Ecdysteroid synthesis and imaginal disc development in the midge Chironomus riparius as biomarkers for endocrine effects of tributyltin
Abstract
Acute effects of the endocrine disruptor bis (tri-n-butyltin) oxide (TBTO) on molting-hormone biosynthesis and imaginaldisc development were investigated in larvae of the midge Chironomus riparius (Meigen). Ecdysteroid synthesis was measured by 24-h incubation of molting-hormone-synthesizing tissues (prothoracic glands) in vitro with or without the addition of TBTO. The amount of ecdysteroids produced was analyzed by radioimmunoassay. Developmental effects in vivo were investigated by determining the developmental phase of the genital imaginal discs before and after a 48-h exposure to TBTO in water. Sex-specific effects were found with both endpoints. Ecdysteroid synthesis was significantly reduced (analysis of variance [ANOVA], p ≤ 0.005) in female larvae at all concentrations (TBTO-Sn at 50, 500, and 5,000 ng/L), whereas a significant elevation of the biosynthesis rate occurred in male larvae in the 500-ng/L treatment (ANOVA, p ≤ 0.05). In vivo experiments with development of the genital imaginal disc within a 48-h exposure period revealed a significantly slower development in female larvae and a significantly faster development in male larvae (contingency tables, p ≤ 0.001) at all concentrations tested (TBTO-Sn at 10, 50, 200, and 1,000 ng/L). These results partly coincided with the in vitro effects on molting-hormone synthesis. The 48-h median lethal concentration (LC50) was 25 μg/L (20–30 μg/L 95% confidence intervals). The combination of in vitro and in vivo methods has proven to be a useful approach for the detection of endocrine effects of TBTO in C. riparius at levels 2,000-fold below the LC50 value. High sensitivity and short test duration suggest that chironomids may have potential as freshwater sentinel organisms for endocrine-disrupting chemicals.
INTRODUCTION
Since the 1950s, organotin compounds have been commonly used as stabilizers for polyvinyl chloride, as industrial chemicals, and as biocides in agriculture and industry. Consequently, these substances have become widespread contaminants in aquatic systems [1]. Although the use of tributyltin in pesticides and antifouling paints has been banned or restricted in most industrial countries during the last 20 years, marine and freshwater species are still endangered by organotin exposure [1, 2]. Because of the substances' lipophilic nature and long-term stability under anaerobic conditions, sediments may serve as sources for resolubilization processes [1, 2]. Tributyltin exhibits acute toxicity at low microgram-perliter concentrations [1, 3], and has been associated with declining gastropod and bivalve populations [4, 5]. Tributyltin at environmentally relevant concentrations of only a few nanograms per liter was demonstrated to interfere with reproductive development in several gastropod species [6]. Considerable evidence indicates that tributyltin acts by interfering with enzymes of the mixed function oxidase (MFO) system, by increasing endogenous testosterone levels in females, which might lead to various degrees of androgenization (imposex or intersex phenomena) [6]. Although not yet fully resolved and not excluding each other, two main hypotheses exist for the mechanism (s) of tributyltin action. Spooner et al. [7] and Bettin et al. [8] suggested inhibition of the enzyme aromatase (CYP19A1), which converts testosterone into estrone or 17β-estradiol, whereas Roonis and Mason [9] found that testosterone elimination was blocked. These effects represent the clearest example of endocrine disruption in invertebrates yet, but more invertebrate groups seem to be affected by tributyltin contamination. Effects on testosterone metabolism have been described in bivalve molluscan [10] and crustacean species [11, 12]; however, examples of endocrine disruption are still rare in other arthropods [13-15].
One objective of the current study was to investigate whether biosynthesis of ecdysteroidal molting hormones in aquatic insects can be altered by tributyltin contamination and may therefore serve as a sensitive endpoint for evaluating endocrine-modulating effects. Although metabolic pathways of ecdysteroidogenesis are yet not fully understood, it is well known that several key enzymes of insect molting-hormone synthesis belong to the cytochrome P450 family and other components of the MFO system [16]. A well-established method to detect effects of endocrine modulators on molting-hormone biosynthesis is the incubation of insect ecdysteroid-synthesizing tissues in vitro [17]. In the present study, this technique was applied to the midge Chironomus riparius (Meigen), which is a standard test organism in aquatic ecotoxicology. The advantage of in vitro ecdysteroid biosynthesis as a biomarker is that it allows a particular test substance to be clearly identified as an endocrine disruptor at the suborganismal level. As a link to higher intergrating levels of toxicity, an additional experiment was set up to evaluate developmental effects on C. riparius in vivo after short-term exposure to low tributyltin concentrations in water. A combination of endpoints at different levels of biological organization may provide information about the ecotoxicological significance of endocrine-modulating substances [13, 15].
