Predicting bioavailability and bioaccumulation with in vitro digestive fluid extraction
Abstract
Bioavailability of sediment-associated contaminants was assessed in parallel tests by means of a bivalve (Macoma nasuta) bioaccumulation assay and a novel in vitro digestive fluid extraction procedure. Digestive fluid was obtained from the deposit-feeding polychaete Arenicola brasiliensis and used to extract sediments from a U.S. Navy facility. Both the digestive fluid extraction and the bivalve bioaccumulation test identified Cd, Pb, high molecular weight polycyclic aromatic hydrocarbons (HPAHs), and polychlorinated biphenyls (PCBs) as the contaminants of concern; both procedures indicated that As, Cu, Hg, Ni, Zn, and low molecular weight polycyclic aromatic hydrocarbons were rarely, if ever, of concern. The only contaminant for which the techniques consistently differed was Cr, a result attributable to constraints on intestinal absorption of the metal by the bivalves. For Cd and Pb, the concentration attained in digestive fluid during a brief extraction was highly correlated with concentration attained in the bivalve after a 28-d exposure; correlation was marginal for HPAHs and was nonsignificant for PCBs. However, bulk sediment concentrations were equally good predictors of bioaccumulation because of minimal differences in bioavailability from the most contaminated sediments. In vitro contaminant extraction with the digestive fluid assay has potential as a screening tool to predict relative bioaccumulation risk, and has several advantages over traditional tests.
INTRODUCTION
Assessment of the ecological risk posed by dredged material and other contaminated aquatic sediments often involves bioaccumulation testing. In freshwater sediments, the oligochaete Lumbriculus variegatus often is used, and in marine sediments, the bivalve Macoma nasuta or the polychaete Neanthes virens are often the test organisms. The long duration of the tests (often 28 d) and their extensive analytical requirements often make bioaccumulation testing the most time-consuming and costly component of sediment testing.
In vitro digestive fluid extraction recently has been proposed as a good predictor of bioavailability for both organic compounds [1, 2] and trace metals [3, 4]. In this procedure, the digestive fluid of a deposit-feeding organism is obtained by dissection and then used as an extractant in in vitro incubation with the sediment of concern. The contaminant concentration attained in the digestive fluid is determined, and can be used to place a maximum limit on the bioavailable contaminant fraction via ingestion and digestion. Unlike conventional chemical extractions, which are intended to achieve complete recovery, digestive fluid extraction provides an estimate of the amount of contaminant solubilized in vivo by digestive processes and thus available for subsequent absorption and bioaccumulation. The fraction of contaminant extracted by digestive fluid is far less than that extracted by conventional organic solvents or strong acids, and far more than is extractable by water [5]. In some instances, a weak acid extraction may yield comparable trace metal solubilization as digestive fluid, although mechanistically the two approaches have little in common because deposit-feeder digestive fluid is often near neutral pH [5]. Extraction with the digestive fluid of deposit-feeding polychaetes (Arenicola brasiliensis or Arenicola marina) has been shown to be a good predictor of polycyclic aromatic hydrocarbon (PAH) bioaccumulation by the polychaete A. brasiliensis [1] and mercury bioaccumulation by the amphipod Leptocheirus plumulosus [3].
This study was designed to compare the relative bioavailability and bioaccumulation of contaminants from a variety of sediments by means of digestive fluid extraction with estimates of these parameters obtained from traditional 28-d bioaccumulation tests with M. nasuta. Thirteen in situ-contaminated estuarine sediments were assessed by both procedures, and the ability of digestive fluid extraction to predict contaminant uptake by M. nasuta was evaluated with respect to PAHs, polychlorinated biphenyls (PCBs), and eight trace metals.
MATERIALS AND METHODS
Sediment collection
Sediments were collected in 1997 at the former Naval Air Station Alameda (now known as Alameda Point) in San Francisco Bay (CA, USA), which was in the process of conversion to civilian use. Two areas of the base, Seaplane Lagoon and the Landfill Wetlands, were evaluated. Seaplane Lagoon is an embayment of San Francisco Bay and the location of two large outfalls that discharged industrial wastewater and storm runoff from the base [6]. The harbor areas in front of both outfalls were sampled, and designated as East Lagoon and West Lagoon. The second area on the base, known as the Landfill Wetlands, was the site of past solid waste disposal but is now a wetland with no visible evidence of historical disposal. Sediments of three ponds at the site (designated as wetland ponds 1, 2, and 3) were sampled. Wetland pond 1 is connected to San Francisco Bay via a culvert. Wetland ponds 2 and 3 have no surface connection with the Bay and vary seasonally between hypersaline and fresh depending on rainfall.
The East and West Lagoon sites were sampled with both a 2-m gravity corer and a 0.025-m2 Ponar grab (Wildco Instruments, Saginaw, MI, USA). The gravity corer penetrated 0.8 to 1.1 m into the substratum, and the entire core length was homogenized before any testing. The Ponar grab penetrated to a depth of 10 cm, and six grabs were composited to create each sample. The wetland ponds were sampled by hand with a 10-cm-diameter core tube pushed into the substratum approximately 15 cm, and 10 cores were composited to create each sample.
Two reference sites were also sampled to put data from the naval base in perspective to locations elsewhere in San Francisco Bay. The first site (Central Bay) was at the entrance to the base's navigation channel and was considered representative of deep waters of San Francisco Bay distant from point sources. The second site, the public marina in Berkeley (CA, USA) was selected because Seaplane Lagoon is most likely to be developed as a marina, and we wanted to compare contaminant levels and bioavailability in lagoon sediments to a nearby, comparable-use facility. Finally, a control sediment was collected from Half Moon Bay along the Pacific Coast south of San Francisco. Macoma nasuta used in bioaccumulation testing also were collected from this location. The bioaccumulation tests were done in two batches and a control was included in each batch. Therefore, data are presented for two control sediments from the same location but collected a few months apart.
