 0.061 | 0.516 | 0.61 | 2.32 | 2.26 | 95 | 61 |
| Down | 0.667 | 3.431 | 0.00* | 2.70 | 2.04 | 95 | 61 | |||
| 90 | Up | −0.767 | −0.836 | 0.40 | 4.44 | 5.21 | 95 | 61 | ||
| Down | 1.653 | 1.863 | 0.06 | 5.54 | 3.89 | 95 | 61 | |||
| Nickel, total (μg Ni/L) | — | 50 | Up | −0.313 | −0.552 | 0.58 | 39.55 | 39.87 | 84 | 54 |
| Down | −1.228 | −1.808 | 0.07 | 38.86 | 40.09 | 84 | 54 | |||
| 90 | Up | −0.001 | −0.001 | 1.00 | 40.64 | 40.64 | 84 | 54 | ||
| Down | 0.003 | 0.004 | 1.00 | 40.44 | 40.44 | 84 | 54 | |||
| Total ammonia (mg NH3-N/L) | — | 50 | Up | 0.160 | 1.483 | 0.14 | 0.24 | 0.08 | 94 | 61 |
| Down | 0.170 | 1.338 | 0.18 | 0.30 | 0.13 | 94 | 61 | |||
| 90 | Up | 0.308 | 1.483 | 0.14 | 0.49 | 0.18 | 94 | 61 | ||
| Down | 0.395 | 1.596 | 0.11 | 0.71 | 0.31 | 94 | 61 | |||
| Phosphorus, total (mg P/L) | — | 50 | Up | −0.013 | −0.309 | 0.76 | 0.23 | 0.24 | 79 | 50 |
| Down | 0.117 | 1.773 | 0.08 | 0.38 | 0.26 | 79 | 50 | |||
| 90 | Up | −0.096 | −0.912 | 0.36 | 0.45 | 0.54 | 79 | 50 | ||
| Down | 0.316 | 2.544 | 0.01* | 0.79 | 0.47 | 79 | 50 | |||
| PH | ± | 50 | Up | 0.006 | 0.123 | 0.90 | 7.99 | 7.98 | 83 | 55 |
| Down | −0.094 | −1.753 | 0.08 | 7.95 | 8.04 | 83 | 55 | |||
| 90 | Up | 0.032 | 0.614 | 0.54 | 8.25 | 8.22 | 83 | 55 | ||
| Down | −0.070 | −1.313 | 0.19 | 8.20 | 8.27 | 83 | 55 | |||
| Total suspended solids (mg/L) | — | 50 | Up | −5.984 | −1.599 | 0.11 | 22.50 | 28.48 | 94 | 60 |
| Down | 0.506 | 0.147 | 0.88 | 24.85 | 24.35 | 94 | 60 | |||
| 90 | Up | −38.779 | −1.516 | 0.13 | 71.07 | 109.85 | 94 | 60 | ||
| Down | −4.010 | −0.270 | 0.79 | 74.55 | 78.56 | 94 | 60 | |||
| Total toxic units | — | 50 | Up | 0.645 | 1.367 | 0.17 | 1.51 | 0.87 | 94 | 59 |
| Down | 1.659 | 2.142 | 0.03* | 2.52 | 0.86 | 94 | 59 | |||
| 90 | Up | 0.854 | 1.222 | 0.22 | 2.07 | 1.22 | 94 | 59 | ||
| Down | 1.769 | 1.853 | 0.07 | 3.22 | 1.45 | 94 | 59 | |||
| Zinc, total (μg Zn/L) | — | 50 | Up | −1.754 | −0.754 | 0.45 | 18.16 | 19.92 | 95 | 60 |
| Down | 4.197 | 2.050 | 0.04* | 21.08 | 16.88 | 95 | 60 | |||
| 90 | Up | 3.818 | 0.480 | 0.63 | 45.55 | 41.73 | 95 | 60 | ||
| Down | 5.907 | 0.533 | 0.59 | 53.82 | 47.91 | 95 | 60 | |||
| % WWTP effluent at mean river flow | — | Up | 2.096 | 2.335 | 0.02* | 4.77 | 2.67 | 94 | 63 | |
| Down | 3.444 | 2.870 | 0.00 | 7.10 | 3.65 | 94 | 63 | |||
| % WWTP effluent at low river flow | — | Up | 12.971 | 1.968 | 0.05* | 59.78 | 46.81 | 94 | 63 | |
| Down | 10.016 | 1.729 | 0.09 | 73.13 | 63.12 | 94 | 63 | |||
- a + indicates that higher value represents higher quality; — indicates that higher value represents lower quality; ± indicates that the direction cannot be determined.