MATERIALS AND METHODS
Experimental animals
Midges (C. riparius) were cultured as described in Hahn et al. [18]. The water used for chironomid culture and in vivo experiments was checked regularly and chemical parameters were found to be in the following ranges: pH, 8.0 to 8.1; NH , 0.2 to 0.5 mg/L; NO
, 0.2 to 0.5 mg/L; NO , 0.0 to 0.1 mg/L; NO
, 0.0 to 0.1 mg/L; NO , < 0.1 mg/L; and total hardness (expressed as CaCO3), 15 to 20 mg/L. Dissolved oxygen content was always higher than 90%. For the determinations, test kits from Macherey and Nagel (Düren, Germany) and pH and oxygen electrodes from Wissenschaftlich Technische Werkstätten (Weilheim, Germany) were used.
, < 0.1 mg/L; and total hardness (expressed as CaCO3), 15 to 20 mg/L. Dissolved oxygen content was always higher than 90%. For the determinations, test kits from Macherey and Nagel (Düren, Germany) and pH and oxygen electrodes from Wissenschaftlich Technische Werkstätten (Weilheim, Germany) were used.
Reagents
If not otherwise stated, all buffers, salts, and solvents were purchased from Merck (Darmstadt, Germany) in analytical quality (solvents in high-performance liquid chromatography quality), and all solutions were prepared in water taken from a MilliQ water purification system (Millipore®, Bedford, MA, USA). Amino acids and cholesterol for Cannon's modified insect medium were obtained from Sigma (Deisenhofen, Germany). The test substance used in our study, bis (tri-n-butyltin) oxide (TBTO), was purchased from Riedel deHaen (Seelze, Germany) in the highest purity available (standard for gas chromatography, Pestanal®, deHaen). A stock solution was prepared in pure ethanol and diluted to the respective concentrations. The initial amount of solvent in all experiments including controls was 0.005% and all TBTO exposure concentrations given in this paper refer to quantities of tin.
Ecdysteroid biosynthesis in vitro
For measurement of in vitro molting-hormone biosynthesis, larvae were sexed and staged [19] and only fourth-stage larvae in the ninth (last) developmental phase of either sex were chosen. Animals were anesthetized by submersion in ice-cold water until they stopped moving. Then they were briefly washed in 70% ethanol, blotted dry, and quickly dissected under a binocular microscope in ice-cold Beadle's Ringer's solution [20]. The head capsules and the abdominal segments were severed and the midgut and salivary glands were removed from the remaining thoracic segments. The segments were then opened dorsally by a longitudinal cut and the body cavity was carefully flushed with Ringer's solution. The first thoracic segment contained the intact complex of cerebral and subesophageal ganglion, corpora allata, corpora cardiaca, and prothoracic glands. Dissected tissues were collected in 1 ml of sterile-filtered Cannon's modified insect medium, which was prepared as described in Ringborg and Rydlander [21]. However, we used a commercial penicillin-streptomycin combination (1:100, v/v, Sigma) instead of penicillin alone, and omitted the addition of phenol red. After 30 min of acclimation at room temperature, thoracic tissues were placed individually in autoclaved 8 × 40-mm glass vials (Merck Eurolab) filled with 80 μl of Cannon's medium in which the respective concentrations of TBTO were dissolved. The tissues were incubated for 24 h at 30°C. Test concentrations of TBTO were 50 ng/L, 500 ng/L, and 5,000 ng/L. Incubation was stopped by the addition of 300 μl of cold methanol. Vials were briefly vortexed, tissues were removed, and samples were stored at −20°C until further analysis.
