Lysosomal response of earthworm (Eisenia fetida) coelomocytes to the fungicide copper oxychloride and relation to life-cycle parameters
Abstract
Juvenile specimens of the earthworm species Eisenia fetida Savigny, 1826, were exposed in the laboratory to a range of concentrations of the fungicide copper oxychloride. After an eight-week exposure period, the neutral-red retention (NRR) times were measured in the coelomocytes of all the worms that had matured. Life-cycle traits (survival, biomass change, cocoon production, cocoon mass, hatching of cocoons, number of hatchlings) were also monitored. Dose-related effects on NRR times, maturation, growth, and reproduction parameters were determined (one-way analysis of variance, p < 0.05). The lowest effect concentration for substrate copper (Cu) measured for the NRR time was 8.9 mg/kg (dry wt). Growth was severely affected at the highest exposure concentration (330 mg/kg substrate dry wt), at which no worms matured and no NRR times could be measured. Negative linear correlations (p < 0.05) were apparent between the NRR times and substrate and body Cu concentrations. Positive linear relationships were found between the NRR times and some life-cycle parameters (p < 0.05). This study showed that the NRR assay can indicate toxic stress due to Cu exposure at an early stage, and that NRR times can be linked to effects on certain life-cycle traits.
INTRODUCTION
The toxicity of copper (Cu) for earthworms was first recognized by Nielson in 1951 [1]. The general toxicity of Cu for these organisms is considered to be low by some authors [2, 3], but others have shown that Cu causes mortality and sublethal injury to these organisms at environmental concentrations much lower than those of lead and zinc [4]. In 1967, Van Rhee [5] showed that the mean density of earthworms was much lower in orchards treated with copper oxychloride than in untreated orchards. Recent studies by Helling et al. [6] and Maboeta [7] have also shown that Cu has a considerable impact on earthworm reproduction and growth rate. Studies on the effects of Cu indicated that it decreased earthworm growth at exposure concentrations of 100 mg/kg and higher and reproduction at concentrations of 53 to 150 mg/kg soil [8-11].
Copper compounds have been applied in the past as effective fungicides to a range of crops at high dose rates [1]. Some of the compounds used seem to be of low toxicity, but because of repetitive application, Cu accumulates and, with time, reaches levels high enough to become toxic to biological systems. Copper oxychloride is widely used as fungicide in South African vineyards and is applied at a rate of 1.25 to 7.5 kg/ha, with several applications per season [6]. Copper concentrations of as much as 50 μg/g have been found in soil immediately after the spraying season [7]. Although these concentrations are well below the median lethal concentration value for Eisenia andrei, which varies between 100 and 1,000 mg/kg substrate Cu (dry wt) [10, 12], they may have adverse effects on the earthworms, as was shown by Helling et al. [6]. This will especially be the case if available Cu accumulates after consecutive seasons of spraying with copper oxychloride. If the beneficial organisms in these soils are to be protected from excessive Cu, it is of the utmost importance to detect toxic stress caused by this metal at the earliest possible time to adjust spraying regimes accordingly.
When an organism is exposed to toxic stress, the cellular level is one of the lowest levels of biological organization to be affected [13]. At the subcellular level, the lysosomal system has been identified as a particular target for the toxic effects of xenobiotics [14, 15]. According to Lu [16], metal complexes enter the cell by passive diffusion and accumulate in the lysosomes, where they degrade and release the metal ions, which can inhibit the proteolytic enzymes in the lysosomes and cause cell injury. Early stages of response to environmental pollutants, therefore, can be revealed by investigating alterations at this subcellular level before integrated cellular damage shifts to the level of organ or whole-animal physiological processes.
Allinson and Young [17] proposed that intravital dyes such as neutral-red be used to stain lysosomes during investigations of cellular condition. The neutral-red retention (NRR) assay used during the present study has been developed as a biomarker of metal toxicity by Lowe et al. [18] and by Lowe and Pipe [19]. It was initially used as a biomarker of pollution stress for marine organisms but, subsequently, was shown to provide a rapid and sensitive indication of cellular responses in earthworms [10, 20-22]. The assay is based on the measurement of change in lysosomal membrane stability. The membrane stability decreases in response to stress as the membrane permeability increases, although the mechanism causing this alteration in membrane integrity is not yet well understood [23].
