Herring gulls and great black-backed gulls as indicators of contaminants in bald eagles in Lake Ontario, Canada
Abstract
In 2000, a pair of bald eagles (Haliaeetus leucocephalus) nested successfully along the shorelines of Lake Ontario in North America for the first time since 1957. However, it is a continuing question whether bald eagles will be able to reproduce successfully as they return to nest on Lake Ontario. Great black-backed gulls (Larus marinus) and herring gulls (L. argentatus) were selected as surrogate species to predict contaminant levels in eggs of bald eagles nesting on Lake Ontario. Because of the suspected overlap in the diets of great black-backed gulls and bald eagles (i.e., fish, gull chicks, and waterfowl), the two species probably occupy a similar trophic level in the Lake Ontario food web and, thus, may have similar contaminant levels. Fresh great black-backed gull and herring gull eggs were collected from three study sites in eastern Lake Ontario in 1993 and 1994 and analyzed for contaminants. Average contaminant levels of dichlorodiphenyldichloroethane (p,p′-DDE), total polychlorinated biphenyls (PCBs), and dieldrin in great black-backed gull eggs were 12.85, 26.27, and 0.27 μg/g, respectively. The mean ratio of contaminant levels in great black-backed gull eggs to contaminant levels in herring gull eggs for these three contaminants was 2.09 (range of means, 1.73–2.38). Predicted levels of contaminants in bald eagle eggs in Lake Ontario would be expected to be similar to the mean levels reported for great black-backed gull eggs. As a comparison, contaminant levels in bald eagle eggs collected from other Great Lakes nesting sites were compared to mean levels reported for herring gull eggs collected from nearby sites in 1986 to 1995. The mean ratio of contaminant levels in bald eagle eggs to contaminant levels in herring gull eggs from these sites for DDE, total PCBs, and dieldrin was 2.40 (range of means, 1.73–3.28). These ratios are very similar to those reported using great black-backed gull eggs, illustrating the apparent similarity in trophic status shared by the two top predator species at these Great Lakes sites. Predicted levels of contaminants in bald eagle eggs at Lake Ontario are similar to levels reported for bald eagles breeding at other Great Lakes sites, suggesting that bald eagles may be able to breed on the shores of Lake Ontario. However, it is unclear at this time what level of breeding success should be expected, given that productivity at other similarly contaminated Great Lakes sites may be below that required to sustain a successful breeding population. The absence of an inland bald eagle population from which bald eagles may begin to colonize the shorelines of Lake Ontario may be delaying initiation of nesting site selection; other factors such as habitat and prey availability would likely not limit reproductive success.
INTRODUCTION
The decline of the bald eagle (Haliaeetus leucocephalus) throughout many parts of North America during the 1960s and 1970s has been well documented [1-3]. High levels of dichlorodiphenyldichloroethane (DDE; a metabolite of DDT) and other chlorinated hydrocarbons found in eggs were associated with eggshell thinning, poor productivity, and local population declines and/or extirpations in many raptorial and fish-eating bird species, including bald eagles [2, 4-7]. As top predators, bald eagles are exposed to elevated levels of persistent contaminants in their diet. Additionally, factors such as habitat loss and human persecution also contributed to the dramatic declines observed in both the number of bald eagles and their reproductive success during this period [8-10].
Bald eagles nesting in the Great Lakes basin were severely affected during this period [1, 2, 11, 12]. Although a common breeder in southern Ontario, Canada, until the 1950s [13], by 1980 only three pairs of bald eagles were reported along the Lake Erie shoreline, none of which were breeding successfully [14]. Eagles nesting along the U.S. shorelines of the Great Lakes were affected similarly, producing only 0.1 young per occupied nest during 1961 to 1970 [1]. After the ban on use of DDT by Canada and the United States in 1972 and the addition of the bald eagle to the endangered species list in 1973, populations slowly began to increase around the Great Lakes [15].
Despite the return of the bald eagle to many parts of the Great Lakes, some populations nesting along various shorelines continue to show impaired reproduction. The distribution of nesting sites is patchy, and the productivity of these populations is not uniform among breeding areas [16, 17]. Levels of contaminants in eggs, nestling blood plasma, and whole nestlings collected from nests on the Great Lakes shorelines are significantly higher than those in comparable tissues collected from nests found further inland [16, 18-21]. Bald eagles breeding on the shorelines also experience lower reproductive success compared to those breeding inland [17]. These differences have been associated with a relatively contaminated Great Lakes forage base, notably fish [16, 22]. A similar spatial pattern of contamination was found by Giesy et al. [23], who noted that fish populations found below dams with access to the Great Lakes had higher contaminant levels than fish populations found above dams without access to the Great Lakes.
Bald eagles breeding on Lake Superior and Lake Erie have higher productivity than birds breeding on Lake Huron and Lake Michigan [16, 17]. Up until 2000, Lake Ontario, recognized as the most contaminated of the Great Lakes [24], was the only lake where bald eagles did not nest [14; P. Nye, personal communication]. Their recent nesting in 2000 confirmed earlier surveys that nesting habitat appeared to be favorable for reoccupation [20; W. Bowerman and D. Weseloh, unpublished data]. Historically, bald eagles have nested on Lake Ontario [25].
It is a continuing question whether a bald eagle population would be self-sustaining once nesting began on the Lake Ontario shoreline. The primary objective of this study was to address this question through use of an appropriate surrogate, same-trophic-level species that currently nests on Lake Ontario. For example, is there a predatory avian species, one trophic level above other fish-eating birds (i.e., gulls, terns, and cormorants) that could be used to predict what contaminant levels might be in bald eagles if they nested on Lake Ontario? If so, would these predicted concentrations permit successful nesting by bald eagles? We selected the great black-backed gull (Larus marinus) as a surrogate species for bald eagles on Lake Ontario. We suggest that great black-backed gulls and bald eagles would have similar diets on Lake Ontario and, therefore, would occupy a similar trophic level. The great black-backed gull, the largest of all North American gulls, is essentially a maritime species, breeding primarily off the coast of eastern North America [26]. However, since the 1970s, they have bred regularly in small numbers (1–12 pairs/site) at several locations in eastern Lake Ontario [27-30; D. Weseloh, personal observation]. To our knowledge, no intensive diet studies for great black-backed gulls nesting in the Great Lakes have been undertaken; however, the appearance of large numbers of gull and tern remains at these nesting sites suggests that colonial waterbirds may form an important part of their diet (D. Weseloh and M. Patrikeev, personal observation). Indeed, studies have documented the predatory nature of this large gull, which feeds on fish, smaller aquatic birds (including herring gulls [L. argentatus]), and mammals [31-38]. Because both herring gulls and ring-billed gulls (L. delawarensis) are abundant at the eastern end of Lake Ontario, these larid species could provide an ample food source for great black-backed gulls, especially during the breeding season, when young are numerous. Bald eagles are also opportunistic predators and feed on fish [39-41], birds (including herring gulls and other larids) [42-44], and mammals [18-20, 45-49]. In winter, they are noted to be opportunistic scavengers, much like gulls, and rely more on waterfowl and mammals [39, 44, 50, 51]. As a result of the suspected overlap in the diets of bald eagles and great black-backed gulls, we suggest that contaminant levels in the eggs of great black-backed gulls may be comparable to those in bald eagles in areas (i.e., lakes) where the two nest sympatrically. However, because no such areas exist in the Great Lakes (great black-backed gulls nest regularly and in sufficient numbers for sampling only on Lake Ontario, and until 2000, bald eagles nested on all Great Lakes expect Lake Ontario), we used contaminant ratios (i.e., contaminant levels in great black-backed gulls to herring gulls in Lake Ontario, and contaminant levels in bald eagles to herring gulls in Lake Erie, Lake Huron, Lake Michigan, and Lake Superior) to validate our model/prediction. We also examined contaminant levels in the eggs of great black-backed gulls nesting on the Great Lakes, for which, to our knowledge, no previous statistical analysis of data has been performed.