For ecdysteroid extraction, sample vials were brought to room temperature, thoroughly vortexed, and sonicated in an ultrasonic bath (Branson Ultrasonics, Danbury, CT, USA) for 2 min. Then they were centrifuged at 1,000 g for 15 min and the supernatants were collected. The remaining pellets were extracted once as described above. The combined supernatants were evaporated to dryness at 55°C under a stream of nitrogen. Each sample was then redissolved in 300 μl of radioimmunoassay buffer (100 mM boric acid-sodium tetraborate, 75 mM NaCl, pH 8.4) and analyzed for ecdysteroids by radioimmunoassay [22]. Tritiated ecdysone (NEN, Zaventam, Belgium), diluted to an activity of 0.1 μCi/ml in radioimmunoassay buffer, was used as the radioactive tracer (specific activity 53 Ci/mmol). The ecdysone-specific antiserum DBL-1 (Trifolio, Lahnau, Germany) was used in a 1:1,000 dilution in radioimmunoassay buffer to which 5% (v/v) normal sheep serum (ICN Biomedicals, Esch-wege, Germany) was added. A standard curve ranging from 13.25 to 5,300 pg was generated with pure ecdysone (Sigma).
To 100 μl of unknown sample or standard, 100 μl of tracer and 100 μl of antibody dilution were added and the samples were incubated overnight at 4°C. Incubation was stopped by the addition of 300 μl of ice-cold saturated ammonium sulfate solution. Samples were placed on ice for 30 min and then centrifuged for 12 min at 20,000 g at 4°C. The supernatants were removed and 25 μl of water and 1.5 ml of Wallac HiSafe2 scintillation fluid (Wallac, Freiburg, Germany) were added to the pelleted antigen-antibody complexes. The antibody-bound radioactivity was determined as counts per minute in a Wallac 1409 scintillation counter. Nonspecific binding, which was always below 10% of the maximal radioactivity bound, was subtracted from all samples and standards. Quantifiable results were in the range from 20 to 70% of maximal bound radioactivity [22], which corresponded to 100 to 1,000 pg of ecdysone. Because of the cross-reactivity of the antiserum with 20-hydroxyecdysone and other free ecdysteroids [22], all results were expressed as picograms ecdysone-equivalents synthesized per hour. For the above procedure, a recovery rate of 99.8 ± 9.4% coefficient of variation (n = 10) was determined by extracting known amounts of ecdysone dissolved in Cannon's medium. All standards and samples were assayed in duplicate. Duplicates that differed by more than 10% from their means were rejected as a criterion of assay validity. Between 8 and 14 samples were analyzed per sex and treatment.
Bis (tri-n-butyltin) oxide effects in vivo
A method for exact determination of sex and developmental stage of fourth-stage chironomid larvae by examining the imaginal discs was described by Wülker and Götz [19]. They characterized nine morphologically distinct phases of equal duration. For the present study, single specimens were collected from the culture vessels and placed dorsal surface down on a microscope slide for sexing and staging. Another slide was carefully placed on top to keep the larva in place. At 50-fold magnification, the genital imaginal discs in the abdomen were easily observable. No symptoms were found that larvae were injured by this procedure. For the experiments, only larvae in phases 5 or 6 were chosen. Each single larva was staged and then placed individually into a 20-ml glass vial filled with 4 ml of control or test medium. Animals were kept for 48 h under culture conditions (20°C, 16:8 h light:dark), but without aeration, sediment, or food. Test concentrations of TBTO were 10 ng/L, 50 ng/L, 200 ng/L, and 1,000 ng/L. Sample sizes per sex and treatment were between 39 and 107 larvae. At the end of the exposure period, each larva was staged again and its individual 48-h development was described by subtracting the value of the larval phase at the start experiment (5 or 6) from its value after 48 h of exposure. None of the larvae pupated during the exposure period.
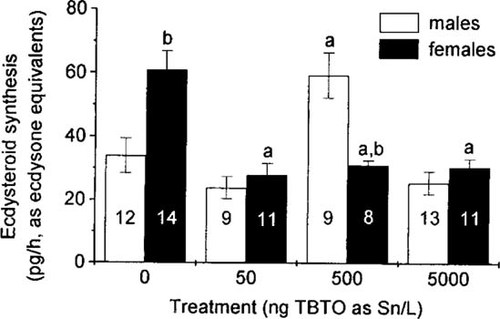
Effects of bis (tri-n-butyltin) oxide (TBTO) on the rates of in vitro ecdysteroid biosynthesis in male and female Chironomus riparius larvae. Numbers in columns show the sample size n, error bars indicate ± standard error, a = significantly different from the respective control; b = significantly different from the respective male group (Scheffé's test, p ≤ 0.05).