Bio accumulation testing
Bioaccumulation testing generally followed the protocol of Lee et al. [7]. Macoma nasuta were held in the laboratory for 10 d in their home sediment (Half Moon Bay), and then 12 to 13 clams were placed in 10-L glass aquaria containing a 4-cm layer of test sediment and 4 L of 1-μm-filtered water (from Bodega Bay, CA, USA). Each sediment was tested in three replicate aquaria. Aquaria were continuously aerated and water was replaced three times per week. Temperature was maintained at 13°C, and the overlying water salinity was 35 practical salinity units (psu). Wetland ponds 2 and 3 were hypersaline (84 and 40 psu, respectively), so interstitial water was reduced to 30 psu by addition of distilled water before bioaccumulation testing and digestive fluid extraction. Interstitial water of all other sediments tested ranged from 28 to 35 psu, and these sediments were used without salinity adjustment.
In an initial test of core samples from the West Lagoon area, about 70% of the exposed clams died within two weeks, and the test was terminated. These West Lagoon sediments were mixed with control sediments in the ratio of six parts control sediment to one part West Lagoon sediment (on a dry wt basis). Mortality rate in the diluted sediments was still high (mean of 27% at 28 d), but enough clams survived to allow measurement of bioaccumulation. In reporting results, the undiluted sediments are designated as West Lagoon cores 1, 2, and 3; the diluted sediments are given a b suffix (i.e., West Lagoon cores 1b, 2b, and 3b).
After the 28-d exposure, clams were placed in the control sediment for 24 h to allow gut evacuation and then frozen until analysis. Clams from each aquarium were composited, but each of the three replicate aquaria was analyzed independently.
Digestive fluid extraction
Digestive fluid was obtained from the deposit-feeding polychaete A. brasiliensis, which was collected intertidally near San Francisco. Worms were held in seawater without sediment for 24 h to evacuate their guts. The digestive fluids were recovered by puncturing the gut wall with a pipette and withdrawing about 1 ml of fluid per individual. The fluid from 140 individuals was composited, centrifuged at 160 g for 5 min to remove residual sediment, and stored at −80°C until use.
Digestive fluid extractions were done at the completion of the bioaccumulation tests, with sediment subsamples that had been held at 4°C since the beginning of the bioaccumulation tests. Sediment aliquots of 1.2 g wet weight were placed in glass centrifuge tubes along with 2 ml of digestive fluid, and two replicate extractions were done for each sediment sample. Extractions used wet sediment to avoid changes in bioavailability relating to drying of sediments, but the 1.2 g of wet sediment corresponded to an average of 0.6 g of dry sediment (range, 0.33–0.95 g), given the water content of the sediments tested. The tubes were vortexed for 10 s and placed on a reciprocating shaker table for 3 h in the dark. Twice during the extraction period, the samples were removed from the shaker table, briefly vortexed, and then returned to the shaker table. After the extractions were complete, the tubes were centrifuged at 4,000 g for 10 min, and the supernatant was frozen at −20°C until analysis.
Digestive fluid of A. brasiliensis is known to contain high concentrations of some metals even in individuals from relatively pristine areas [8], so an aliquot of the digestive fluid was analyzed for preexisting contaminants before test sediment extraction. These values were subtracted from postextraction concentrations when calculating the percentage of contaminant solubilized.
To determine if solubilization of the contaminants of interest was constrained by saturation of the digestive fluid, one of the most contaminated sediments (West Lagoon core 1b) was used for a series of extractions with varying sediment to digestive fluid ratios. The amounts of West Lagoon core 1b wet sediment (0.45–1.2 g wet wt; equivalent to 0.37–0.96 g dry wt) and digestive fluid (1–6 ml) were varied to obtain dry sediment to fluid ratios of 0.06 to 0.96, a range that included the ratios of 0.17 to 0.48 g dry sediment/ml used in extractions of all other sediments.
Chemical analyses
Trace organic and metal analyses were conducted at the Skidaway Institute of Oceanography (Savannah, GA, USA) with methods given in Maruya et al. [9] and Smith and Maruya [10]. All organic solvents used in the extraction and analysis of trace organic analytes were pesticide grade or better (Optima grade, Fisher Scientific, Fair Lawn, NJ, USA). Silica gel and Florisil were chromatography grade (Fisher Scientific). Both PAH and PCB standards for instrument calibration were purchased from the National Institute of Standards and Technology (Gaithersburg, MD, USA). Surrogate (recovery) standards (>99% purity) were purchased from ULTRA Scientific (North Kingstown, RI, USA). Reagent and instrument gases were of high-purity grade or better (>99.99% pure). All acids and other chemical reagents were American Chemical Society grade or better. Deionized water was double glass distilled before use. All glassware was detergent washed, rinsed with tap water, air or oven dried, and fired in a ceramic kiln at approximately 550°C overnight.
For analysis of organic compounds, sediment samples were freeze-dried and wet tissue samples were homogenized with kiln-fired Na2SO4 before extraction. Sediment and tissue samples were Soxhlet (Millville, NJ, USA) extracted with 400 ml of CH2Cl2 for more than 16 h and Soxhlet extracts were reduced and exchanged to hexane in a glass flask-Snyder column apparatus (LABGLASS, Vineland, NJ, USA) placed in a heated water bath. Sediment extracts were treated for sulfur with acid-activated copper powder and fractionated on 2.0 g of silica gel activated at 130°C overnight and slurry packed with hexane into a glass chromatography column. Polar and lipid interferences in tissue extracts were removed with Florisil (18.0 g, 1.0% water deactivated) column chromatography. Two fractions of increasing polarity were collected. More than 99.9% of the PCB eluted in the first (nonpolar) fraction with pure hexane and the majority of PAHs eluted in the second (slightly polar) fraction with a mixture of CH2Cl2 and hexane (with the remainder of the PAH eluting in fraction 1). Extracts were reduced and exchanged to hexane with a TurboVap II (Zymark, Hopkington, MA, USA).