The weak cationic dye neutral-red penetrates the cell membranes by nonionic diffusion and accumulates intracellularly in the lysosomes [24, 25]. When the lysosomal membrane integrity is affected by the presence of a toxic substance, the accumulated dye in the lysosomal vacuole diffuses into the cytosol, staining it light red. The NRR time is calculated by determining the time needed for the dye to leak into the cytosol in 50% of the cells observed.
The development of the NRR assay for earthworms by Weeks and Svendsen [20] involved the exposure of adult (clitellate) worms for a period of time before the measurements were made. The same procedure of exposing adult worms and measuring the effects after several weeks was subsequently followed by Svendsen and Weeks [10, 21] and by Reinecke and Reinecke [22] using coelomocytes from the coelomic fluid of earthworms. However, based on the findings of Van Gestel et al. [26] and Spurgeon and Hopkin [27] that juvenile worms are more sensitive than adult worms to pollutants, it was decided to start the exposure period during the present experiment with newly hatched worms. These worms were exposed until they reached maturity (clitellate), and thereafter for another four weeks, before the NRR counts were made. This approach makes it possible to relate NRR times to other parameters such as growth and maturation. Previous studies have indicated that this technique is responsive to heavy metals in the laboratory [22, 28], and especially to Cu [10, 20, 21]. However, it is important to establish clear links between the NRR response and ecologically important life-cycle endpoints such as growth, maturation, and fecundity if this biomarker is to be utilized in field work as a practical tool for prediction of higher-level effects.
The aim of this study was to evaluate possible use of the NRR assay as a biomarker of stress in E. fetida resulting from exposure to Cu as an ingredient of the fungicide copper oxychloride. Furthermore, this study also aimed to determine whether any relationships between the NRR times and life-cycle parameters existed and, therefore, whether the NRR assay as biomarker could be related to ecologically relevant endpoints.
MATERIALS AND METHODS
Experimental set-up
Eisenia fetida Savigny, 1826, was chosen for this study because it is an accepted model test species for toxicity testing [29]. Its growth and reproduction characteristics are also well documented [27, 30-32].
Cocoons from an uncontaminated culture of E. fetida, maintained in our laboratory, were incubated and the hatchlings used for this experiment. Hatchlings of the same age were used for all replicates and exposure concentrations. Plastic containers (dimensions, 12 × 15 × 20 cm) with tight-fitting, perforated lids were used for all exposures. The substrate used consisted of urine-free cattle manure that was previously dried, ground, and sieved to a particle size of between 100 and 500 μm. The substrate was prepared by thoroughly mixing 75 g of dried, sieved cattle manure with distilled water, containing the specific concentration of the fungicide, to obtain a moisture content of 78%. The fungicide Viricop® (Sentrachem-Agrihold, 67/00258/06, Formchem, Kempton Park, South Africa; active ingredient, 850 g/kg of copper oxychloride containing 500 g/kg of Cu metal) was used to obtain the following exposure concentrations: 3.3, 10, 33, 100, and 330 mg/kg substrate Cu (based on the amount of Cu metal in the fungicide). The exposure concentrations, therefore, represent the amount of Cu that was mixed in, calculated from the nominal amounts present in the fungicide. Four replicates were prepared for each exposure concentration, as were four control replicates. These were left for 2 d to stabilize before adding the worms at the start of the experiment. Ten hatchlings were placed into each container. The mean biomass per hatchling of all the hatchlings used (n = 200) at the beginning of the experiment was 32.98 ± 20.9 mg (mean ± standard deviation). The individual weights ranged from 3 to 142 mg, but they normally reach a very similar weight within approximately 20 d [6]. The containers with worms were kept for eight weeks in a climate-controlled room at 25°C. The pH and moisture content in the substrates were measured weekly. The pH varied between 6.8 and 7.2, and the moisture content varied between 75% and 80%. From the fifth week onward, 30 g of cattle manure substrate, treated with the corresponding Cu concentrations at the start of the experiment, were added as food and mixed into the substrate. All concentrations are expressed in dry weight.
Life-cycle parameters
During the exposure period, the worms were handsorted from the substrate every week to determine survival, which was found to be 100% for all exposure concentrations for the duration of the experiment. Each week, the worms were also weighed and observed for clitellum development. After signs of maturation were observed, cocoons were removed by hand-sorting and weighed as reported by Helling et al. [6]. The cocoons were placed individually in separate chambers on moist filter paper in multiwell plates to incubate at 25°C. The cocoons were observed daily, and the emergence of hatchlings was noted.