- b Up indicates that the monitoring site is upstream from a wastewater treatment plant (WWTP) discharge point; Down indicates that the monitoring site is downstream from a WWTP discharge point.
- * p < 0.05.
At mean flow, the mean percent cumulative effluent in urban areas was roughly twice that of rural areas. However, at low flow, between 46 and 73% of river flow could be attributed to effluent from both urban and rural areas. According to Diamond and Daley [15], biotic integrity is typically impaired downstream of WWTPs that consistently discharge effluents that fail whole effluent toxicity tests and have ≥80% river water composed of effluent. Birge et al. [16] and Dickson et al. [17] have illustrated that ambient toxicity may be more relevant to aquatic communities than whole effluent toxicity test results or accounting for percent effluent. While our study did not have whole effluent toxicity or ambient toxicity test results, the use of total toxic units from ammonia and metals provided a surrogate approach to assess the potential of WWTP-derived effects. Downstream urban sites had significantly greater total (median) toxic units than rural sites. Toxic units were derived via benchmarks to U.S. EPA water quality criteria values for metals and ammonia. Exceedence of a toxic unit of 1 is indicative of the potential for adverse effects. Exceedences of the median and 90th-percentile-based statistics per urban river segment indicated that these areas routinely receive metals and ammonia concentrations that together may address the decrease in biotic integrity at downstream sites. However, only the 90th-percentile values exceeded a toxic unit of 1 in rural areas.
| High population density area | Low population density area | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paired differences | Paired differences | |||||||||||
| Variable | Unit | Sign | Mean | SDb | t | df | p | Mean | SD | t | df | P |
| Index of biotic integrity | + | −1.71 | 5.35 | −3.375 | 110 | 0.00* | −0.06 | 6.95 | −0.071 | 70 | 0.94 | |
| Darter and sculpin species | Count | + | −0.35 | 1.40 | −2.606 | 110 | 0.01* | 0.07 | 1.64 | 0.347 | 70 | 0.73 |
| Darter species | % | + | −0.33 | 1.32 | −2.594 | 110 | 0.01* | 0.02 | 1.58 | 0.082 | 70 | 0.94 |
| Fish per sample | Count | + | −46.96 | 590.99 | −0.837 | 110 | 0.40 | −8.07 | 602.14 | −0.113 | 70 | 0.91 |
| Fish with deformities, eroded fins, lesions, or tumors | % | − | 0.38 | 3.12 | 1.283 | 110 | 0.20 | 0.05 | 3.86 | 0.119 | 70 | 0.91 |
| Intolerant fish species | Count | + | −0.08 | 0.96 | −0.907 | 110 | 0.37 | 0.10 | 1.00 | 0.811 | 70 | 0.42 |
| Minnow species | Count | + | −0.22 | 2.22 | −1.040 | 110 | 0.30 | 0.25 | 2.09 | 1.004 | 70 | 0.32 |
| Omnivores | % | − | 1.33 | 14.53 | 0.964 | 110 | 0.34 | 1.78 | 11.02 | 1.359 | 70 | 0.18 |
| Round-bodied suckers | % | + | −1.34 | 9.26 | −1.524 | 110 | 0.13 | 1.83 | 8.79 | 1.758 | 70 | 0.08* |
| Sensitive species | Count | + | −0.40 | 2.17 | −1.938 | 110 | 0.06 | 0.87 | 2.42 | 3.045 | 70 | 0.00* |
| Sucker species | Count | + | −0.06 | 1.04 | −0.603 | 110 | 0.55 | 0.37 | 1.17 | 2.641 | 70 | 0.01* |
| Sunfish species | Count | + | −0.36 | 1.21 | −3.166 | 110 | 0.00* | 0.10 | 1.18 | 0.727 | 70 | 0.47 |
| Tolerant fish species | % | − | 0.06 | 17.53 | 0.039 | 110 | 0.97 | −2.62 | 20.88 | −1.058 | 70 | 0.29 |
| Top carnivores | % | + | 0.26 | 8.38 | 0.330 | 110 | 0.74 | 0.54 | 5.16 | 0.881 | 70 | 0.38 |
| Total species richness for the sampling pass | + | −0.99 | 4.46 | −2.352 | 110 | 0.02* | 0.97 | 4.75 | 1.725 | 70 | 0.09* | |
| Weight of fish per distance | Kg | + | 3.65 | 51.42 | 0.747 | 110 | 0.46 | 4.81 | 45.43 | 0.892 | 70 | 0.38 |
- a + indicates that higher value represents higher quality; — indicates that higher values represents lower quality; ± indicates that the direction cannot be determined, in which case a two-tailed p value was calculated.