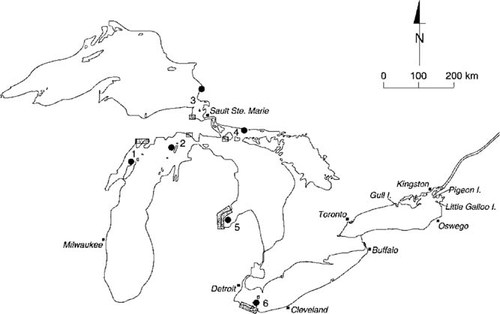
Locations of the great black-backed gull and herring gull colonies on Lake Ontario in North America, from which eggs were collected in 1993 and 1994. Shaded box areas denote bald eagle nesting sites on each of the Great Lakes, and filled circles denote adjacent herring gull Annual Monitor Colonies (i.e., those located within 100 km of bald eagle nesting sites). Numbers correspond to herring gull Annual Monitor Colonies and are as follows: 1, Big Sister Island; 2, Gull Island; 3, Agawa Rock; 4, Double Island; 5, Channel-Shelter Island; and 6, Middle Island.
MATERIALS AND METHODS
Study sites and egg collections
Three study sites located at the eastern end of Lake Ontario—Little Galloo Island (USA), Pigeon Island (Canada), and Gull Island (Canada)—were selected (Fig. 1), because these were the only three islands where great black-backed gulls had been reported to nest regularly on Lake Ontario [27-29]. Little Galloo Island (45°53′N, 76°26′W) is 17 ha in size and is located approximately 40 km south of Kingston, Ontario, Canada, on the U.S. side of Lake Ontario, approximately 23 km west of Sackets Harbor (NY, USA). In 1993, seven great black-backed gull nests and 41 herring gull nests (partial count) were found on Little Galloo Island; in 1994, eight great black-backed gull nests and approximately 300 herring gull nests were found on the Island (D. Weseloh, unpublished data). Pigeon Island (44°04′N, 76°33′W) is approximately 0.5 ha in size and is located 25 km south of Kingston. Five great black-backed gull nests were found on Pigeon Island in 1993; herring gull nest counts were not performed at Pigeon Island in that year. Four great black-backed gull nests and 73 herring gull nests were observed there in 1994 (D. Weseloh, unpublished data). Gull Island (44°0′N, 77°40′W) is 17 ha in size and located 4 km south of Brighton, Ontario, Canada. Two great black-backed gull nests and 60 herring gull nests were found there in 1993 (D. Weseloh, unpublished data). This island was not visited in 1994; therefore, no nest count surveys were conducted.
Egg collections were made (under permit) in 1993 and 1994. All eggs had been freshly laid and were collected by taking one egg (at random) from a recently completed clutch. On April 28 and 29, 1993, 1 to 10 great black-backed gull and herring gull eggs were collected from each of the three study sites. On May 3, 1994, 2 to 11 great black-backed gull and herring gull eggs were collected from Pigeon Island and also from Little Galloo Island; two weeks later, a second set of two great black-backed gull eggs was collected from Pigeon Island. No eggs were collected from Gull Island in 1994. Eggs were transported in a padded container from each site and stored at 4°C within 48 h of collection before chemical analyses.
Chemical analyses
Organochlorine pesticide and polychlorinated biphenyl (PCB) analyses were performed on all gull eggs at the Canadian Wildlife Service National Wildlife Research Centre (Hull, PQ, Canada) according to the methods of Peakall et al. [52] and Won et al.[53]. Before analysis, eggs were thawed to room temperature. Egg contents were emptied into hexanerinsed jars and then homogenized. Aliquots of individual eggs were then pooled by species and site. A subsample of the pooled egg homogenate was dehydrated by grinding with an excess of anhydrous sodium sulfate (6:1, w/w) and extracted with hexane on a column. The extract was then cleaned up and separated into three fractions by Florisil chromatography (J.T. Baker, Phillipsburg, NJ, USA). Organochlorine compounds and PCB concentrations were quantified by gas chromatography using a Hewlett-Packard 7673A GC (Avondale, PA, USA) equipped with a splitless injector port and a 60-m DB-5 fused silica capillary column. Organochlorine pesticides examined included p,p′- DDE, mirex, heptachlor epoxide, dieldrin, hexachlorobenzene, and oxychlordane. Total PCB concentrations were estimated by determining the sum concentration of 42 individual PCB congeners measured. These individual PCB congeners (International Union of Pure and Applied Chemistry number) were as follows: 28, 31, 42, 44, 49, 52, 60, 64, 66, 70, 74, 87, 97, 99, 101, 105, 110, 118, 128, 129, 137, 138, 141, 146, 149, 151, 153, 158, 170, 171, 172, 174, 180, 182, 183, 185, 194, 195, 200, 201, 203, and 206 [54]. Organochlorine residues and PCBs are expressed on a wet-weight basis (μg/g). Detection limits for wet-weight organochlorine residues and PCBs were 0.0025 and 0.005 μg/g, respectively.
Data analyses
Mean contaminant levels for each species and year were determined by weighting the contaminant concentration measured at each site by the number of eggs in the pool. Similarly, a grand mean contaminant level for each species for both years together was determined by weighting the mean contaminant concentration for each study year by the total number of eggs analyzed for that year. To predict contaminant levels in the eggs of bald eagles on Lake Ontario, we calculated a grand mean ratio of contaminant levels in the eggs of great black-backed gulls to levels in the eggs of herring gulls for all sites and both years on Lake Ontario. This ratio was then multiplied by the grand mean contaminant level determined for herring gull eggs for the Lake Ontario sites in 1993 and 1994. Note that “grand mean” is an arbitrary term used to describe a mean value for a variable calculated for both/all years of study. Nonparametric statistics were selected due to small sample sizes and heterogeneity of variances. Contaminant levels in great black-backed gull and herring gull eggs were compared using a one-tailed Mann-Whitney U-test, because levels were expected to be higher in great black-backed gull eggs relative to herring gull eggs [55].