Neutral-red retention test
After the exposure period of eight weeks, the clitellate worms were removed from the substrate, washed with distilled water, and blotted dry on filter paper. Coelomocytes were collected from individual worms as described by Reinecke and Reinecke [22], and the test to determine the NRR times was executed as described by Weeks and Svendsen [20] and by Reinecke and Reinecke [22]. The NRR times of between 20 and 30 worms were determined for each exposure concentration.
Metal analysis
At the end of the experiment, a representative sample of four to six worms was removed from each of the four containers per exposure, and these worms were allowed to depurate their gut contents on moist filter paper. The worms were then frozen for analysis of metal concentration. Worms and substrate samples were acid digested and analyzed with a Varian atomic absorption (AA)-1275 flame atomic spectrophotometer (Varian, Walnut Creek, CA, USA). These methods were previously described by Reinecke and Reinecke [22] and by Helling et al. [6].
Statistical analysis
The data were analyzed for statistical differences using a one-way analysis of variance, followed by an all pairwise multiple comparison procedure (Student-Newman-Keuls method). Linear regression and Spearman correlation analyses were performed using a Jandel Scientific Sigmastat software package (Jandel Scientific, San Rafael, CA, USA).
RESULTS
Substrate Cu concentrations
The final Cu concentrations, as determined by the AA analysis, were higher than the adjusted administered exposure concentrations. Instead of the amounts of 3.3, 10, 33, 100, and 330 mg/kg Cu that were added, the following concentrations were measured, respectively, at the end of the experimental period: 8.92, 15.92, 39.47, 108.72, and 346.85 mg/kg Cu (dry wt). These concentrations represented the (unmeasured) initial background concentrations plus the additions of manure throughout the study. In the control substrate, where no copper oxychloride was added, a background level of 4.02 mg/kg Cu (dry wt) was measured after the eight-week period. In the present paper, the administered or absolute dose concentrations are referred to as the exposure concentrations. The measured Cu concentrations are also provided with the graphs (see Figs. Fig. 1., Fig. 3.).
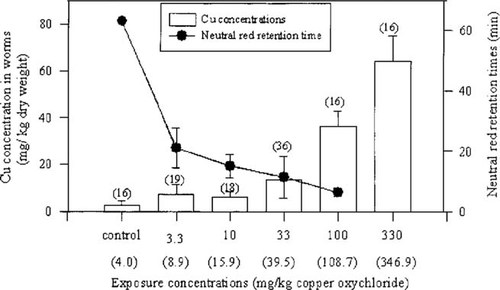
Bar chart: Mean ± standard deviation (SD; error bars) body (tissue) Cu concentrations of Eisenia fetida (mg/kg dry wt) measured after eight weeks of exposure to different sublethal concentrations of copper oxychloride in a pH range of 6.8 to 7.2. Line graph: Mean ± SD (error bars) neutral-red retention times (min) measured for earthworms after exposure to copper oxychloride for eight weeks. Numbers (n) given in brackets above bar graphs. Numbers in brackets on the x-axis: Measured Cu content in substrate after eight weeks.
Neutral-red retention times and Cu concentrations
The bar chart in Figure 1 represents the mean Cu concentrations in all the worms that were used for the NRR time measurements. The two lowest ambient exposure concentrations (3.3 and 10 mg/kg) resulted in corresponding low mean body concentrations of Cu (14.6 and 12.4 mg/kg dry wt, respectively) accumulating in the tissues of the worms and did not differ significantly (p < 0.05). The body Cu concentrations of these two groups of worms were, however, significantly different (p < 0.05) from all other exposure groups as well as from the control. The Cu concentrations of all other groups of worms also differed significantly (p < 0.05) from each other.
The line graph in Figure 1 represents the mean NRR times for E. fetida, calculated for all the worms measured and plotted against the Cu exposure concentrations. A substantial difference was found between the mean NRR time of the worms in the control substrate and that of the first exposure group (exposure concentration, 3.3 mg/kg). A statistically significant decrease (p < 0.05) in NRR times with exposure to increasing substrate Cu concentrations was recorded for the other exposure concentrations. The NRR time for control worms was in excess of 60 min, which decreased to 6.25 + 1.0 min at the 100 mg/kg exposure. At the highest Cu exposure concentration (330 mg/kg), no worms reached maturity, and no reproduction took place. No clitellate worms, therefore, were available for determination of NRR times, and no reproduction data were available.
