Temporal and spatial variation in plasma thyroxine (T4) concentrations in juvenile alligators collected from lake Okeechobee and the northern Everglades, Florida, USA†
Presented at the 21st Annual Meeting, Society of Environmental Toxicology and Chemistry, Nashville, Tennessee, USA, November 12–16, 2000.
Abstract
We examined variation in plasma thyroxine (T4) in juvenile American alligators (Alligator mississippiensis) collected from three sites within the Kissimmee River drainage basin (FL, USA). Based on historical sediment data, Moonshine Bay served as the low contaminant exposure site, Water Conservation Area 3A served as an intermediate contaminant exposure site, and Belle Glade served as the high contaminate exposure site. In May 1999, alligators (n = 22) from Water Conservation Area 3A exhibited higher T4 concentrations than animals from both Belle Glade (n= 22; p =0.0003) and Moonshine Bay (n = 33; p = 0.001). In May 2000, alligators (n = 29) Water Conservation Area 3A again exhibited higher T4 concentrations than those from Belle Glade (n = 49; p = 0.02) but not those from Moonshine Bay (n= 40). No sexual dimorphism was observed among mean T4 concentrations within any of the sites during either year (p > 0.05). Animals within all sites exhibited higher T4 concentrations in May 2000 when compared to May 1999. When variance was examined, animals from Water Conservation Area 3A exhibited higher variance in plasma T4 concentrations than those from either Moonshine Bay or Belle Glade. We concluded that mean plasma T4 concentrations did not match the sediment contaminant mixture data presently available to us, whereas variance seems to be a more reliable indicator of contaminant exposure.
INTRODUCTION
Thyroid function in vertebrates is associated with various aspects of growth, reproduction, differentiation, and metabolism. Thyroid hormones produce changes in target tissues, thus making them more responsive to other hormones, neural stimulation, and environmental stimuli [1]. Thyroid hormones act in a permissive manner, through nuclear receptors, by directing the synthesis of adenylate cyclase, regulating the availability of adenosine triphosphate through action on the mitochondria, or inducing the synthesis of receptor proteins for other hormones [1].
Non-endocrine-induced variation in thyroid hormone concentrations has been documented in fish, amphibians, reptiles, and mammals, thus stressing the complex relationship between thyroid activity and environmental factors. Thyroid hormone concentrations have been demonstrated to vary seasonally [2-11], with both photoperiod and temperature influencing plasma concentrations. Temperature influences thyroid activity in reptiles, although activity generally is lower than that observed in mammals [12]. In lizards, thyroid activity has been demonstrated to be highest during the warmer seasons, with activity decreasing when the animals are placed at cooler temperatures [13]. Snakes exhibit the same pattern, with the highest thyroid activity coinciding with the warmer seasons and the lowest activity being observed during hibernation [14, 15]. Exceptions to this pattern exist in some lizards inhabiting warmer climates in which lower temperatures lead to increases in thyroid activity [16, 17]. The situation is further complicated by the fact that thyroidectomy and injection of thyroxine (T4) affected metabolic rate in lizards (Trachydosaurus rugosus) acclimated at 30 to 32°C but had no effect at 20 to 22°C [12].
The nutritional status of an animal also influences thyroid hormone concentrations in several vertebrate classes, with differences existing among taxa. In rats, fasting has been demonstrated to depress circulating triiodothyronine (T3) and T4 [1]. Deprivation of food led to a decrease in T4 in green sea turtles but had no effect in Kemp's ridley turtles [18]. Increases in feeding did not influence plasma T4 in either species. In spiny mice, overnutrition (from a sucrose-rich diet) led to an increased conversion of T3 to T4 and an overall decrease in T4 [19]. In female domestic nutria, food restriction induced an increase in T4 and adenosine 3′,5′-cyclic monophosphate [20]. These examples illustrate that thyroid hormones are potential mediators of the effects of nutritional stress and should be taken into account when using thyroid hormones as biomarkers of contaminant exposure.
The influence of organochlorine compounds on thyroid function has become an issue of increasing concern. A number of studies associated polychlorinated biphenyls (PCBs), organochlorine pesticides, or heavy metals with various thyroid abnormalities found in humans and wildlife species [21-25]. Polychlorinated biphenyls, p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE), and dioxin all have been associated with decreases in T4 and or T3 in marine mammals, along with various thyroid abnormalities (reviewed by Rolland [25]). Pentachlorophenol and lindane have been shown to lower serum T4 in ewe lambs [26]. The results of the study suggest that pentachlorophenol acts directly on the thyroid gland because of the reduction in thyrotropin response. Another recent study has demonstrated that hydroxylated PCBs compete for transthyretin with the same affinity as T4, whereas the same compounds compete for the human nuclear thyroid receptor with an affinity 10,000 times lower than that of T3 [27]. This suggests that in vivo these compounds are more likely to bind to serum-binding proteins than to the thyroid receptor [27]. Competition between endogenous thyroid hormones and hydroxylated PCBs could offer a plausible explanation for the reduced plasma thyroid hormones associated with elevated PCB exposure reported in the literature.