To validate our model for Lake Ontario and because herring gulls, great black-backed gulls, and bald eagles do not nest together in the same part of any of the Great Lakes, we calculated the ratios of contaminant levels in the eggs of bald eagles to levels in the eggs of herring gulls from other Great Lakes where both species are known to nest. Addled bald eagle eggs had been collected previously from the U.S. side of the Great Lakes on Lake Michigan, Lake Huron, Lake Superior, and Lake Erie from 1986 to 1995. During this period, bald eagle contaminant monitoring was undertaken by state and federal agencies as recovery tasks in the Northern States Bald Eagle Recovery Plan [56]. For the purpose of this study, bald eagle nesting sites were selected based on their proximity to herring gull annual monitor colonies [57-59]; that is, nests were within 100 km of each other (Fig. 1). Data for fresh herring gull eggs from the following annual monitor colonies were used: Agawa Rock (eastern Lake Superior, Canada), Gull Island and Big Sister Island (Lake Michigan, USA), Channel-Shelter Island (Lake Huron, USA), Double Island (Lake Huron, Canada), and Middle Island (western Lake Erie, Canada). Mean contaminant levels in bald eagle and herring gull eggs at sites on Lake Michigan, Lake Huron, Lake Superior, and Lake Erie were determined first by calculating an annual mean for each site sampled (where multiple eggs were collected), an annual mean for all sites within a lake, and then an average for all years sampled (1986–1995) for each lake. Grand mean ratios (± standard deviation [SD]) were determined using annual mean contaminant levels in bald eagle eggs to annual mean levels in herring gull eggs for all four of the Great Lakes together. These ratios were then compared to grand mean ratios determined for great black-backed gulls and herring gulls on Lake Ontario.
Bald eagle eggs were analyzed for a number of contaminants, including DDE, dieldrin, and total PCBs. Contaminant data for herring gull eggs were obtained from the Canadian Wildlife Service's extensive database of contaminants found in colonial waterbird eggs collected around the Great Lakes [57-59]. To ensure that contaminant levels in addled bald eagle eggs and fresh herring gull eggs were comparable, contaminant levels in addled bald eagle eggs were corrected for moisture loss (i.e., to fresh wt). Chemical analyses of all bald eagle eggs followed the procedure of Cromartie et al. [60] with the one exception: The extract was separated into four fractions rather than three to enable separation of dieldrin and endrin from the rest of the pesticides. Total PCBs were estimated from 1986 to 1995 in bald eagle eggs using two different methods: By determining the sum of all nine chlorinated homologues, and by determining the sum of all Aroclors (Aroclor 1242, 1248, 1254, and 1260). Estimates of total PCBs using these different methods were generally comparable, and they were also comparable to estimates of PCBs determined using the sum of all 42 individual congeners as performed for analyses of herring gull eggs (J. Moore, personal communication). Detection limits for wet-weight organochlorine residues and PCBs in bald eagle eggs were 0.01 and 0.05 μg/g, respectively; in cases where contaminant levels were less than the detection limit, a value of half the limit was used in the statistical analyses [61, 62]. Before 1988, total PCBs in herring gull eggs collected from the Great Lakes were not quantified using the sum-concentration-of-individual-congeners method but, instead, were quantified using a 1:1 (w/w) mixture of Aroclor 1254:1260. Total PCB concentrations, however, could be estimated in eggs collected in 1986 and 1987 using the relationship between measured concentrations of PCBs determined using this mixture and measured concentrations of total PCBs determined for herring gull eggs specific to each of the Great Lakes [63]. Because comparisons of contaminant levels in eggs of bald eagles and herring gulls did not meet the conditions of parametric testing, Spearman rank tests were performed to determine if correlations existed between levels of contaminants in eggs of bald eagles and eggs of herring gulls collected from Great Lakes nesting sites. Statistical analyses were performed using the SAS® statistical package [64] with a significance level of p < 0.05.
| Year | Site | Species | n | DDEb | Mirex | Heptachlor epoxide | Dieldrin | Oxychlordane | Hexachloro-benzene | Total PCBsc |
|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | Little Galloo Island | GBBG | 7 | 14.17 | 2.20 | 0.13 | 0.39 | 0.17 | 0.12 | 28.83 |
| HERG | 10 | 6.33 | 0.94 | 0.06 | 0.17 | 0.11 | 0.04 | 11.17 | ||
| 1993 | Pigeon Island | GBBG | 4 | 10.24 | 1.55 | 0.10 | 0.17 | 0.14 | 0.08 | 22.68 |
| HERG | 6 | 5.54 | 0.76 | 0.06 | 0.13 | 0.10 | 0.05 | 10.11 | ||
| 1993 | Gull Island | GBBG | 1 | 21.13 | 3.32 | 0.14 | 0.34 | 0.32 | 0.11 | 36.80 |
| HERG | 10 | 6.64 | 0.91 | 0.07 | 0.14 | 0.12 | 0.05 | 10.96 | ||
| 1993 | Mean | GBBG | 12 | 13.44 | 2.08 | 0.12 | 0.31 | 0.17 | 0.11 | 27.44 |
| HERG | 26 | 6.27 | 0.89 | 0.06 | 0.15 | 0.11 | 0.05 | 10.84 | ||
| 1994 | Little Galloo Island | GBBG | 5 | 14.88 | 2.43 | 0.14 | 0.30 | 0.17 | 0.09 | 29.68 |
| HERG | 11 | 6.97 | 1.15 | 0.11 | 0.19 | 0.16 | 0.06 | 13.49 | ||
| 1994 | Pigeon Island | GBBG | 8.31 | 1.68 | 0.09 | 0.13 | 0.11 | 0.05 | 17.58 | |
| 1) | 2 | 8.78 | 1.49 | 0.06 | 0.10 | 0.15 | 0.05 | 19.40 | ||
| 2) | 2 | 5.85 | 1.10 | 0.07 | 0.11 | 0.11 | 0.06 | 12.15 | ||
| HERG | 9 | |||||||||
| 1994 | Mean | GBBG | 9 | 12.06 | 2.05 | 0.11 | 0.22 | 0.15 | 0.07 | 24.71 |
| HERG | 20 | 6.47 | 1.13 | 0.09 | 0.15 | 0.14 | 0.06 | 12.89 | ||
| 1993 & 1994 | Grand mean range | GBBG | 21 | 12.85 | 2.07 | 0.12 | 0.27 | 0.16 | 0.09 | 26.27 |
| 8.31–21.13 | 1.49–3.32 | 0.06–0.14 | 0.10–0.39 | 0.11–0.32 | 0.05–0.12 | 17.58–36.80 | ||||
| HERG | 46 | 6.36 | 0.99 | 0.07 | 0.15 | 0.12 | 0.05 | 11.73 | ||
| 5.54–6.97 | 0.76–1.15 | 0.06–0.11 | 0.11–0.19 | 0.10–0.16 | 0.04–0.06 | 10.11–13.49 |
- a Mean contaminant levels for each year and species represent weighted means. The number of eggs in the pool is represented by n. Grand means for each contaminant represent weighted means for both study years combined.