The scatter plot (Fig. 2) represents the NRR times for each individual earthworm plotted against its corresponding body Cu concentration. A statistically significant relationship (p < 0.05) between body Cu concentration and decreased NRR time was apparent, and the NRR times decreased with increasing body concentrations of Cu. Three groups can be distinguished in the figure. The values for the control worms cluster in the upper left-hand corner, the values for the worms exposed to the lower ambient Cu concentrations (3.3, 10, and 33 mg/kg) in the lower left, and those for the worms exposed to 100 mg/kg in the lower right of the graph.
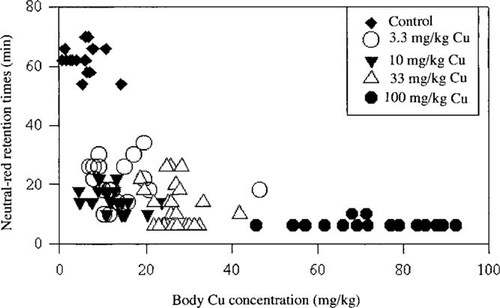
Neutral-red retention times (min) plotted against corresponding earthworm body Cu (tissue) concentrations (mg/kg dry wt) for Eisenia fetida exposed to increasing substrate Cu concentrations for eight weeks (95 individuals plotted). The different symbols represent the different exposure concentrations.
It is clear from both Figures Fig. 1., Fig. 2. that negative linear relationships existed between NRR times and both substrate and body Cu concentrations in worms that were exposed to higher-than-normal (control) exposure concentrations. Statistical analysis showed these relationships to be strongly negative (R2 = 0.811 and 0.807, respectively; p < 0.05).
Neutral-red retention times and life-cycle traits
Survival was 100% for all exposure concentrations for the duration of the experiment. The effects on cocoon production are presented as bar graphs in Figure 3 for the total number produced by all 40 worms in each exposure group over the duration of the experimental period. At the highest exposure concentration (300 mg/kg), no worms matured, and no cocoons were produced. The decrease in total numbers of cocoons between the control and the 3.3 mg/kg exposure, the 3.3 mg/kg and 10 mg/kg exposures, and the control and 10 mg/kg exposure were statistically significant (p < 0.05). No statistically significant differences occurred, however, between the numbers of cocoons obtained among the 10, 33, and 100 mg/kg exposure concentrations.
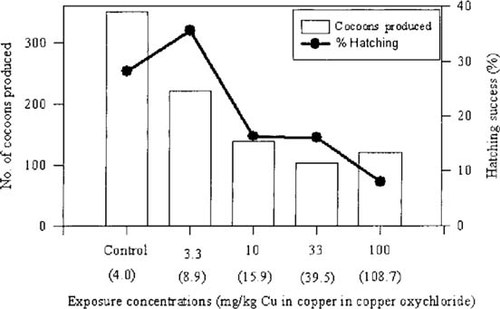
Data on cocoon production and hatching success of Eisenia fetida after exposure to a series of concentrations of copper oxychloride. Bar chart: Total number of cocoons produced by 40 worms per exposure group; line graph represents the hatching success of these cocoons. Numbers in brackets on x-axis: Measured Cu content in substrate after eight weeks.
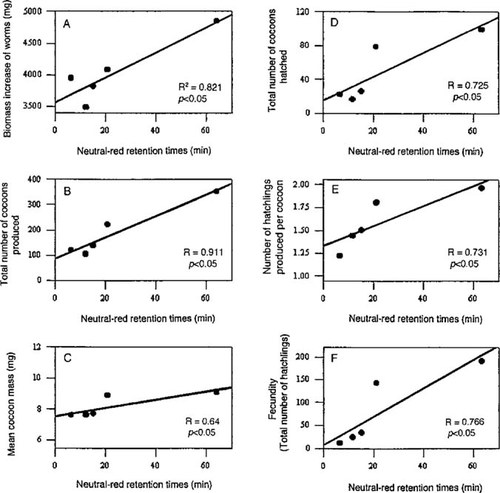
Relationships between mean neutral-red retention times (min) of Eisenia fetida coelomocytes and means of different life-cycle parameters for four exposure concentrations of copper oxychloride (3.3. 10, 33, and 100 mg/kg) and control. A. Biomass increase. B. Total number of cocoons produced by all exposed worms. C. Mean mass of cocoons produced by the worms in each exposure concentration. D. Total number of cocoons produced that hatched. E. Number of hatchlings produced per cocoon. F. Fecundity (total number of hatch-lings produced). Number of individual measurements (n) for each datapoint = between 17 and 29.