Digestive fluid samples (∼2 ml) were transferred into solvent-rinsed glass graduated tubes, spiked with recovery surrogates, and extracted twice sequentially. The first extraction solvent mixture was 3.2 ml of CH2Cl2 and 2.4 ml of methanol and the second mixture was 2.4 ml each of CH2Cl2 and methanol. The tubes were shaken vigorously for several minutes and centrifuged at 10,000 rpm for 5 min. The bottom (CH2Cl2) layers from each extraction aliquot were combined, gently evaporated to near dryness with N2, and then brought up with 1 ml of hexane for gas chromatography (GC) analysis. Extracts were analyzed for PCBs with a Varian 3400CX gas chromatograph with dual electron-capture detectors (ECDs). Extracts were analyzed for PAHs with a Varian 3400CX gas chromatograph coupled to a Saturn 3 ion trap mass spectrometer (GC-MS, Varian, Walnut Creek, CA, USA). The GC-ECD columns were 30 m × 0.25-mm DB-5 and DB-1701. The GCMS column was a DB-XLB of similar dimensions. All analytical columns were purchased from J&W Scientific (Folsom, CA, USA).
The following 36 PCB congeners (some as coeluting pairs) were quantified and their sum was reported as total PCBs: 8, 18, 28, 29, 44, 50, 52, 66/95, 77/110, 87, 90/101, 105, 118, 126, 128, 138/163/164, 153/132, 154, 170/190, 180, 187/182/159, 188, 194, 195, 201, 206, and 209 (congener numbers follow numbering convention of International Union of Pure and Applied Chemistry). The following PAH analytes were quantified, with their sum reported as total low molecular weight PAH (LPAH): naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, biphenyl, 2,6-dimethylnaphthalene, acenaphthylene, acenaphthene, 2,3,5-trimethylnaphthalene, fluorene, phenanthrene, anthracene, and 1-methylphenanthrene. The following PAH analytes were quantified, with their sum reported as total high molecular weight PAH (HPAH): fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, perylene, indeno[1,2,3-c,d]pyrene, dibenz-[a,h]anthracene, and benzo[g,h,i]perylene.
Throughout the text, only class sums are reported (ΣLPAH, ΣHPAH and ΣPCB). In obtaining class sums, class constituents that were undetected or present below the method detection limit (MDL) were treated as zero. Sediment and tissue MDLs were approximately 0.1 μg/kg for PCBs and approximately 3 μg/kg for PAHs. The MDLs for digestive fluid were approximately 0.001 μg/ml for PCBs and approximately 0.005 μg/ml for PAHs.
Trace metal analytes included zinc (Zn), arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and mercury (Hg). Approximately 250 mg of dry sediment was extracted in an acid-cleaned Teflon® beaker with 10 ml each of HNO3 and HF and 5 ml of HClO4. After equilibrating overnight the samples were heated on a hot plate at 120°C just until dryness. The residue was dried twice more after adding 1 ml of HNO3 to drive off chlorides. The resulting residue was dissolved in 20 ml of 1% HNO3.
Approximately 0.5 g of freeze-dried tissue or an equivalent wet tissue was digested with 6 ml of nitric acid with a Teflon bomb-microwave oven (CEM model MDS-2100, CEM, Matthews, NC, USA). The resulting solution was diluted to 20 ml. A 0.5-ml aliquot of digestive fluid was treated with 6 ml of nitric acid as described for tissue samples. The resulting solution was diluted to 20 ml.
Analyses were done by inductively coupled plasma-mass spectroscopy (ICP-MS). The ICP-mass spectrometer was a VG Elemental PQII Plus equipped with a Gilson model 222 autosampler and a high-performance interface (VG Elemental, Cambridge, UK). Mercury was analyzed by isotope dilution and detection by ICP-MS as described by Smith [11]. Sediment and tissue MDLs were approximately 1 μg/g for most metals; MDLs for digestive fluid were 0.002 μg/ml for Hg and approximately 1 μg/ml for other metals.
A comprehensive quality assurance-quality control program was instituted and included the analysis of procedural blanks, spiked matrices, and standard reference materials; guidelines for initial and continuing instrument calibration; and requirements for the recovery of surrogate compounds spiked into each sample before extraction. Some analytes were detectable in procedural blanks; however, their concentrations were less than the reported MDL in all cases. Recoveries of target analytes in National Institute of Standards and Technology standard reference materials 1941a (sediment) and 1974a (bivalve tissue) ranged between 78 ± 17% for PAHs in M. nasuta tissue to 102 ± 14% for PAHs in sediment. Surrogate recoveries (perdeuterated PAH and dibromooctafluorobiphenyl) ranged between 63 ± 11% and 98 ± 19% for dibromooctafluorobiphenyl in sediment and digestive fluid samples, respectively. Initial instrument calibration was based on a five-point calibration curve with a minimum correlation coefficient (r) of 0.99 for all analytes. Instrument calibration remained within ±15% of the initial calibration based on daily injections of a midlevel continuing calibration standard.
Total organic carbon was measured on a Fisons NA1500 Elemental Analyzer (Fisons Instruments, Milan, Italy), after acid vapor treatment to remove inorganic carbon. Grain size distribution was determined by wet sieving on stacked sieves with the pan weight (< 63μg) reported as total silt and clays.
RESULTS
Sediment characteristics
Alameda Point test sediments were generally sandy mud with organic carbon contents typically between 1 and 4% (Table 1). Reference sites elsewhere in San Francisco Bay were comparable in their silt and clay content, although they contained somewhat less organic carbon (∼1%). Two exceptions to the generally muddy character of the sediments were wetland pond 3 and the control sediments from the Half Moon Bay home site of M. nasuta. Both areas were sandy, with about 0.4% organic carbon.