- b DDE = dichlorodiphenyldichloroethane.
- c PCBs = polychlorinated biphenyls.
RESULTS
Contaminant levels in great black-backed gull eggs
Contaminant levels in pooled egg samples of great black-backed gulls nesting on Lake Ontario in 1993 and 1994 ranged as follows: DDE, 8.31 to 21.13 μg/g; mirex, 1.49 to 3.32 μg/g; dieldrin, 0.10 to 0.39 μg/g; and total PCBs, 17.58 to 36.80 μg/g (Table 1). In 1993, the single great black-backed gull egg collected from Gull Island had the highest levels of total PCBs and all organochlorine pesticides (except dieldrin and hexchlorobenzene). In 1994, eggs were not collected from Gull Island, and Little Galloo Island consistently had the highest levels of all organochlorine pesticides and total PCBs in great black-backed gull eggs. Eggs from Pigeon Island had the lowest levels of all contaminants for both study years. For the two study sites sampled in both 1993 and 1994 (Little Galloo Island and Pigeon Island), an increase in contaminant levels in great black-backed gull eggs was observed between years in 36% (5/14) of the comparisons. Great black-backed gull eggs contained significantly higher levels of all contaminants (except dieldrin) relative to herring gull eggs (Mann-Whitney U-test, n1 = 5, n2 = 5, U > 21, α1 = 0.05).
Contaminant levels in herring gull eggs
Contaminant levels in pooled egg samples of herring gulls nesting on Lake Ontario in 1993 and 1994 ranged as follows: DDE, 5.54 to 6.97 μg/g; mirex, 0.76 to 1.15 μg/g; dieldrin, 0.11 to 0.19 μg/g; and total PCBs, 10.11 to 13.49 μg/g (Table 1). In 1993, herring gull eggs collected from Gull Island had the highest concentrations for all organochlorine pesticides, with the exception of mirex, dieldrin, and total PCBs; in 1994, Little Galloo Island had the highest levels of all contaminants measured in herring gull eggs. Eggs collected from Pigeon Island also had the lowest organochlorine pesticide levels and total PCBs for both years; the only exception to this was at Little Galloo Island in 1993, where hexachlorobenzene levels were the lowest recorded in this study. For the two study sites sampled in both 1993 and 1994 (Little Galloo Island and Pigeon Island), an increase in contaminant levels in herring gull eggs was observed between years in 93% (13/14) of the comparisons.
Contaminant ratios and predicted contaminant levels in bald eagles
Ratios of contaminant levels in eggs of great black-backed gulls to levels in eggs of herring gulls for each site sampled in 1993 and 1994 are reported in Table 2. All ratios were greater than one, except at Pigeon Island in 1994, where levels of hexachlorobenzene in eggs of herring gulls (0.06 μg/g) were slightly higher than levels in great black-backed gulls (0.05 μg/g). For both years together, total PCBs had the highest grand mean ratio ± SD (2.38 ± 0.67), and oxychlordane had the lowest grand mean ratio (1.57 ± 0.64). On average (± SD) for all contaminants, great black-backed gull eggs had approximately 1.95 (±0.69)-fold the contaminant load of herring gull eggs.
Using a surrogate species, we attempted to predict contaminant levels that would have been observed in bald eagle eggs if these birds had been nesting on the shorelines of Lake Ontario in 1993 and 1994. This was achieved by multiplying the grand mean ratio for each contaminant (Table 2) by the grand mean concentration (and range) found in herring gull eggs for both years (Table 1); the results are portrayed in Table 3.
| Year | Site | DDEb | Mirex | Heptachlor epoxide | Dieldrin | Oxychlordane | Hexachloro-benzene | Total PCBsc |
|---|---|---|---|---|---|---|---|---|
| 1993 | Little Galloo Island | 2.24 | 2.34 | 2.17 | 2.29 | 1.55 | 3.00 | 2.58 |
| 1993 | Pigeon Island | 1.85 | 2.04 | 1.67 | 1.31 | 1.40 | 1.60 | 2.24 |
| 1993 | Gull Island | 3.18 | 3.65 | 2.00 | 2.43 | 2.67 | 2.20 | 3.36 |
| 1993 | Mean | 2.14 | 2.34 | 1.89 | 2.09 | 1.87 | 2.29 | 2.53 |
| 1994 | Little Galloo Island | 2.13 | 2.11 | 1.27 | 1.58 | 1.06 | 1.50 | 2.20 |
| 1994 | Pigeon Island | 1.46 | 1.44 | 1.07 | 1.05 | 1.18 | 0.83 | 1.52 |
| 1994 | Mean | 1.86 | 1.81 | 1.22 | 1.47 | 1.12 | 1.17 | 1.92 |
| 1993 & 1994 | Grand mean | 2.17 ± 0.64 | 2.32 ± 0.82 | 1.64 ± 0.47 | 1.73 ± 0.61 | 1.57 ± 0.64 | 1.83 ± 0.82 | 2.38 ± 0.67 |
- a Mean ratios for each year were calculated using the weighted means of contaminant levels for each species, as shown in Table 1. Grand mean (± standard deviation) represents the mean of all ratios at all sites for both years.
- b DDE = dichlorodiphenyldichloroethane.
- c PCBs = polychlorinated biphenyls.
Model validation
Mean fresh-weight contaminant levels in addled bald eagle eggs from selected nests on Lake Erie, Lake Huron, Lake Michigan, and Lake Superior from 1986 to 1995 and in fresh herring gull eggs from the nearest annual monitor colonies for the same year are presented in Table 4. In all cases, mean contaminant levels were greater in bald eagle eggs than in herring gull eggs; thus, all ratios were greater than one. In bald eagle eggs collected from the four Great Lakes, mean DDE levels ranged from 3.60 to 11.66 μg/g, mean dieldrin levels from 0.34 to 0.69 μg/g, and mean total PCB levels from 12.91 to 42.32 μg/g. Mean DDE and dieldrin levels were greatest in Lake Michigan, and mean total PCB levels were greatest in Lake Huron. In herring gull eggs collected from sites in close proximity to bald eagle sites, mean DDE levels ranged from 2.20 to 7.28 μg/g, mean dieldrin levels from 0.13 to 0.32 μg/g, and mean total PCB levels from 6.26 to 17.80 μg/g. Mean DDE levels in herring gull eggs were greatest in Lake Michigan, mean dieldrin levels in the one Lake Superior egg collected, and mean total PCBs in eggs from Lake Huron. Predicted levels of DDE, dieldrin, and total PCBs in bald eagle eggs from Lake Ontario (see above) were slightly higher, lower, and intermediate, respectively, relative to mean concentrations observed in bald eagle eggs at other Great Lakes nesting sites.