Forty-eight—hour lethal concentration
Similar exposure conditions were applied to determine the 48-h median lethal concentration (LC50). In addition to the controls, the concentrations of TBTO that were used were 10 μg/L, 20 μg/L, 30 μg/L, and 100 μg/L. For each treatment. 9 to 11 larvae of either sex were used. Again, all larvae were in phases 5 or 6.
Data analysis
Data from the in vitro incubations were tested for normal distribution and equality of variances. If necessary, data were transformed with a suitable transformation exponent estimated by explorative data analysis. Results were then subjected to a one-way analysis of variance (ANOVA) procedure. When significant differences were found, an appropriate post hoc procedure suitable for unequal sample sizes was applied, as suggested by Sokal and Rohlf [23] (Scheffé's test). The effects of TBTO on the development of chironomid larvae were analyzed with contingency tables. Independency within the experimental groups for the factors treatment and sex were tested by computing the likelihood quotient with the G test procedure [23]. Significant differences meant that independency was not given and results were influenced by either treatment or sex. Statistical analyses were performed with SPSS® 10.0 (SPSS, Chicago, IL, USA). The LC50 and its 95% confidence interval (CI) from the 48-h acute toxicity experiments were computed as described by Gelber et al. [24].
RESULTS
Ecdysteroid biosynthesis in vitro
In males (Fig. 1), the ecdysteroidogenic activity showed a statistically significant increase compared to the control level in the 500-ng/L treatment (p = 0.026). No significant differences from the control group were observed in the other two test concentrations. A different effect of TBTO was revealed in females (Fig. 1). In all three groups exposed, ecdysteroid production was signifcantly lowered by about 50% compared to the control group (p values between 0.005 and <0.001). Additionally, mean ecdysteroid-synthetic rates differed between male and female control groups.
| Nominal concentration (μg TBTO as Sn/L) | |||||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 100 | |
| Males | 100 | 80 | 100 | 0 | 0 |
| Females | 100 | 90 | 100 | 0 | 0 |
Forty-eight—hour lethal concentration
Survival rates for 48-h acute toxicity of TBTO are given in Table 1. From these data, an LC50 value of 25 μg/L (20— 30 μg/L 95% CI) was computed. No difference in susceptibility between male and female larvae was found.
Forty-eight—hour developmental effects
Analyses of fourth larval instar development within a 48-h exposure revealed a sex-specific influence of TBTO. The proportions of larvae in distinct developmental phases at the end of the exposure period exhibited significantly different patterns depending on TBTO contamination in both males and females (p = 0.001 andp < 0.001, respectively). As illustrated in Figure 2, exposed male larvae (Fig. 2A) developed faster, compared to the control group (i.e., more larvae reached a higher developmental phase within 48 h). In contrast, TBTO-treated females (Fig. 2B) clearly showed slower development relative to the controls.
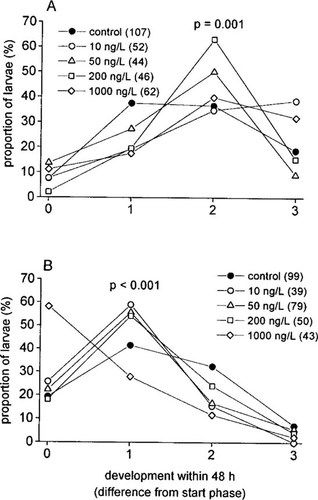
Development of fourth larval instar Chironomus riparius within a 48-h period of exposure to different concentrations of bis (tri-n-butyltin) oxide (TBTO) (A, males; B, females). Legend numbers in parentheses show the sample size n; p values indicate the statistical differences of TBTO influence on the proportions of developmental phases (contingency table tests).