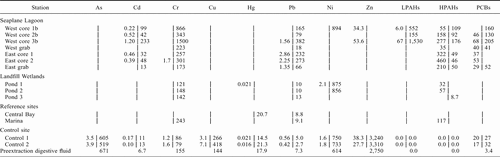
| Station | TOC (%) | Silt and clay (%) | As | Cd | Cr | Cu | Hg | Pb | Ni | Zn | LPAHs | HPAHs | PCBs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seaplane Lagoon | |||||||||||||
| West core 1 | 4.29 | 30 | 15 | 585 | 2,850 | 239 | 1.59 | 1,240 | 119 | 1,330 | 23,400 | 6,210 | 4,480 |
| West core 2 | 2.93 | 68 | 11 | 208 | 959 | 135 | 0.89 | 413 | 124 | 672 | 8,470 | 6,640 | 2,310 |
| West core 3 | 2.80 | 25 | 25 | 764 | 1,940 | 216 | 1.78 | 860 | 115 | 1,100 | 33,300 | 13,800 | 7,660 |
| West core 1b | 1.11 | 16 | 8 | 82 | 355 | 40 | 0.26 | 238 | 42 | 179 | 3,020 | 1,530 | 647 |
| West core 2b | 0.85 | 23 | 6 | 32 | 161 | 26 | 0.14 | 76 | 44 | 116 | 1,110 | 1,360 | 336 |
| West core 3b | 0.87 | 16 | 21 | 97 | 394 | 30 | 0.17 | 128 | 59 | 209 | 4,780 | 2,170 | 1,100 |
| West grab | 1.69 | 83 | 9 | 2 | 146 | 58 | 0.52 | 57 | 78 | 148 | 226 | 2,600 | 135 |
| East core 1 | 3.08 | 57 | 9 | 49 | 223 | 81 | 0.76 | 216 | 89 | 207 | 846 | 5,680 | 530 |
| East core 2 | 2.50 | 65 | 10 | 38 | 232 | 82 | 0.69 | 181 | 91 | 193 | 256 | 3,640 | 434 |
| East grab | 2.56 | 51 | 10 | 18 | 193 | 102 | 0.94 | 223 | 83 | 260 | 453 | 6,830 | 314 |
| Landfill Wetlands | |||||||||||||
| Pond 1 | 3.05 | 51 | 11 | 1.6 | 145 | 69 | 0.52 | 54 | 93 | 173 | 215 | 1,180 | 111 |
| Pond 2 | 2.66 | 60 | 8 | 1.6 | 118 | 52 | 0.52 | 49 | 78 | 142 | 339 | 2,340 | 122 |
| Pond 3 | 0.35 | 4 | 2 | 0.2 | 55 | 10 | 0.09 | 9 | 31 | 28 | 357 | 104 | 11.2 |
| Reference sites | |||||||||||||
| Central Bay | 0.96 | 56 | 11 | 0.4 | 115 | 34 | 0.21 | 20 | 66 | 89 | 285 | 1,740 | 34.1 |
| Marina | 1.58 | 90 | 16 | 0.5 | 149 | 70 | 0.35 | 37 | 100 | 152 | 255 | 2,450 | 34.8 |
| Control site | |||||||||||||
| Control 1 | 0.47 | 12 | 10 | 0.4 | 49 | 12 | 0.02 | 11 | 34 | 45 | 16 | 230 | 8.5 |
| Control 2 | 0.38 | 13 | 7 | 0.2 | 53 | 10 | 0.02 | 10 | 34 | 41 | 90 | 270 | 6.7 |
- a LPAH = low molecular weight polycyclic aromatic hydrocarbon; HPAH = high molecular weight polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyl.
Compared to contaminant concentrations at the Central Bay and Marina reference sites, all analytes except As and Ni showed substantial increases in sediment concentrations in Seaplane Lagoon (Table 1). The most dramatic increases were seen for Cd (∼1,000-fold higher in Seaplane Lagoon relative to reference sites) and PCB and LPAH (both about 100-fold greater). The west area of Seaplane Lagoon was clearly the most contaminated area for all analytes. West Lagoon Cd concentrations reached 764 mg/kg and PCB concentrations reached 7,660 μg/kg. After dilution with control sediment, the contaminant concentrations of the diluted West Lagoon core samples were comparable to the undiluted sediments from East Seaplane Lagoon, although concentrations in both sets of Seaplane Lagoon samples were still well above those of the reference areas. The West Lagoon grab sample, which represented only the top 10 cm of the sediment column, was far less contaminated than the West Lagoon core samples, which contained sediments collected to about 100 cm depth, suggesting that the deeply buried material was more contaminated than nearsurface sediments. Concentrations in the deeply buried material would have been even higher than shown in Table 1 had the deep material not been diluted by the cleaner surface material during homogenization of the core samples. The buried material was visibly contaminated by oil and contained paint chips, which comprised about 4% of the mass of some samples.
Landfill Wetlands sediments generally showed little increase in contaminant levels above the reference sites. Only Hg and PCB showed any appreciable increase (two- to threefold), and even these elevated levels were still well below concentrations seen in Seaplane Lagoon. Wetland pond 3 was the least contaminated of the Landfill Wetland sites, presumably because of the sandy sediment that was low in organic carbon.
Control sediments were the least contaminated of all sediments tested, as would be expected given their sandy character and distance from significant pollutant point sources.
Bioaccumulation and digestive fluid extraction results
West Lagoon core sediments retained some toxicity to M. nasuta even after dilution with control sediments. Over the 28-d exposure period, mortality rates ranged from 6% (West Lagoon core 1b) to 48% (West Lagoon core 3b). In comparison, mortality in all samples other than West Lagoon area cores was limited to a single individual in one control aquarium.
The control sediments used were from the M. nasuta collection site in Half Moon Bay. After the 28-d laboratory exposure to control sediment, body burdens for all analytes were similar to concentrations found in the clams at time of collection, indicating no unintentional contaminant exposure during collection and holding (Table 2; initial concentration data not shown). Two control sediment exposures were done; tissue concentrations attained in both were very similar.