Spearman rank correlations of contaminant levels in eggs of bald eagles and herring gulls collected from sites in close proximity to one another on four of the five Great Lakes were significant for DDE only (rs = 0.64, p = 0.002) (Table 5); the strength of this correlation was increased when contaminant data for eggs of great black-backed gulls and herring gulls from Lake Ontario were included in the correlation (rs = 0.63, p = 0.0005). A significant correlation was not observed for PCBs between bald eagles and herring gulls (rs = 0.36, p = 0.10); however, a marginally significant correlation was found when contaminant levels in eggs of great black-backed gull eggs and herring gulls were included (rs = 0.37, p = 0.06). A comparison of the grand mean ratio of contaminant levels in eggs of bald eagles to herring gulls (collected from Lake Superior, Lake Michigan, Lake Huron, and Lake Erie in 1986–1995) with contaminant levels in eggs of great black-backed gulls to herring gulls (collected from Lake Ontario in 1993–1994) for DDE, dieldrin, and total PCBs is shown in Table 5. Comparisons of ratios using bald eagles and great black-backed gulls were also more similar for DDE and total PCBs relative to dieldrin (8% and 20%, respectively, vs 90%). On average, for all three contaminants, addled bald eagle eggs had approximately 2.40-fold the contaminant load of fresh herring gull eggs, and fresh great black-backed eggs had approximately 2.09-fold the contaminant load of herring gull eggs; the difference between these two values is 15% relative to the ratio calculated using great black-backed gull eggs. Given the general similarities in grand mean ratios calculated using bald eagles and great black-backed gulls, predicted levels of DDE, dieldrin, and PCBs in bald eagle eggs can also be calculated in a manner similar to that determined using great black-backed gull eggs (as outlined above) That is, the observed grand mean ratio of contaminant levels in eggs of bald eagles to herring gulls from the four Great Lakes where they are known to nest (Table 5) is multiplied by the grand mean level of these contaminants in herring gulls on Lake Ontario (Table 1). Predicted levels of DDE, dieldrin, and PCBs for bald eagles using this method are 11.00, 0.49, and 25.81 μg/g, respectively. These predicted levels for DDE, dieldrin, and PCBs are similar to those determined above using great black-backed gull eggs (13.80, 0.26, and 27.92 μg/g, respectively), suggesting further that bald eagles and great black-backed gulls may, indeed, occupy similar trophic levels in the Lake Ontario food web.
| Contaminant | Level |
|---|---|
| Total PCBsa | 27.92 μg/g (24.06–32.11) |
| Mirex | 2.30 μg/g (1.8–2.7) |
| Oxychlordane | 0.19 μg/g (0.16–0.25) |
| Hexachlorobenzene | 0.09 μg/g (0.07–0.11) |
| DDEb | 13.80 μg/g (12.0–15.1) |
| Dieldrin | 0.26 μg/g (0.19–0.33) |
| Heptachlor epoxide | 0.11 μg/g (0.10–0.18) |
- a PCBs = polychlorinated biphenyls.
- b DDE = dichlorodiphenyldichloroethylene.
DISCUSSION
Contaminants in eggs
To our knowledge, only two other studies have examined contaminant levels in great black-backed gull eggs. Levels (mean ± SD) of DDE and PCB for eggs of great black-backed gulls collected from Appledore Island in Maine in 1977 were 8.66 ± 4.67 μg/g (wet wt) and 30.96 ± 22.3 μg/g (wet wt), respectively (n = 28 eggs) [65]. Hatching success of great black-backed gulls on Appledore Island during this period was high, at 76% (SAS Institute [64]), leading them to suggest that levels of these residues were not high enough to impair great black-backed gull reproduction on this Island. In contrast, Cooke [66] found that mean levels of DDE and PCBs reported for great black-backed gull eggs collected from Skomer Island in Wales in 1972 were one-tenth to one-quarter of the mean levels found in the present study. Contaminant levels in herring gull eggs collected from Pigeon Island in 1993 and 1994 were comparable to levels detected in eggs from a nearby colony on Snake Island, a site that has been monitored annually by the Canadian Wildlife Service since 1977 [57-59].
| Lake | Species | n | DDEb | Dieldrin | Total PCBsc |
|---|---|---|---|---|---|
| Erie | BAEA | 10 | 3.60 ± 2.37 | 0.44 ± 0.15 | 29.02 ± 13.58 |
| HERG | 10 | 2.20 ± 0.28 | 0.13 ± 0.05 | 17.44 ± 2.60 | |
| Superior | BAEA | 1 | 9.54 | 0.51 | 12.91 |
| HERG | 1 | 3.13 | 0.32 | 6.26 | |
| Michigan | BAEA | 7 | 11.66 ± 8.79 | 0.69 ± 0.64 | 31.40 ± 13.19 |
| HERG | 7 | 7.28 ± 1.27 | 0.29 ± 0.13 | 11.99 ± 2.32 | |
| Huron | BAEA | 4 | 7.62 ± 3.03 | 0.34 ± 0.19 | 42.32 ± 22.08 |
| HERG | 4 | 5.05 ± 2.06 | 0.13 ± 0.06 | 17.80 ± 10.53 | |
| Ontario | BAEA* | — | 13.80 | 0.26 | 27.92 |
| HERG | 2 | 6.36 | 0.15 | 11.73 |
- a Bald eagle nests from each of the Great Lakes were selected based on their proximity to herring gull (HERG) Annual Monitor Colonies; that is, nests were within 100 km of each other. The same years were compared for both species. Predicted contaminant levels in BAEA eggs nesting on Lake Ontario, Canada (*), using great black-backed gull and HERG eggs collected in 1993 to 1994 are also shown for comparison; observed grand mean contaminant levels in HERG eggs collected in 1993 to 1994 from sites on Lake Ontario are also shown. The number of years used to calculate the mean is represented by n.
- b DDE = dichlorodiphenyldichloroethane.
- c PCBs = polychlorinated biphenyls.
The two predicted DDE levels in bald eagle eggs in Lake Ontario (11.00 and 13.80 μg/g) are similar to levels commonly measured in bald eagle eggs at nesting sites that are considered to be contaminated. Kubiak and Best [16] found that mean DDE levels in addled bald eagle eggs collected from U.S nesting sites on Lake Huron and Lake Michigan were similar to each other (20.5 and 20.1 μg/g, respectively) and substantially higher than mean levels found on Lake Erie and Lake Superior (3.4 and 4.5 μg/g, respectively) from 1986 to 1990. In the present study, DDE levels appear to be elevated at Lake Superior relative to Lake Erie and more similar to mean levels at Lake Huron and Lake Michigan (Table 4). This apparent discrepancy can be explained by the fact that only one egg was included in the Lake Superior analysis based on its close proximity to the herring gull Annual Monitor Colony at Agawa Rock, whereas the mean levels reported by Kubiak and Best [16] were estimated using the entire collection of eggs from along the U.S. Lake Superior shoreline. Therefore, spatial patterns observed for bald eagle eggs in the present study are not representative of lake-wide bald eagle contaminant patterns. Nonetheless, they serve to examine similarities in spatial patterns observed in herring gull eggs and in the ratio between levels of contaminants in great black-backed gulls and bald eagles. Predicted levels of DDE for Lake Ontario bald eagles are also similar to levels found in addled bald eagle eggs collected in the early 1980s from the most contaminated sites in Maine, Oregon, and one site in Wisconsin on Lake Michigan, USA [67]. Fresh bald eagle eggs collected during the 1980s from the Columbia River estuary [68], a region exposed to a wide variety of contaminants, had a mean DDE level of 9.70 μg/g (range, 4–20 μg/g), which is less than the values predicted in the present study.