Map of southern Florida, USA, showing the three study sites that were investigated (Moonshine Bay, Belle Glade, and Water Conservation Area 3A).
Previous work by our laboratory has demonstrated that male alligators collected from Lake Okeechobee (FL, USA), a lake with a history of contamination from municipal and agricultural runoff, have elevated T4 concentrations when compared to a relatively pristine reference population living in Lake Woodruff (FL, USA), which has no known history of a chemical spill or exposure to agricultural runoff [28]. Furthermore, female and male juvenile alligators from Lake Woodruff exhibited a clear negative relationship between body size and plasma T4 concentration, whereas females and males from Lake Okeechobee did not show such a relationship [28].
This study investigated spatial and temporal variation in T4 concentrations in juvenile alligators collected from three sites within the Everglades watershed. Based on historical sediment contaminant data, Moonshine Bay served as a low contaminant exposure site, Water Conservation Area 3A served as an intermediate contaminant exposure site, and Belle Glade served as the high contaminant exposure site (see Fig. 1). We chose to measure T4 because it tends to have a longer biological half-life (∼7 d in humans [1]) than T3 (∼24 h in humans [1]) and, therefore, should serve as a more stable indicator of thyroid activity than T3. From this information, we hope to determine whether T4 is a useful marker of exposure to a mixture of contaminants.
MATERIALS AND METHODS
Animal collection
Juvenile alligators (0.9–1.5 m) were caught by hand from each of the three study sites. Matched samples were collected from Moonshine Bay, Belle Glade, and Water Conservation Area 3A on consecutive nights in May of 1999 and 2000, so as to minimize variation between the sites due to weather, temperature, seasonal variation, and diurnal variation (Moonshine Bay: 1999, n = 33, 2000, n = 40; Belle Glade: 1999, n = 22, 2000, n = 49; Water Conservation Area 3A: 1999, n = 22, 2000, n = 29) [29]. Sex, body weights, snout-vent lengths (SVLs), and total lengths were recorded for each animal. Blood samples were collected immediately upon capture from the vertebral vein, placed in lithium heparin vacutainers, and stored on ice (∼4°C). Samples were spun down the next morning (at 2,000 g for 30 min) and the plasma was flash frozen in liquid nitrogen. The plasma was assayed for T4 by means of standard T4 radioimmunoassay techniques validated from alligator plasma [28].
Study sites
The three sites were chosen based on historical sediment concentrations of pesticides in south Florida, with Belle Glade having higher concentrations of organochlorine pesticides relative to Moonshine Bay (lower) and Water Conservation Area 3A (intermediate between the two sites; refer to Fig. 1 and Table 1). Historically, Lake Okeechobee and its tributaries have been the catch basins of agricultural runoff containing pesticides and animal wastes [30, 31]. The ban on DDT production in 1973 triggered numerous pesticide-monitoring efforts nationwide. The south end of the lake (Belle Glade) has three islands (Kreamer Island, Torry Island, and Rita Island). Torry and Rita islands have had documented levels of organochlorines (DDT, DDE, and dichlorodiphenyldichloroethane [DDD]) ranging from 2,200 to 110,000 μg/kg sediment (DDT is not shown in Table 1) [31]. This is not surprising because of the heavy agricultural activities (muck farming) that have traditionally taken place on these islands. The south end of the lake presently is surrounded by sugar cane farming activity. More recent reports show that sediment samples collected from the south end of the lake contain detectable levels of DDD (near detection limits to 24 μg/kg) and DDE (7.4–47 μg/kg; Table 1) [32, 33]. Detection limits ranged from 0.86 to 0.88 μg/kg (Table 1). Historically, lower organochlorine concentrations have been observed in the Moonshine Bay area, with detectable sediment levels of DDE (1.2 μg/kg) and DDD (2.4 μg/kg) present [31]. This would be expected given the fact that limited agricultural activity takes place on the west shores of the lake, adjacent to this area. More recent sampling at the mouth of Fish Eating Creek (located near Moonshine Bay) has shown DDD and DDE to be nondetectable in sediments [32, 33]. Historically, Water Conservation Area 3A (located south of the sugar cane farming activity around the south end of Lake Okeechobee) has had reported levels of DDE ranging from 3.8 to 150 μg/kg and DDD ranging from 0.1 to 62 μg/kg [31]. More recent sampling in and around Water Conservation Area 3 A has shown DDD levels to be at or a little above the minimum detection limit, whereas DDE levels ranged from a little above the minimum detection limit to 21 μg/kg sediment [32, 33]. All the samples collected from the sites included in our study showed atrazine to be present in surface waters at approximately the times we collected animals (Table 1; minimum detection limits are listed in the table). The highest atrazine levels recorded in May 2000 were at the site showing 5.7 μg/L (Florida ground water guidance concentration limit is 3 μg/L), an area in close proximity (north) to where we collected animals in Water Conservation Area 3A [32].