Body burdens attained upon exposure to test sediments are shown in Table 2 for those analytes at stations where tissue concentrations exceeded the control. We considered the test sediment body burdens to exceed the control if the distribution of the replicates was completely nonoverlapping (i.e., the highest body burden among the control replicates was less than the lowest body burden among the test sediment replicates). Two contaminants did not exceed control tissue levels in any test sediment (As and Cu), and five contaminants exceeded control tissue levels only slightly in a few sediments (Cr, Hg, Ni, Zn, and LPAHs). Substantial increases in body burdens relative to the controls were only seen for Cd, Pb, HPAHs, and PCBs, and the increases were largely limited to Seaplane Lagoon. Exposure to sediments from the Landfill Wetlands and reference sites rarely led to any increase in body burdens relative to the controls.
- a LPAH = low molecular weight polycyclic aromatic hydrocarbon; HPAH = high molecular weight polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyl.
| Contaminant | Seaplane Lagoon | Landfill Wetlands | Reference sites | Controls |
|---|---|---|---|---|
| As | 1.2 | <0 | 1.2 | 0.5 |
| (<0–3.1) | (<0-<0) | (<0–2.4) | (<0–0.9) | |
| Cd | 0.6 | 1.4 | 1.9 | 5.8 |
| (0.3–1.5) | (0.3–2.8) | (1.1–2.7) | (4.0–7.6) | |
| Cr | 0.5 | 0.2 | 0.4 | <0 |
| (0.2–0.9) | (0.1–0.3) | (0.0–0.7) | (<0-<0) | |
| Cu | 1.1 | 0.3 | 1.1 | 4.9 |
| (0.2–1.9) | (0.0–0.9) | (0.8–1.3) | (3.1–6.6) | |
| Hg | 1.6 | 4.9 | 0.2 | 0.0 |
| (<0–9.5) | (<0–14.6) | (<0–0.3) | ||
| Ni | 1.6 | 3.4 | 1.3 | 1.6 |
| (0.6–2.3) | (2.6–4.5) | (0.6–1.9) | (1.4–1.7) | |
| Pb | 0.4 | 0.1 | 0.1 | <0 |
| (0.2–0.8) | (0.1–0.2) | (0.1–0.1) | (<0-<0) | |
| Zn | 2.2 | 2.2 | 1.6 | 5.1 |
| (1.2–3.9) | (0.5–4.6) | (0.1–3.0) | (5.1–5.1) | |
| LPAHs | 25.2 | 0.0 | 0.0 | 0.0 |
| (0.0–81.2) | (0.0–0.0) | (0.0–0.0) | (0.0–0.0) | |
| HPAHs | 11.7 | 7.3 | 0.6 | 0.0 |
| (0.0–23.3) | (0.0–21.9) | (0.0–1.2) | (0.0–0.0) | |
| PCBs | 81.2 | Data not presented | Data not presented | Data not presented |
| (23–194) |
- a LPAH = low molecular weight polycyclic aromatic hydrocarbon; HPAH = high molecular weight polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyl.
Contaminant concentrations attained in digestive fluid after extraction of test sediments also are shown in Table 2 for those analytes at stations where digestive fluid concentrations exceeded the control (i.e., both digestive fluid replicates were higher than both control replicates). Good agreement with bioaccumulation results is evident. Digestive fluid extracted little or no additional As, Cu, Hg, Ni, and Zn from test sediment above the amounts extracted from the control sediments. Increases occurred in digestive fluid concentrations of Cd, Pb, LPAHs, HPAHs, and PCBs after extraction of test sediments but largely only in Seaplane Lagoon. The one noteworthy inconsistency between M. nasuta bioaccumulation and digestive fluid extraction is evident in the Cr results. Extraction of every test sediments resulted in Cr concentrations in the digestive fluid that were greater than from the control. In contrast, Cr bioaccumulation by M. nasuta in excess of the control was seen for only one sediment (East Lagoon core 2) and even this increase was minimal.
Digestive fluid extraction also was compared to the total amount of contaminant initially in the sediment (Table 3). For any given metal, the vast majority of the sediment-associated fraction was not extractable by digestive fluid, with solubilization efficiencies typically only 0 to 2%. Much higher extraction efficiencies were observed for organic compounds in some instances. Both LPAH and HPAH extraction from Seaplane Lagoon sediments averaged 25.2 and 11.7%, respectively, largely because of high values from the diluted West Lagoon core samples. The PCB extraction efficiencies are shown in Table 3 only for the Seaplane Lagoon sediments where PCB concentrations in digestive fluid were highest. In the other areas, PCB sediment concentrations were relatively low (< 120 μg/kg) and, thus, very few PCB congeners were quantifiable in digestive fluid after extraction of these sediments. In Seaplane Lagoon, an average of 81% of the PCB was solubilized, although this value is inflated by two of the seven samples in which extraction efficiencies exceeded 100% (129 and 194%). These values greater than 100% were obtained in extraction of some of the least PCB-contaminated sediments of the Seaplane Lagoon sites (West Lagoon grab, 135 μg/kg; West Lagoon core 2b, 336 μg/kg) and again reflect the difficulties of quantifying low levels of PCBs. Excluding these two values, the PCB extraction efficiency from the remaining five Seaplane Lagoon sediments averaged 49%.
Arsenic and Hg extractions were noteworthy in that their concentrations in digestive fluid after sediment extraction were often less than their initial concentrations in the fluid (671 and 17.9 ng/ml for As and Hg, respectively). Negative solubilization values (a decrease in concentrations in the fluids after extraction) were measured for As in one or both replicates of 13 out of 14 sediments extracted, and for Hg in one or both replicates of 12 out of the 14 sediments. Negative solubilization values were rare for all other analytes.
Digestive fluid saturation
Desorption of the PAHs by digestive fluid from sediments highly contaminated with PAHs has been shown to be limited by saturation of the fluid [12]. Test sediments in our study had two orders of magnitude less PAHs than those of Voparil and Mayer [12], but to test if saturation was attained for any of the analytes, an extraction series was done in which the sediment to fluid ratio was manipulated. The effect on contaminant desorption of manipulating this ratio is shown (Fig. 1) for all analytes for which appreciable extraction occurred (Table 2; Cd, Cr, Pb, PAHs, and PCBs). West Lagoon core 1b was used in this extraction series and should be considered a worst-case sediment because it had the highest concentration of Pb, the second highest concentrations of Cd, Cr, PCBs, and the fourth highest concentration of PAHs.