| Contaminant | n | rs | p | Grand mean bald eagle: herring gull | Grand mean great black-backed gull: herring gull | % Difference |
|---|---|---|---|---|---|---|
| DDEb | 22 | 0.64 | 0.002 | 1.73 ± 1.05 | 2.17 ± 0.64 | +20% |
| DDE including GBBGc data | 27 | 0.63 | 0.0005 | — | — | — |
| Dieldrin | 22 | 0.24 | 0.30 | 3.28 ± 2.00 | 1.73 ± 0.61 | −90% |
| Dieldrin including GBBG data | 27 | 0.20 | 0.40 | — | — | — |
| Sum PCBsb | 22 | 0.36 | 0.10 | 2.20 ± 1.05 | 2.38 ± 0.67 | + 8% |
| Sum PCBs including GBBG data | 27 | 0.37 | 0.06 | — | — | — |
- a Correlations between levels of contaminants in eggs of bald eagles and of herring gulls including contaminant level data from great black-backed gulls and herring gulls nesting on Lake Ontario, Canada are also shown, n, rs, and p denote the number of means for a given year and site, Spearman rank correlation coefficients, and levels of significance, respectively. A comparison of the grand mean ratio of contaminant levels (± standard deviation [SD]) in eggs of bald eagles to eggs of herring gulls (collected from four of the Great Lakes in 1986–1995) and the grand mean ratio of contaminant levels (±SD) in eggs of great black-backed gulls to eggs of herring gulls (collected from Lake Ontario in 1993–1994) is also shown. Percentage difference refers to differences in the two ratios relative to the grand mean great black-backed gull: herring gull ratio.
- b DDE = dichlorodiphenyldichloroethane.
- c GBBG = great black-backed gull.
- d PCBs = polychlorinated biphenyls.
High levels of mirex are typically observed in wildlife from Lake Ontario relative to that of the other Great Lakes as a result of its release from two historical sources located upstream from Lake Ontario [69]. To our knowledge, mirex levels in great black-backed gull eggs are among the highest ever detected in fish-eating birds nesting on the Great Lakes in the 1990s [58, 59]. The predicted level of mirex in bald eagle eggs (2.30 μg/g) in Lake Ontario is at least 16-fold greater than mean levels in bald eagle eggs collected from similar sites as herring gull egg collection sites on the Great Lakes from 1986 to 1995 (W. Bowerman et al., unpublished data); this predicted level is also at least fourfold greater than levels detected in bald eagle eggs collected during the early 1980s throughout the United States [67].
The two predicted total PCB levels (25.81 and 27.92 μg/g) in eggs of bald eagles on Lake Ontario are intermediate relative to the mean levels found by Kubiak and Best [16] for four of the Great Lakes in 1986 to 1990 (highest mean, 76.7 μg/g at Lake Huron; lowest mean, 10.1 μg/g at Lake Superior). Also, these levels for PCBs in Lake Ontario bald eagle eggs are higher than those for 97% of the eggs (102/105 eggs) collected in the United States during the 1980s [67]. Comparably, on the Pacific coast of Canada, where bald eagles do not show impaired reproduction, mean levels of total PCBs in fresh eggs of bald eagles [70], quantified using methods similar to those for herring gull eggs, were at least 80% less than that predicted for fresh eggs in Lake Ontario. Overall, predicted levels of DDE, mirex, and total PCBs in Lake Ontario bald eagles appear to be similar to levels found at sites that have been considered to be contaminated (see the following section for how levels of these three contaminants might affect bald eagle productivity).
Whereas high levels of dieldrin have been demonstrated to be very toxic to birds [71], levels of dieldrin in great black-backed gulls (measured) and bald eagles (predicted) on Lake Ontario do not appear to be high enough to impair reproduction. The two predicted dieldrin levels (0.26 and 0.49 μg/g) are similar to, if not lower than, the mean levels reported for other Great Lakes bald eagle eggs presented in the present study (Table 4). They are also intermediate to the mean dieldrin levels determined at other Great Lakes nesting sites in 1986 to 1990 (highest mean, 1.32 μg/g at Lake Michigan; lowest mean, 0.25 μg/g at Lake Superior) [16]. These levels are also intermediate to those reported for eggs of bald eagles collected throughout the United States from 1980 to 1984 [67]. These predicted dieldrin levels in eggs are also approximately three- to fivefold the concentration cited as causing no adverse effect in bald eagles (0.1 μg/g) [7, 72] and 51 to 74% less than the concentration cited as having a significant impact on reproduction (1.0 μg/g for golden eagles [Aquila chrysaetos]) [71]. In golden eagles from West Scotland in 1966 to 1968, a mean concentration of dieldrin in eggs of 0.34 μg/g was associated with 69% nest success [71]. In the present study, we detected no significant difference between dieldrin concentrations in eggs of great black-backed gulls and herring gulls when all Lake Ontario sites and years were pooled together.
Predicted levels of heptachlor epoxide (0.11 μg/g), oxychlordane (0.19 μg/g), and hexachlorobenzene (0.09 μg/g) likely are not high enough to have a significant impact on reproduction in bald eagles. Generally lower levels of heptachlor epoxide and oxychlordane than those found in the present study were reported in bald eagle eggs in the United States during the early 1980s [67]. The mean heptachlor epoxide level measured in bald eagle eggs collected throughout Ontario from 1965 to 1972 [73] was approximately twofold greater than the predicted level in the present study. The predicted level of hexachlorobenzene falls in the range of that found in eggs of white-tailed sea eagles (H. albicilla), a closely related species in Sweden (range of means, 0.08–0.12 μg/g) [74]. Helander et al. [74] suggested that levels in this range were too low to affect reproductive success.
Aquatic birds that feed on other birds would be expected to have higher contaminant levels than birds that feed on fish exclusively [18, 68]. Ratios of greater than one and, on average, of equal to two, as reported for great black-backed gulls in the present study, illustrate the biomagnification of these contaminants through the food web, whereby the top predator contains the highest concentration of contaminants. Szaro et al. [65] also noted higher levels of DDE and PCBs in eggs of great black-backed gulls compared to levels in eggs of herring gulls collected from the same island in Maine. Ratios for mean DDE to mean total PCB levels in the two species were 4.46 and 3.99, which differ nearly twofold from the ratios estimated in the present study. This apparent discrepancy likely is due to differences in prey availability between the two populations. Biomagnification factors determined for bald eagle eggs and fish in three Michigan rivers were considerably larger, being equal to 22 and 28 for DDE and total PCBs, respectively [22]. The ability of contaminants to biomagnify and, thus, to become more concentrated at higher trophic levels puts top predators such as great black-backed gulls and bald eagles at risk in terms of their survival and reproductive success. It has been suggested that the poor productivity observed in bald eagle populations at some locations in North America has been exacerbated by these birds feeding on herring gulls, waterfowl, and colonial waterbirds [18, 46].