Also, developmental patterns of male and female larvae at the same exposure concentration were found to be different at each concentration tested. The male-female differences in all TBTO-treated groups were highly significant (p ≤ 0.001). In all exposed groups, about 40 to 60% of the males developed at least two phases, whereas in females, 25 to 60% developed only one phase or did not show any change in developmental phase at all. However, a significant difference between sexes was also observed in the controls (p = 0.011), where most larvae developed one or two phases (about 30–40% each). Mortality in the experiment was low (2.5%) and was not correlated with TBTO concentration or sex.
DISCUSSION
Ecdysteroid biosynthesis in vitro
Insect endocrinology is a well-understood field compared to the knowledge of hormonal processes in other invertebrate groups [25]. However, relatively little work has yet been directed to possible impacts of endocrine-disrupting chemicals on aquatic insects [3, 18, 26]. Measurements of the in vitro biosynthesis of ecdysteroids previously have been employed in investigations on the regulation of ecdysteroidogenesis in blowfly larvae [17] and, therefore, may also serve as a biomarker for endocrine disruption in chironomids. Because Laufer et al. [27] showed that titers of free ecdysteroids in chironomid larval hemolymph strictly depended on age and sex, it was crucial for the current study that experimental animals in all groups were of the same sex and developmental phase. This was ensured by sexing and staging the animals with the method of Wülker and Götz [19]. The in vitro experiments clearly evidenced a TBTO-induced alteration of ecdysteroid production in C. riparius. An impact on the metabolism of arthropodan-specific molting hormones, as demonstrated here, might have important implications for other aquatic insect and crustacean species.
The reactions of male and female larvae upon TBTO exposure were different. Females exhibited reduced ecdysteroid synthesis rates, to approximately 50% of the control at all exposure concentrations, whereas males responded only in the 500-ng/L treatment. Here the biosynthetic activity almost doubled. We do not believe that this is only a spurious result due to poor experimental technique. The experiments were performed over several weeks and the larvae used for tissue preparation originated from several different generations. Test concentrations and controls were applied in random order to the tissue cultures and radioimmunoassay-based analysis was validated by a number of standard samples. Therefore, it is more likely that ecdysteroid-metabolizing enzymes or signaling pathways either were affected differently in males and females, or that the same impact exhibited different reactions in the two sexes.
Comparing male and female control groups, the mean ecdysteroid synthesis rates from male tissues was found to be approximately one half of that of females (33.9 and 60.9 pg/h, respectively). At all TBTO concentrations, this ratio of ecdysteroid production was either reduced or completely reversed (500 ng/L) relative to the control. These results suggest that ecdysteroid metabolism is regulated by different mechanisms in male and female late fourth-stage chironomid larvae. This is supported by the data of Laufer et al. [27], who detected much higher ecdysteroid titers in untreated female pupae than in male pupae, which they attributed to gender-specific differences in the levels and function of sex-hormone-like ecdysteroids in adult insects. Differences in ecdysteroid titer and metabolism have been reported for numerous insect species and are discussed in context with sex-hormone-like functions of ecdysteroids, including vitellogenesis, gonad maturation, and pheromone production [28].
In the in vitro experiments, no dose-response curve was found over the concentration scale investigated. A somehow similar effect was reported by Oberdörster et al. [29] in a study on testosterone metabolism in a marine prosobranch snail. The authors found a tributyltin-chloride-induced increase in reductase activities only at low exposure concentrations, whereas no differences from control activities were found at higher concentrations. Recently, Dickerson et al. [30] discussed the hypothesis that dose-response curves in the endocrine system might sometimes differ from traditional toxicology paradigms, because of multiple target sites and various mechanisms of action.
Possible mechanisms that could result in the reported effects in C. riparius are direct TBTO interactions with cytochrome P450 enzymes involved in ecdysteroid synthesis or elimination, or indirect effects on these enzymes by affecting their electron transport systems, the NADPH-dependent cytochrome P450 reductases, cytochrome b5, or a combination of these [16]. Both mechanisms have been discussed in the context of organotin contamination [10]. Furthermore, insect ecdysteroid synthesis has been shown to be second-messenger regulated and dependent on Ca2+ [31]. Cima and Ballarin [32] reported conformational change of the intracellular calcium regulator protein calmodulin upon tributyltin contamination. Thus, TBTO influence on ecdysteroidogenesis by interfering with calcium regulation cannot be excluded. Finally, Oberdörster and Cheek [13] reported the induction of imposex in a prosobranch snail by a neuropeptide. This suggests involvement also of the neuronal system in tributyltin-induced imposex formation in gastropods.