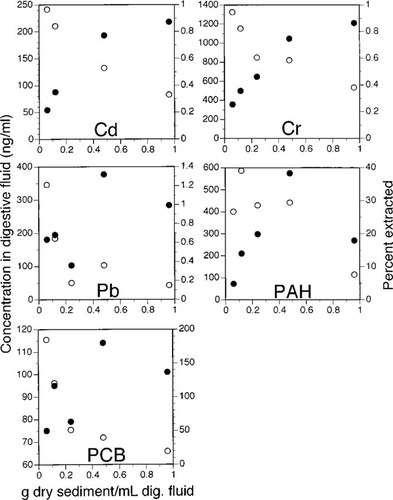
The effect of manipulating the sediment to fluid ratio used in digestive fluid extractions on the contaminant concentration in digestive fluid (filled circles) or the percentage of contaminant solubilized (open circles). An average ratio of 0.30 (range, 0.17–0.48) was used in all other extractions throughout this study; PAH = polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyl.
No saturation of the fluid was evident with respect to Cd. Cr, and PAHs up to a sediment to fluid ratio of 0.48, the maximum used in other extractions in this study. An increase in the amount of sediment led to an increase in the concentrations attained in digestive fluid (Fig. 1). For PCBs and Pb, the relationship was less obvious, but analysis of the data is suggestive of a similar trend. For most contaminants, an apparent leveling off or decreasing of concentrations occurred at the highest sediment to fluid ratio (0.96), suggesting either an approach to saturation of the relevant ligands or other alternatives discussed further below.
Even though the fluid was not saturated for all or most extractions in these series, a decrease in extraction efficiency with increasing sediment to fluid ratios does seem to occur, beginning even at the lowest ratio. For example, the proportion of Cd and Cr extracted decreased from about 1% to 0.4% over the range of ratios tested. The PCB extraction efficiencies, although in some cases elevated because of the analytical difficulties discussed earlier, also decreased with increasing sediment to fluid ratios.
Comparison of digestive fluid with bivalve bioaccumulation
The ability of in vitro extractions to predict relative differences in bioaccumulation by M. nasuta among the sediments was evaluated by testing correlations (Pearson product-moment) between digestive fluid concentrations attained after sediment extraction and tissue concentrations achieved at the end of the 28-d exposure period (Fig. 2). This comparison was done for the contaminants that showed increased concentrations in both fluid and tissue (Cd, Pb, HPAHs, and PCBs). All sediments tested were included in correlations for Cd, Pb, and HPAHs. For PCBs, the correlation analysis was limited to sediments from the Seaplane Lagoon site because of difficulties in reliably quantifying the low PCB concentrations in digestive fluid extractions from the other sites. For two of the four contaminants, Cd and Pb, a highly significant (p < 0.01) correlation was found between concentrations in digestive fluid after extraction of Seaplane Lagoon sediments and the tissue concentrations reached in M. nasuta after a 28-d exposure to these same sediments. For HPAHs, the relationship was suggestive, but not significant at the α < 0.05 level. For PCBs, for which demonstration of significance was made more difficult by elimination of the least contaminated samples and the reduced sample size, the relationship was not significant.
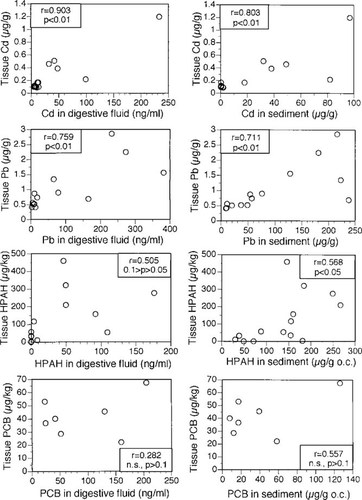
The relationship between contaminant concentration in digestive fluid and Macoma nasuta tissue as alternative measures of bioaccumulation potential. Data points are all sediments tested for Cd. Pb, and PAHs, and the seven sediments from Seaplane Lagoon (San Francisco Bay, CA, USA) tested for PCBs. Significance was tested by Pearson's product-moment correlation. For comparison, correlations of body burdens with bulk sediment concentrations are also shown. PAH = polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyl; HPAH = high molecular weight polycyclic aromatic hydrocarbon.
For purposes of comparison, Figure 2 also illustrates the correlations between contaminant concentrations in the bulk sediment and tissue concentrations in M. nasuta after the bioaccumulation test for the same four contaminants that showed appreciable bioaccumulation (Cd, Pb, HPAHs, and PCBs). Sediment concentrations for the organic contaminants were organic carbon-normalized for this analysis, in recognition of the strong role that organic carbon plays in mediating their bioaccumulation [13]. Sediment concentrations produced slightly worse correlations with body burden for the trace metals; they were slightly improved for the organic compounds. However, overall, the value of the bulk sediment concentrations in predicting bioaccumulation was comparable to that of the digestive fluid.
DISCUSSION
Comparison of digestive fluid extraction and traditional bioaccumulation testing
The primary value of the digestive fluid extraction procedure in this data set was identification of those contaminants that potentially were available for bioaccumulation. Concentrations of contaminants such as Cu, Hg, and Zn were increased at some of the sampling sites by 20- to 90-fold relative to control locations, yet the inability to extract these substances in digestive fluid suggested that they were not bioavailable, and, in fact, no bioaccumulation by M. nasuta was observed. The in vitro extraction identified Cd, Cr, Pb, HPAHs, and PCBs as the potentially bioavailable contaminants in the majority of the samples. Macoma bioaccumulation results supported this conclusion, with the exception of Cr. Digestive fluid extraction provides an indication of the potential for desorption in the gut environment, generally a necessary step for bioaccumulation, but it does not insure that solubilized contaminants will be subsequently absorbed. We believe that the absence of Cr bioaccumulation by M. nasuta was due to unique constraints on dietary absorption of that metal (discussed further below).