- b SD = standard deviation.
- * p < 0.05.
| High population density area | Low population density area | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paired differences | Paired differences | |||||||||||
| Variable | Unit | Signa | Mean | SDb | t | df | p | Mean | SD | t | df | p |
| Invertebrate community index | + | −2.26 | 12.21 | −1.787 | 92 | 0.08 | 1.96 | 12.17 | 1.173 | 52 | 0.25 | |
| Caddisfly | Count | + | −0.50 | 2.87 | −1.755 | 100 | 0.08 | 0.75 | 2.12 | 2.786 | 62 | 0.01* |
| % | + | 0.48 | 10.43 | 0.442 | 92 | 0.66 | 0.07 | 11.91 | 0.045 | 52 | 0.96 | |
| Dipteran taxa | Count | + | −0.48 | 6.20 | −0.785 | 100 | 0.43 | 0.81 | 7.47 | 0.865 | 62 | 0.39 |
| % | + | −5.00 | 20.20 | −2.385 | 92 | 0.02* | 2.50 | 21.99 | 0.829 | 52 | 0.41 | |
| Ephemoptera, plecoptera, and trichoptera | Count | + | −1.34 | 5.03 | −2.689 | 100 | 0.01* | 0.22 | 4.98 | 0.347 | 62 | 0.73 |
| Invertebrate taxa | Count | + | −1.47 | 14.90 | −0.992 | 100 | 0.32 | 2.84 | 15.26 | 1.475 | 62 | 0.15 |
| Mayfly taxa | Count | + | −0.73 | 3.62 | −2.023 | 100 | 0.05* | 0.90 | 3.39 | 2.115 | 62 | 0.04* |
| % | + | −1.24 | 13.89 | −0.864 | 92 | 0.39 | 0.55 | 19.10 | 0.209 | 52 | 0.84 | |
| Oligochaete taxa | % | − | 3.06 | 13.60 | 2.168 | 927 | 0.03* | −1.62 | 11.01 | −1.071 | 52 | 0.29 |
| Other taxa | % | − | 4.68 | 24.92 | 1.811 | 92 | 0.07 | −2.50 | 27.49 | −0.663 | 52 | 0.51 |
| Qualitative taxa | Count | + | −3.23 | 12.64 | −2.567 | 100 | 0.01* | −0.19 | 12.20 | −0.123 | 62 | 0.90 |
| Tanytarsini taxa | % | + | −3.57 | 17.50 | −1.967 | 92 | 0.05* | 2.19 | 13.66 | 1.166 | 52 | 0.25 |
| Total macroinvertebrates | Count | + | 1,022 | 8,576 | −1.198 | 100 | 0.23 | 2,033 | 9,311 | 1.733 | 62 | 0.09* |
- a + indicates that higher value represents higher quality; — indicates that higher value represents lower quality; ± indicates that the direction cannot be determined, in which case a two-tailed p value was calculated.
- b SD = standard deviation.