Similarities in contaminant ratios using eggs of great black-backed gulls versus those of herring gulls and of bald eagles versus those of herring gulls (Table 5) likely reflect similarities in diet and position in the aquatic-based food web for the two species. Slight differences between the ratios may be due to small differences in prey items selected during the breeding season, differences in winter diet, and differences between species in their ability to metabolize persistent contaminants [75]. It is important to point out that predicted contaminant levels for bald eagles in the present study are for fresh eggs. Mean contaminant levels found in addled bald eagle eggs (Table 4), although corrected for moisture loss, might be slightly biased toward higher contaminant levels, because these eggs were selected due to their failure to hatch [67]. Consequently, ratios of contaminant levels in addled bald eagle eggs to those in fresh herring gull eggs may be slightly biased toward higher values relative to comparisons of levels in fresh eggs between species. Estimates of fresh wet-weight contaminant concentrations based on moisture loss, on the other hand, tend to be biased toward lower contaminant levels, because moisture loss, which is corrected to 100% egg volume, does not take into account the presence of an air cell. Another potential source of bias is that predicted levels of contaminants for bald eagles may actually be higher than that predicted using the surrogate species, because bald eagles have been reported to prey on great black-backed gulls [47]. Alternately, we suspect that these gulls might feed more often on eggs, young, and adults of nearby nesting fish-eating birds than on fish relative to bald eagles (D. Weseloh, personal observation). If this is the case, then great black-backed gulls would have a more contaminated diet and, if accumulation rates are similar in the two species, therefore might show higher ratios compared to those of bald eagles.
Relationship of contaminants to bald eagle productivity on Lake Ontario
Lake Ontario has a history of being one of the most contaminated of the Great Lakes. Extensive biological monitoring has shown contaminant-related effects in herring gulls, double-crested cormorants (Phalacrocorax auritus), black-crowned night herons (Nycticorax nycticorax), and common terns (Sterna hirundo) [76-80]. Following a decline in levels of a number of contaminants in the Lake Ontario food web since the mid-1970s, the reproductive success of most avian species has improved considerably [69].
Although bald eagles have exhibited a remarkable recovery in North America relative to population estimates in the 1950s and 1960s, populations along shorelines of the Great Lakes continue to show impaired reproduction [16, 17]. Depressed productivity in bald eagles nesting along the Great Lakes and throughout North America has been associated with high levels of contaminants, notably DDE and PCBs, in eggs, nestling blood plasma, and whole nestlings [2, 7, 16, 18-21, 67, 68; P. Hunter, unpublished data]. In 2000, a pair of bald eagles nested successfully on Lake Ontario for the first time since a nesting on Amherst Island in 1957 [25].
A review of the literature suggests that the predicted contaminant levels (as determined in the present study) of DDE, PCBs, and possibly, mirex in eggs of bald eagles nesting on Lake Ontario would exceed those at which the population could be sustained. An annual production of 0.7 young per occupied nest is required to ensure a stable bald eagle population [1], and Wiemeyer et al. [7] have suggested that 1.0 young per occupied nest is associated with a healthy population unaffected by contaminants. Wiemeyer et al. [67] also reported that levels of DDE greater than 6.3 μg/g are associated with a large drop in reproductive success; the two predicted DDE levels for bald eagles in the present study (11.00 and 13.80 μg/g) are approximately twofold higher than this critical level and are associated with 13.7 to 14.6% eggshell thinning in fresh eggs. Additionally, Wiemeyer et al. [67] detected a significant relationship between DDE levels in addled bald eagle eggs and mean five-year production of young in populations sampled across the United States. Using this regression equation, a predicted DDE level of 13.80 μg/g in an addled bald eagle egg corresponds to a mean five-year productivity equal to 0.28 young per occupied nest, which is far less than that required to support a bald eagle population. Kubiak and Best [16] also found a significant relationship for DDE levels and productivity of bald eagles nesting on the Great Lakes. In this case, at the predicted level cited above, productivity would be equal to 0.67 young fledged per nest.
Differences in the predicted productivities between the two studies could be due to differences in the methodology for estimating productivity between them. Although high DDE levels have been frequently cited as a major contributor to low productivity in bald eagles [2, 7, 67], high PCB levels may pose an additional threat to productivity, because these compounds have been implicated in both embryonic mortality and developmental deformities observed in many avian species. Bill defects consistent with the presence of PCBs and related compounds have been observed in bald eagle nestlings on the Great Lakes [81]. The no-observable-adverse effect concentration for total PCBs in bald eagle eggs has been predicted to be 4 μg/g [7, 22, 72]. This threshold concentration is approximately one-sixth to one-seventh of the two predicted total PCB levels (25.81 and 27.92 μg/g) in Lake Ontario bald eagles and as much as one-tenth that predicted for Lake Huron bald eagles (Table 4). These predicted levels are also at least 30% less than the critical concentration of PCBs (40 μg/g) cited as having a significant effect on reproduction in white-tailed sea eagles [74].
With regard to mirex, high dietary levels (100–600 μg/g) ingested by laying hens and mallard ducks (Anas platyrhychos) were associated with reduced hatching rates and survival of the young [82, 83]. Relatively lower levels of dietary mirex (5–160 μg/g), on the other hand, did not affect egg production, egg weight, or shell thickness in white leghorn chicken (Gallus gallus) or Japanese quail (Coturnix japanica) [84]. Dietary mirex (8 μg/g) fed to captive American kestrels (Falco sparverius), however, was associated with a marked decline in sperm concentration [85]. Mirex levels reported in great black-backed gull eggs in this study (mean, 2.07 μg/g in 1993 and 1994) may not be high enough to impair reproductive behavior, because nesting numbers of great black-backed gulls on Lake Ontario have been increasing slowly since the early 1980s [86; D. Weseloh, personal observation]. It is unclear to what extent bald eagles might be affected by levels as high as predicted for Lake Ontario.
In summary, given the literature evidence reported above and the levels and reproductive success observed at U.S. Lake Michigan and Lake Huron sites [16], we would expect that the elevated levels of DDE, PCBs, and possibly, mirex predicted in the present study would have an adverse effect on bald eagle productivity at the population level. The predicted levels appear to be too high to support a viable, self-sustaining bald eagle population. It would be difficult to discern conclusively the effects of each of these contaminants individually, because as is the case in many field studies, contaminant levels are often co-correlated [67].