The percentage hatching success is indicated as a line graph in Figure 3. Some fluctuation was found, but overall, a downward trend from the control to the highest exposure concentration occurred. The hatching rate was higher for the lowest exposure concentration than for the control and was lowest at the 100 mg/kg concentration. In all exposure concentrations higher than 3.3 mg/kg, the hatching success was significantly (p < 0.05) lower than that of the control.
Linear relationships between NRR times and life-cycle parameters are presented in Figure 4. Statistically significant positive relationships, using the mean values and including the control group, were apparent in all cases. The mean increase in biomass of the worms in the different treatments over the eight-week exposure period ranged from 4,079 mg at the lowest exposure concentration (control) to 210 mg at the highest exposure concentration (300 mg/kg). In Figure 4A, the linear relationship for biomass increase is shown. In Figures 4B to F, the correlations between the NRR times and different reproduction parameters (total number of cocoons produced by all worms in each group, mean cocoon mass, total number of cocoons that hatched, number of hatchlings produced per cocoon, and total number of hatchlings) are given. All correlated positively with the biomarker response.
DISCUSSION
The results of the present study support the findings of previous researchers that the NRR assay, as used for earthworm coelomocytes, is a reliable, dose-related assessment of the effects of the heavy metal Cu on these organisms [10, 20, 21]. The assay was very sensitive for the detection of responses by the worms to elevated Cu concentrations. This was evident from a lowest-observed-effect concentration of less than 8.9 mg/kg Cu (dry wt) measured in the exposure substrate during the present study. This is a much lower value than the lowest-observed-effect concentration of 50 mg/kg Cu (dry wt) found by Scott-Fordsmand et al. [33] in a laboratory study with E. fetida in Cu-spiked sandy clay soil.
The clear threshold for the NRR lysosomal reaction between exposure concentrations of 40 and 80 mg/kg Cu found by Weeks and Svendsen [20] and by Svendsen and Weeks [10] was not distinct during the present study (Fig. 1). A threshold was evident between the control (effective Cu concentration, 4 mg/kg) and 3.3 mg/kg exposure (effective Cu concentration, 8.9 mg/kg) (Figs. Fig. 1., Fig. 2.). These values are much lower than those obtained by Svendsen and Weeks [10], indicating a more sensitive response. This could be attributed to the fact that Cu was administered in the form of copper oxychloride in a cattle manure substrate during our study, whereas Svendsen and Weeks [10] have used copper chloride in a sandy forest soil. Speciation of metals is influenced by soil characteristics such as pH and organic matter content [34], and these factors differed between our study and those mentioned above. This prohibits a direct comparison, because the bioavailability of Cu is expected to differ.
Weeks and Svendsen [20] and Svendsen and Weeks [10] were also able to measure NRR times at concentrations higher than 100 mg/kg Cu, because the worms they used reached maturity at these exposures. No comparison could be made with the results of the present study at such high concentrations, however, because no worms reached adulthood at concentrations higher than 100 mg/kg Cu in our study. The differences in bioavailability of Cu due to different pH and organic matter values, as well as the different feeding regimes, could have influenced growth and maturation differently. The food added during the present study was spiked with Cu at the same concentration as that of each specific exposure concentration, whereas the food added during the above-mentioned studies [10, 20] was uncontaminated. The worms in these previous studies [10, 20], therefore, could feed on this uncontaminated substrate, whereas the worms in our study could not avoid the Cu. At the highest exposure concentration in our study (330 mg/kg), the worms probably fed at a much lower rate, because earthworms are sensitive to Cu and tend to avoid it [5, 7], causing growth and maturation to be affected detrimentally.
Harreus et al. [35], who used an extrusion fluid to obtain coelomocytes for the NRR assay from adult specimens of the earthworm species Aporrectodea rosea, could not collect enough cells to do the counts at Cu concentrations of 160 and 320 mg/kg. These findings indicate that the worms are definitely affected detrimentally at these higher exposure concentrations, whether at the cellular level (numbers of coelomocytes) or at the level of whole-organism physiology (influencing growth and maturation). This was also substantiated by the very low NRR times measured by Svendsen and Weeks [10] for the higher Cu exposure concentrations. The highest exposure concentration during the present study (330 mg/kg) was more than threefold greater than the preceding lower exposure concentration of 100 mg/kg. The differences in body concentrations between the worms from these two exposures, measured after eight weeks, were nevertheless small when viewed against the exposure concentrations (Fig. 1). Although earthworms are able to regulate Cu and do not accumulate very high body concentrations at high exposure concentrations, they are still negatively affected, as evidenced by the NRR assay results and the effects on growth and reproduction. This correlates with the findings of Streit [36], who observed that earthworms do not accumulate high Cu concentrations even from heavily contaminated soils. The significant correlations between the NRR times and Cu concentrations obtained for the five exposure groups that did mature provide further evidence of the dose-response relationship between NRR times and Cu concentrations.