- * p < 0.05.
| High population density area | Low population density area | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paired differences | Paired differences | |||||||||||
| Variable | Signa | Percentile | Mean | SDb | t | df | p | Mean | SD | t | df | p |
| Biochemical oxygen demand, 5 d, 20°C (mg/L) | − | 50 | −0.29 | 19.90 | −0.107 | 53 | 0.92 | 0.33 | 2.40 | 0.900 | 42 | 0.37 |
| 90 | −0.29 | 20.62 | −0.103 | 53 | 0.92 | 0.51 | 3.75 | 0.887 | 42 | 0.38 | ||
| Cadmium, total (μg Cd/L) | − | 50 | 0.05 | 0.18 | 2.498 | 94 | 0.01* | −0.07 | 0.62 | −0.910 | 60 | 0.37 |
| 90 | 0.13 | 0.50 | 2.565 | 94 | 0.01* | −0.06 | 0.62 | −0.766 | 60 | 0.45 | ||
| Copper, total (μg Cu/L) | − | 50 | 0.33 | 2.19 | 1.453 | 94 | 0.15 | −0.07 | 1.41 | −0.378 | 60 | 0.71 |
| 90 | 0.80 | 4.73 | 1.646 | 94 | 0.10 | 0.10 | 6.10 | 0.127 | 60 | 0.90 | ||
| Dissolved oxygen (mg O2/L) | + | 50 | −0.37 | 2.27 | −1.432 | 76 | 0.16 | 0.38 | 2.41 | 1.128 | 49 | 0.26 |
| 90 | −0.55 | 3.48 | −1.375 | 76 | 0.17 | 0.16 | 3.97 | 0.287 | 49 | 0.78 | ||
| Hardness, total (mg CaCO3/L) | − | 50 | −3.05 | 49.34 | −0.599 | 93 | 0.55 | −5.15 | 66.84 | −0.591 | 58 | 0.56 |
| 90 | 3.34 | 70.62 | 0.458 | 93 | 0.65 | −3.89 | 83.03 | −0.360 | 58 | 0.72 | ||
| Lead, total (μg Pb/L) | − | 50 | 0.38 | 1.30 | 2.864 | 94 | 0.01* | −0.23 | 0.84 | −2.103 | 60 | 0.04* |
| 90 | 1.10 | 6.23 | 1.720 | 94 | 0.09 | −1.32 | 8.12 | −1.271 | 60 | 0.21 | ||
| Nickel, total (μg Ni/L) | − | 50 | −0.69 | 5.99 | −1.055 | 83 | 0.29 | 0.23 | 1.18 | 1.404 | 53 | 0.17 |
| 90 | −0.19 | 5.76 | −0.310 | 83 | 0.76 | −0.20 | 3.44 | −0.423 | 53 | 0.67 | ||
| Total ammonia (mg NH3-N/L) | − | 50 | 0.06 | 1.26 | 0.461 | 93 | 0.65 | 0.05 | 0.22 | 1.810 | 60 | 0.08 |
| 90 | 0.22 | 2.43 | 0.866 | 93 | 0.39 | 0.13 | 0.58 | 1.759 | 60 | 0.08 | ||
| Phosphorus, total (mg P/L) | − | 50 | 0.15 | 0.39 | 3.371 | 78 | 0.00* | 0.02 | 0.36 | 0.372 | 49 | 0.71 |
| 90 | 0.34 | 0.82 | 3.724 | 78 | 0.00* | −0.07 | 0.86 | −0.570 | 49 | 0.57 | ||
| pH | ± | 50 | −0.04 | 0.23 | −1.524 | 82 | 0.13 | 0.06 | 0.27 | 1.732 | 54 | 0.09 |
| 90 | −0.05 | 0.31 | −1.438 | 82 | 0.15 | 0.05 | 0.33 | 1.198 | 54 | 0.24 | ||
| Total suspended solids (mg/L) | − | 50 | 2.36 | 20.78 | 1.100 | 93 | 0.27 | −4.13 | 24.27 | −1.318 | 59 | 0.19 |
| 90 | 3.48 | 88.72 | 0.380 | 93 | 0.70 | 31.29 | −192.43 | −1.260 | 59 | 0.21 | ||
| Total toxic units | − | 50 | 1.00 | 5.54 | 1.757 | 93 | 0.08 | −0.01 | 0.33 | −0.225 | 58 | 0.82 |
| 90 | 1.14 | 7.57 | 1.465 | 93 | 0.15 | 0.23 | 3.48 | 0.506 | 58 | 0.61 | ||
| Zinc, total (μg Zn/L) | − | 50 | 2.92 | 11.74 | 2.419 | 94 | 0.02* | −3.04 | 15.63 | −1.504 | 59 | 0.14 |
| 90 | 8.27 | 69.70 | 1.157 | 94 | 0.25 | 6.18 | 51.62 | 0.928 | 59 | 0.36 | ||
- a + indicates that higher value represents higher quality; - indicates that higher value represents lower quality; ± indicates that the direction cannot be determined, in which case a two-tailed p value was calculated.