Although the evidence for the above conclusion seems unequivocal, recent data from western Lake Erie presents a conundrum. Donaldson et al. [87], working with bald eagles nesting on the Canadian shoreline, found that mean DDE and PCB levels in failed eggs collected from 1989 to 1994 were as high as 10.8 and 26.4 μg/g, respectively, or just slightly lower than those predicted levels reported for Lake Ontario bald eagle eggs, yet mean productivity of greater than 1.00 young produced per nest was reported during this time period. Whereas this level of productivity may be associated with a healthy population, only one banded fledgling from this population was subsequently observed reproducing. Possible explanations include that this population has become less sensitive to the effects of contaminants over time; that the high abundance of prey available in western Lake Erie may compensate for the effects of the contaminants expected, because chicks are not food-stressed; and/or, as suggested by Donaldson et al. [87], exposure to persistent organic compounds in ovo may be affecting adult reproductive potential. Alternatively, the relatively high productivity rates could have resulted from a high proportion of the adults originating from clean chicks that were not exposed in ovo and were fostered into the Lake Erie population. Clearly, the role of many factors, including prey availability and habitat quality and availability, may influence bald eagle reproductive success and must be considered (see below).
High levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and dioxin-like compounds, including non-ortho-substituted PCBs, have been linked to reproductive impairment observed in fish-eating birds breeding in the Great Lakes [22, 88, 89]. Biota from Lake Ontario contain relatively high levels of TCDD due to local input sources found upstream on the Niagara River. For example, herring gull eggs collected from Snake Island (Lake Ontario, Canada) and Saginaw Bay (Lake Huron, USA) had mean TCDD levels that were generally at least twofold higher than those at other Great Lakes colonies from 1981 to 1991 [90]. Although levels of these chlorinated hydrocarbons were not measured in the present study, their contribution to overall toxicity should not be overlooked. Elliott et al. [70] examined the relationship between levels of total PCBs and non-ortho-substituted PCB congeners 126 and 77 (which elicit toxic responses similar to TCDD) in bald eagle eggs from the Pacific coast. Based on the predicted total PCB level of 27.92 μg/g in the present study, we estimate that concentrations of PCB 126, of PCB 77, and the sum of these two congeners expressed as toxic equivalents (TCDD-EQs; using toxic equivalency factors derived for avian species according to the World Health Organization, Paris, France) to be 4.43, 2.01, and 0.54 ng/g, respectively. This TCDD-EQ concentration, without considering the contribution of other non-ortho PCBs, mono-ortho PCBs, and dioxins to toxicity, is nearly threefold the lowest-observable-effect limit, defined as 0.21 ng/g in bald eagle eggs [91]. Mean levels of polychlorinated dibenzofurans in herring gull eggs collected from the Lake Ontario colony in 1984 to 1991 were similar to levels found at other Great Lakes colonies with the exception of Saginaw Bay, where levels were generally higher [90]. Indeed, levels of TCDD and PCB congeners exhibiting similar toxic responses to TCDD are important in determining bald eagle reproductive success. Giesy et al. [22] have further suggested that the total concentration of TCDD-EQs, contributed primarily from non-ortho PCBs, is the most important contaminant affecting bald eagle productivity in the Great Lakes.
In summary, predicted contaminant levels of DDE, PCBs, and mirex for bald eagles nesting on Lake Ontario appear to exceed levels associated with a healthy bald eagle population. However, what is clear from the present study is that predicted levels for bald eagles on Lake Ontario are generally similar to levels reported for bald eagles breeding at other Great Lakes sites [16, 87] (Table 3). Therefore, provided that other conditions are favorable and conducive to nesting (see below), bald eagles may be able to breed on the shores of Lake Ontario. What is unclear at this time is the level of success that should be expected, given that productivity at the most contaminated Great Lakes sites (Lake Huron, 0.59 young fledged per nest; Lake Michigan, 0.68 young fledged per nest) [16] is below that required to sustain a successful breeding population.
Other factors affecting bald eagle reproductive success
A number of other factors must be considered when investigating the absence of nesting bald eagles on Lake Ontario. The presence of suitable habitat and availability is crucial before nesting is initiated. An aerial survey of bald eagle nesting habitat on the Canadian and U.S shorelines of Lake Ontario revealed that approximately 8 to 18% of the shoreline was classified as good-excellent for bald eagle nesting [20; D. Weseloh, unpublished data]. The use of artificial nesting platforms has also been successful in attracting bald eagles to nesting areas along the north shores of Lake Erie where preferred supercanopy trees were absent [92]. Bald eagles are sensitive to human disturbance, especially during the incubation period, and ultimately, they may abandon their nest if disturbance continues [9]. Prey availability has also been identified as an important factor influencing bald eagle density and reproductive success [19, 93, 94]. Dykstra et al. [95] suggested that low productivity observed for bald eagles nesting along the shores of Lake Superior (a highly oligotrophic lake) may be related, at least in part, to suspected low prey availability. Bowerman [20] suggested that the high productivity of Lake Erie has likely contributed to the success of bald eagles nesting there. Similar to Lake Erie, Lake Ontario is also a eutrophic lake, where prey availability may not be limited. Also, during the winter and early spring at Lake Ontario, food availability (and, hence, female body condition) may be less likely to be influenced by ice conditions, because this lake seldom freezes over [96], unlike Lake Erie, where there may be more substantial ice cover in winter [50]. Therefore, preliminary evidence suggests that habitat quality and availability as well as prey availability likely would not adversely affect bald eagles nesting on Lake Ontario.
The major consideration here is that, at this time, no substantial inland Lake Ontario population exists in either Canada or the United States from which bald eagles can begin to colonize Lake Ontario. The presence or re-establishment of a population depends on the proximity of a healthy, expanding founder population of eagles. It is encouraging that, in 2000, 51 nesting pairs were recorded in New York State, and that the number of nests in this state has been increasing by 10 to 15% per year since 1987 (P. Nye, personal communication). Remarkably, in the summer of 2000, the first Lake Ontario nest was located in New York State: One young fledged (and was banded), and one egg was collected for contaminant analysis. Eventually, bald eagles may continue to colonize the prime nesting areas along the U.S. shores of Lake Ontario once a large base inland population has become established. Studies of bald eagle reproductive success and survival rates on Lake Ontario will be necessary at that time, because there has been some speculation that, at the more contaminated Great Lakes sites (Lake Michigan and Lake Huron), younger, relatively uncontaminated inland eagles may be replacing those found nesting along the shorelines [17, 18]. In assessing the health of a bald eagle population, it is imperative to continue monitoring the productivity of these individuals over the course of their entire lifetime and, as suggested by Best et al. [17], to determine whether productivity decreases as bald eagles increase in age.
Acknowledgements
The authors would like to thank S. Postupalsky. J. Holt, J. Papp, B. Richardson, and A. Bath for assistance in collecting data that contributed to the model validation portion of this study. K. Pettit assisted in collections of great black-backed gull eggs on Lake Ontario. L. Shutt, D. Stewart, and W.W. Bowerman provided helpful comments on this manuscript.