For the four contaminants that were bioaccumulated by M. nasuta (Cd, Pb, HPAHs, and PCBs), a brief extraction in digestive fluid was a good predictor of bioaccumulation for two (Cd and Pb), and of marginal value for a third (HPAHs). However, bulk sediment concentrations were of essentially equal value in predicting bioaccumulation as the digestive fluid extraction. This result implies that bioavailability differences were minimal among the sediments (i.e., bioaccumulation factor approaching a constant). The correlations between Macoma bioaccumulation and either digestive fluid or sediment contaminant concentrations were driven largely by the five to six most contaminated sites, generally in Seaplane Lagoon. All the Lagoon sites were within 0.5 km of one another, thus minimizing differences in sediment minerology, organic matter quality, and other factors that could have led to more pronounced bioavailability differences among the sediments. A more heterogeneous suite of contaminated sediments may have provided a poorer correlation between sediment concentration and body burden, and a better test of digestive fluid extraction.
The concordance between the results of digestive fluid extraction and Macoma bioaccumulation in estimating the bioavailability and bioaccumulation potential of many analytes is notable because digestive fluid from a polychaete was useful in predicting contaminant uptake by a bivalve. Bivalves, including M. nasuta, are capable of digestively processing ingested particles by two mechanisms [14, 15]. Some particles transit the intestine and are subject to extracellular digestion in the gut lumen (intestinal pathway). Other particles are routed to the digestive gland, where they may be digested intracellularly (glandular pathway). The in vitro digestive fluid extraction technique most obviously mimics the extracellular digestive processes operating in the gut lumen, but the technique was predictive of bioaccumulation in spite of the multiple digestive pathways utilized by bivalves.
Taxa vary widely in how they perceive the bioavailability of sediment-associated contaminants because of differences in the ability of their digestive fluids to solubilize particle-bound contaminants in the gut [5]. Therefore, neither bioaccumulation by M. nasuta nor concentration attained in an in vitro digestive fluid extraction provides a single best measure of absolute bioavailability. However, when comparing multiple sediments, relative differences among the sediments seem robust across taxa. Relative bioavailability estimates across six sediments were consistent whether extractions were done with A. brasiliensis digestive fluid or fluids from the echiuran Urechis caupo [16]. Measures of methylmercury bioavailability based on polychaete (A. marina) digestive fluid extraction and bioaccumulation by an amphipod (Leptocheirus plumulosus) were consistent across a range of sediment organic contents [3]. Therefore, we have employed digestive fluid to evaluate sediments in a relative or correlative sense (herein and in Western and Mayer [1]). Far more validation would be necessary before in vitro extraction could be used to predict absolute body burden in any taxon because of differences among taxa in contaminant solubilization within the gut [5] and taxonspecific biotransformation or elimination capabilities.
Bioavailability, as measured by the proportion of extractable contaminant, was low for all trace metals, generally in the range of 0 to 2%. Extraction efficiencies of metals from Alameda Point sediments were slightly lower, but not dramatically different, than previously reported extraction efficiencies with digestive fluid from another arenciolid polychaete, Arenicola marina, (∼10% for Cu [17, 18]; 1–5% for inorganic Hg [3]). The digestive fluids of A. brasiliensis and many other invertebrates previously have been shown to have a near-neutral pH, and have been shown to be capable of extracting only a small fraction of the metal that would be obtained through a conventional strong-acid extraction [5]. Low extraction efficiencies also may be attributable to the chemical form of the metals in Seaplane Lagoon sediments. The core sediments were anoxic, and some of the trace metals at the site (Cd and Zn, but not Pb and Cr) were present primarily in monosulfide phases [19]. Such metal sulfides are relatively resistant to extraction by digestive fluids [4]. In addition, West Lagoon core sediments contained large quantities of paint chips, from which metals may be less biologically extractable than from natural sediments.
Confounding factors in interpretation of extraction results
The most striking discrepancy between bioaccumulation by M. nasuta and solubilization by digestive fluid was seen for Cr. Digestive fluid extraction of most sediments tested yielded higher fluid Cr concentration than did extraction of control sediment. In extraction of the West Lagoon core 3b sediment, the digestive fluid concentration was nearly 20-fold greater than achieved in the control sediment extraction. However, bioaccumulation of Cr to levels that exceeded the control was observed in only one sediment (East Lagoon core 2), and even there the increase in tissue concentrations was minimal. We suspect this disparity is due to poor gut absorption of Cr and the inability of the in vitro solubilization technique to account for absorptive constraints on bioaccumulation. X-ray absorption spectroscopy has shown that the Cr of Alameda Point sediments was present as Cr3+ [19]. Because of its poor digestive absorption in most animals, Cr3+ is widely used as a conservative tracer in digestive uptake studies [20]. Assimilation efficiencies for Cr in aquatic invertebrates are the lowest of all metals that have been evaluated [21]. Negligible Cr3+ absorption from intestinal contents occurs in Macoma balthica, and appreciable absorption of Cr3+ occurs only from that portion of ingested material routed through the glandular digestive pathway [14, 22]. Absorption of Cr3+ from sediments by M. balthica has been estimated to be only 1 to 3%, the lowest of several metals studied [22, 23]. When intestinal solubilization is correlated with assimilation, as has been shown for some metals in bivalves [24], digestive fluid solubilization may be predictive of bioaccumulation, but it is not applicable to Cr3+ or other contaminants for which absorption rather than solubilization is likely to limit uptake.