- b SD = standard deviation.
- * p < 0.05.
So why are aquatic communities in urban downstream sites so negatively affected by municipal wastewater compared to rural sites? Several generalizations may be possible. First, streams in urban areas are already stressed, and the addition of wastewater and contaminants leads to significant adverse responses. Supporting this assessment are upstream urban sites having mean toxic units > 1 and having a greater percent cumulative effluent than rural sites. While this linkage of calculated parameters to aquatic communities is not equivalent to assessing causality, it does represent a potential toxicological explanation for the adverse responses. Second, municipal wastewater is more potent coming from urban inputs compared to rural inputs. Significant increases in cadmium, copper, lead, phosphorus, and toxic units were observed downstream in urban settings, whereas these were not found to be significant in rural areas.
On the flip side of the adverse effects of urban WWTPs, adverse effects did not appear to be delivered via rural WWTPs. Certainly, municipal effluent represents complex mixtures of nutrients and contaminants, including residual concentrations of consumer product chemicals. Given the large numbers of unmeasured factors in effluent and that the mean cumulative percentage effluent at low flow for 63 sites exceeded 63%, one might have expected to observe adverse biological responses, albeit less than urban areas. However, this was not observed. Our analysis in fact may confirm Diamond and Daley's [15] observation, where the percentage effluent is less important than it is to be consistently toxic and comprises greater than 80% of the river flow. Even so, caution is needed to prevent overinterpretation. According to the Ohio EPA [13], IBI scores ranging from 34 to 42, and ICI scores ranging from 34 to 38 satisfy ecoregion-based criteria for a Warmwater Habitat classification. This classification denotes the lowest 25th-percentile value of the reference conditions based on sample type and ecoregion [18]. The mean IBI and ICI scores at the upstream sites for both urban and rural areas were 36 and 36, respectively. Hence, the mean integrity of the biological communities in this analysis can be considered on the edge of being in good ecological condition.
CONCLUSIONS
The ultimate purpose of this exercise was to identify broad relationships that may have relevance for the risk assessment of chemicals and materials that are associated with municipal effluents (e.g., consumer product ingredients). This analysis clearly showed that while the some of the biological differences between urban and rural areas could be attributed to river size/flow, they were not sufficient to account for directional changes in metrics indicative of adverse impacts. Adverse effects from wastewater in urban areas could be generically inferred. In contrast, significant adverse effects on biological communities downstream of rural WWTPs could not be determined. Significant differences between urban and rural sites for contaminants and total toxic units suggest that waste-water in urban areas is more potent than in rural areas and/or correlates with other sources, such as storm-water discharges and nonpoint sources. Even so, caution is needed so as not to overinterpret the statistical significance of the water chemistry data with the biological trends so as to establish cause-and-effect relationships.
Considering that all municipal wastewaters receive contributions from domestic sources, perhaps these data indicate that management of other sources (industrial, nonpoint) may provide an efficient cost-benefit in order to maintain the ecological integrity of the nation's receiving waters.
Acknowledgements
The authors express their thanks to the Ohio EPA (Chris Yoder and Dennis Mischne) for providing much of the data for this analysis. Major appreciation is extended to Charlotte White-Hull for assembling the data, calculating the cumulative effluent values for all of Ohio, and reviewing of this paper.