Moore and Stebbing [37] exposed coelenterates to low levels of Cu, cadmium, and zinc, and they concluded that lysosomes from these organisms were affected by lower exposure concentrations of metals than affected growth. This seems also to be the case for earthworms in our study. Because the NRR times during our study were only measured after an eight-week exposure period, they could only be correlated with the outcome of growth over the whole period. The correlation plotted for the biomass increases from the start to the end of the experiment (Fig. 4A), indicating a significant positive relationship between biomass change and NRR times (R2 = 0.821). However, this statistically significant correlation is heavily dependent on the single control point, and when that is removed, no real correlation is found (R2 = 0.09). Although no statistically significant correlation between growth and NRR times for the various exposure concentrations was obtained, an effect on growth was evident from the differences between the nonexposed and exposed earthworms with regard to Cu. This is in agreement with the threshold effect mentioned above. Svendsen and Weeks [10] also noted a reduction in growth at the higher exposure concentrations, and they suggested a fairly uniform weight gain at exposures of as much as 80 mg/kg and a weight loss at higher concentrations of Cu. This indicates that the detrimental influence of exposure to high concentrations of Cu, which initially affects the animal on the subcellular level, could also affect growth.
Of all the reproduction parameters measured and plotted against the NRR times during the present study (Fig. 4B to F), the total cocoon production (by 40 worms in every exposure group except the highest) seemed to correlate most significantly with the NRR times (Fig. 4B). However, as was the case with biomass change (Fig. 4A), the control point distorted the picture. Without the control point, the R2 value was much lower (R2 = 0.676 vs 0.911), and a Spearman rank order correlation indicated no significant relationship (p < 0.05). Spurgeon et al. [12] and Spurgeon and Hopkin [27] also indicated that cocoon production by E. fetida is one of the sensitive endpoints of the effects of Cu and other heavy metals. From our data, Cu also has an effect on cocoon production, but a significant correlation with NRR times cannot be established.
The other four reproduction parameters studied correlated positively with NRR times (Fig. 4C to F). Linear regressions on the datapoints without the control datapoint also showed positive correlations (p < 0.05). For the number of hatchlings per cocoon (Fig. 4E), the correlation was even stronger than that with the control point included (R2 = 0.97 without the control point). For the correlations of the other three parameters (mean cocoon mass, number of cocoons that hatched, and number of hatchlings) (Fig. 4, C, D, and F), the R2 values were very similar when calculated without the control datapoint. Spearman rank order correlations on the data from these four parameters also indicated positive correlations in all these cases. Thus, NRR times were significantly reduced at all concentrations in which lower cocoon mass, numbers of cocoons that hatched, hatching success, and fecundity were found. Hatching rate is dependent on the viability of cocoons, which can be influenced by fertilization success. In turn, fertilization success is dependent on the integrity of the gametes, and heavy metals such as lead and manganese can cause damage to sperm cells in earthworms [38]. This type of cellular damage has not yet been shown for Cu in earthworms, but it is conceivable that this could have contributed to the low hatching success during the present study.
Spurgeon et al. [39] recently reported the effects of zinc on NRR times and life-cycle parameters, and they stressed the importance of establishing relationships between this biomarker and life-cycle endpoints such as length of juvenile period and fecundity. Our results indicate significant links between the biomarker response and the life-cycle parameters studied, which provided a high degree of ecological realism. For an ecotoxicological test to fulfill the requirements of ecological realism, the measured responses must be ecologically relevant and indicative of the fitness of the individual and its functioning in ecological processes [40]. Growth and reproduction are such responses. The significant correlations shown in our study between these responses and the NRR times imply that the measuring of effects using this biomarker could provide very good predictions with regard to the effects of contaminants on the reproduction of organisms in the environment. The toxicity of Cu to earthworms was recognized in field studies [5], and the NRR assay is able to indicate toxic stress at a very early stage, making it a potential tool for assessing this toxicity.
Acknowledgements
This study was supported by the National Research Foundation of South Africa and the University of Stellenbosch.