Neither Hg nor As was accumulated appreciably by M. nasuta, and the digestive fluid after sediment extraction had lower Hg and As concentrations than before sediment extraction. Rather than the expected desorption, adsorption was on to the sediment of the Hg and As that were previously present in the fluid. Why negative solubilization values were generally limited to Hg and As is not clear from our results. Sediment adsorption of solubilized metal in A. marina digestive fluid has previously been reported for Hg [3], and has been reported for Cd, Cu, and Pb with digestive fluid of both A. marina and two echinoderms [4]. Our observations were limited to a single time point after 3 h of extraction because previous studies with this procedure have used extractions lasting 2 to 4 h [1, 3-5, 12, 16-18]. However, kinetic studies have sometimes shown desorption of metal from the sediments in the early phase of the extraction (∼1 h), then readsorption as the extraction continues. This phenomenon has been attributed to the formation of surface-reactive metal-ligand complexes and either the precipitation of these complexes or their adsorption to sediment by hydrophobic or electrostatic mechanisms [4]. In such instances, this phenomenon has resulted in an underestimate of the initial metal solubilization if extraction efficiency was reported based on a single later time point, but it has, as in our study, sometimes led to reported negative solubilization efficiencies.
In evaluating sediments by digestive fluid extraction, the potential for fluid saturation must be considered, particularly when using highly contaminated sediments. In our experiments in which the sediment to fluid ratio was manipulated with one of the most contaminated sediments, no indication was found that saturation had occurred up to a 0.5 sediment to fluid ratio, well above the more typical 0.3 ratio in all other extractions. However, at greater sediment to fluid ratios, we observed that contaminant concentration in the fluid was relatively independent of the sediment to fluid ratio, a condition one would want to avoid if the concentration in the extractant is to be used as a measure of risk. We have referred to this condition as saturation, and in fact it may have been due to complete utilization of ligands such as the surfactant micelles and large proteins with hydrophobic regions that are important solubilizing agents for hydrophobic compounds [12], or proteinaceous materials capable of acting as ligands for trace metal complexation [8, 18]. However, other mechanistic explanations are equally possible. As the sediment to fluid ratio used in extractions rises, it is increasingly difficult to maintain a slurry of suspended sediment. Thorough and constant mixing of sediment and fluid is hindered, which potentially contributed to a reduced extraction efficiency. Alternatively, equilibrium partitioning can result in dissolved phase concentrations that are relatively insensitive to changes in the sediment to fluid ratio when only a small fraction of the total contaminant is desorbed.
Given the dependence of extraction efficiency on sediment to fluid ratios, this result indicates a need to keep the sediment to fluid ratio constant when comparing multiple sediments. In our comparative extractions, we maintained the wet sediment to fluid ratio constant at 0.6, but differences in water contents resulted in the dry sediment to fluid ratio varying from about 0.2 to 0.5. In the future, adjusting the amount of sediment used based on solids content and maintaining a constant ratio on a dry weight basis, rather than wet weight, would be preferable.
This study also shows the need for analytical refinements to allow quantification of organic contaminants in digestive fluid that are often in the low parts per billion range and near or below the MDL. Although rarely an issue for trace metals, quantifying PAHs and PCBs in digestive fluid is difficult when sediments with low contaminant concentrations are extracted. For future assessments with relatively low levels of organic contaminants, increasing the amount of sediment and digestive fluid in the extractions or operating in more sensitive GC-MS modes (e.g., selective ion monitoring) may improve the range of the technique. However, as a practical matter, these less contaminated sediments are unlikely to be of concern in risk assessments, so the fact that the concentrations of organic contaminants in the extraction fluid are too low to quantify is itself an assurance of minimal bioaccumulation potential.
CONCLUSIONS
In vitro digestive fluid extraction is a tool that can be used to predict the relative potential for bioaccumulation of contaminants by quantifying the potential for intestinal solubilization. With further development, the in vitro technique may be a useful surrogate for bioaccumulation testing, or at least serve as a screening tool to reduce the number of bioaccumulation tests.
In vitro extraction offers several advantages over traditional bioaccumulation testing with deposit feeders. First, it can be done much faster (exposure time of several hours vs 28 d, plus equivalent analytical time for both), making data available sooner, and reducing the potential for bioavailability changes during the course of a long test. Second, the costs of digestive fluid extraction should be less than for bioaccumulation testing, which includes the costs of maintaining the exposure systems for nearly a month. Third, digestive fluid extractions eliminate the potential confounding effects of biotransformation. Finally, extractions allow evaluation of sediments by a consistent procedure that is applicable over a wider range of conditions (e.g., temperature, salinity, and grain size), and it is not limited by the tolerance limits of any single species. For example, Landfill Wetland ponds 2 and 3 were hypersaline, with salinities of 84 and 40 psu, respectively. It was necessary to reduce salinity of interstitial waters from these pond samples to a level appropriate for survival of M. nasuta (∼30 psu), but this dilution most likely increased the bioavailability of trace metals relative to in situ conditions [25, 26]. However, these sediments could have been tested by digestive fluid extraction without modification.
Digestive fluid extraction is not applicable to some contaminants, such as those for which intestinal absorption rather than solubilization limits uptake (e.g., Cr) or those for which ingestion of contaminated particles is not likely to be the primary route of uptake (e.g., hydrophilic organic compounds [27]). However, the dietary pathway is important for the uptake of many metals [28] and hydrophobic organics [27, 29], and the approach has been successfully applied to these contaminants [1-4, 16].
The primarily constraint to broad application of the approach is its dependence upon natural populations of depositfeeding organisms as a source of digestive fluid. We currently have work in progress to better understand the solubilization mechanisms involved, and to develop a synthetic fluid to mimic in vivo digestive processes with commercially available surfactants or enzymes. The goals of future work are to provide a widely available extractant, to allow the use of lower sediment to fluid ratios to allay saturation concerns, and to allow fluid composition to be manipulated to mimic species varying in digestive intensity.
Acknowledgements
This work was funded by a contract from the U.S. Navy to the University of California (Berkeley, CA, USA). We thank Ralph Smith and Tina Walters for assistance in sample analysis. Larry Mayer, Deborah Penry, and two anonymous reviewers provided comments that improved the draft manuscript.