In our experiments, complete thoracic tissues with intact neural complexes of cerebral ganglia, corpora allata, corpora cardiaca, and prothoracic glands were exposed to the test substance. Because it cannot be definitely decided whether one or more of the discussed TBTO effects led to the results reported here, further work is required for a better elucidation. However, the presence of higher nervous and endocrine centers in the exposed tissues allowed the assessment of effects on various levels within the hierarchy of the endocrine system by using in vitro ecdysteroidogenesis as a single endpoint.
Forty-eight—hour lethal concentration
The LC50 for acute mortality within 48 h of TBTO to midfourth-stage larvae was found to be 25 μg/L (20–30 μg/L 95% CI). This was at least 2,000-fold higher than the concentrations in the 48-h development experiments, at which developmental effects were observed. Fargasova [3] reported a 96-h LC50 value for mortality of only 50 ng/L in a comparable experiment on larvae of another chironomid species, C. plumosus (no sediment or food, mid-fourth instars). Because she did not explicitly refer to quantities of tin, TBTO at 50 ng/L expressed as TBTO-tin approximately equals 25 ng/L, which is exactly 1,000-fold below the results obtained in the present study. Whether differences in species' sensitivity or, more probable, prolonged incubation time caused this huge difference cannot be decided.
Forty-eight—hour developmental effects
Influences of sublethal pesticide concentrations on larval development have been shown for a variety of aquatic insects, usually causing a delay in emergence [33, 34]. Although many of these substances are suspected of being endocrine disruptors [35], no clear evidence for a hormonal mode of action has been established. In the current study, a sex-specific pattern of TBTO influence on larval development was revealed, in which exposed male larvae tended to develop faster than the controls, whereas a delay was observed in females. Protandry (i.e., males start emerging slightly before females) and a sexual difference in larval growth rate are typical of members of the family Chironomidae [19, 36]. This phenomenon probably enhances mating success in species with short-lived adults when abundant males await female emergence [36]. In the developmental experiments, TBTO exposure increased the asynchrony in larval development. Whether this is of ecological relevance for chironomids or other insect species cannot be judged from the current results. Except for molluscs [6], no clear evidence exists to date for effects of tributyltin or other endocrine-disrupting chemicals in invertebrates at the population or community level [15]. However, sex-specific impacts of TBTO on molting-hormone production and larval development in aquatic insect larvae let it seem possible that aquatic systems could be affected.
The current results from in vitro and in vivo experiments hint at a connection between molting-hormone production and larval development. In females, decreased ecdysteroid synthesis coincided with slower development, whereas the opposite pattern occurred in males, at least in part. Larvae showed faster development upon exposure, which was consistent with the elevation of in vitro ecdysteroid synthesis in the 500-ng/L treatment, but not with the results from the groups exposed to 50 and 5,000 ng/L. Whether these effects are connected in a way such that alterations in molting-hormone titers directly influenced larval development is difficult to decide. Results from in vitro and in vivo experiments incorporate different levels of biological organization and previous results, obtained with the ecdysteroid agonist tebufenozide in long-term chironomid standard test procedures [18], gave no hint of a molting-hormone influence on development time. Nevertheless, the current study presents proof for sex-specific effects of TBTO at nanogram-per-liter concentrations in an aquatic insect. Employing imaginal-disc development and ecdysteroid synthesis as test endpoints has provided evidence of endocrine effects on basic developmental processes in vitro and in vivo. Generally, the use of invertebrate species that occupy key positions in food webs (e.g., chironomids [36]) as sentinel organisms in endocrine-disruptor research may provide information on hazards of chemicals in a wide variety of ecosystems [37].
Acknowledgements
This work was supported by a scholarship from the German Federal Environmental Foundation. We are also indebted to the Gregor-Louisoder Environmental Foundation and the Gillet Foundation for their generous support. We thank Kamilla Schenk and Axel Conrad for excellent technical assistance, and Martin Streloke and two anonymous reviewers for their valuable comments on earlier versions of the manuscript.