| Compound | Moonshine Bayb | Bell Gladeb | WCA 3A/WCA 2b | MDL | BCF |
|---|---|---|---|---|---|
| DDD (μg/kg) | 3,173 | ||||
| 1977 | <0.1–2.4 | Islands, 580–13,000 | <0.1–62 | ||
| Lake, <0.1–120 | |||||
| 1999 | —c | 1.5–5.1 Id | — | 0.88 | |
| 2000 | — | 3.3 I-24 | 1.7 I | 0.86 | |
| DDE (μg/kg) | 2,887 | ||||
| 1977 | <0.1–1.2 | Islands, 4,800–9,300 | 3.8–150 | ||
| Lake, 6.4–550 | |||||
| 1999 | — | 7.4–19 | — -21 | 0.88 | |
| 2000 | — | 11–22 | 5.0 I-10 | 0.86 | |
| Atrazine (μg/L) | 86 | ||||
| 1999 | 0.13 | 0.32–0.38 | 0.1–0.38 | 0.0094 | |
| 2000 | 0.044 I | 0.46–0.50 | 0.04 I-5.7e | 0.0094 | |
| Ametryn (μg/L) | 33 | ||||
| 1999 | — | — -0.015 I | 0.011–0.02 I | 0.0094 | |
| 2000 | — | 0.013–0.014 I | 0.013–0.05 I | 0.0094 | |
| PCB 1260 (μg/kg) | |||||
| 1999 | — | — | — | 8.8 | |
| 2000 | — | — | 30 I | 8.6 | |
| Simazine (μg/L) | 221 | ||||
| 1999 | — | 0.023 I | — | 0.019 | |
| 2000 | — | 0.038–0.044 I | 0.013–0.037 I | 0.0094 | |
| Metalochlor (μg/L) | 18 | ||||
| 2000 | — | — | 0.061–0.063 I | 0.057 | |
| Hexazinone (μg/L) | 2 | ||||
| 1999 | — | — | 0.039 I | 0.019 | |
| 2000 | — | — | 0.11 | 0.019 | |
| Atrazine desisopropyl (μg/L) | |||||
| 2000 | — | 0.014 | 0.022–0.036 I | 0.0094 | |
| Atrazine desethyl (μg/L) | |||||
| 2000 | — | 0.036–0.043 I | 0.013 I-0.27 | 0.0094 |
- a WCA = Water Conservation Area; DDD = dichlorodiphenyldichloroethane; DDE = dichlorodiphenyldichloroethylene; PCB = polychlorinated biphenyl; MDL = minimum detection limit; BCF = bioconcentration factor (ratio of concentration of a contaminant in an aquatic organism to that found in the water) [32].
- b Sediment and water concentrations may be from areas close to but not immediately next to these areas.
- c — = below MDL.
- d I = notation used by Pfeuffer [31, 33] and Pfeuffer and Matson [32] to designate a value that is less than the minimum quantification limit, and greater than or equal to the MDL.
- e Florida groundwater guidance concentration is 3 μg/L.
In terms of organochlorine contamination, Moonshine Bay is a relatively low exposure site, Water Conservation Area 3A is an intermediate exposure, and Belle Glade is a relatively high exposure site. When all contaminants detected are considered, a different pattern is apparent. Water Conservation Area 3 A and its surrounding areas have a complex mix of low, yet detectable, levels of compounds that include ametryn, PCB 1260, simazine, metalochlor, hexazinone, atrazine desisopropyl, and atrazine desethyl. Most of these compounds were not detected in the Moonshine Bay area, whereas some were detected at the south end of Lake Okeechobee (Table 1). Mercury has also been demonstrated to be elevated in alligators living in south Florida, especially the Everglades [34-36]. Thus, although absolute levels of single compounds are probably highest at Belle Glade, Water Conservation Area 3A has low concentrations of more compounds.
Thyroxine radioimmunoassay
Plasma T4 concentrations were measured by means of a technique validated for alligator plasma as described previously [28]. Briefly, 100 μl of unextracted plasma was added to each sample tube. Assay buffer (0.2 M borate buffer [12.5 g/L boric acid, 0.5 g sodium azide, pH 8.5]; 100 μl), and 200 μl of bovine serum albumin (BSA)-7-globulin-8-anilino-1-naphthalene-sulfonic acid (ANS) buffer (0.2 M borate buffer-BSA [0.4% final]-7-globulin [1.25 mg/ml final]-ANS [0.8 mg/ml final]) were then added to each tube. Antibody (100 μl; T4 antisera, Endocrine Sciences, Calabasas Hills, CA, USA) was added to a final dilution of 1:2,000 (v/v). L-[125I]-Thyroxine (1,250 μCi/μg; New England Nuclear, Boston, MA, USA) was then added to the tubes for a final activity of 50,000 cpm/tube. Standards were mixed in assay buffer and substituted for samples as mentioned above. In addition, 100 μl of stripped plasma was added to each standard tube. Stripped plasma was prepared by stirring juvenile alligator plasma with activated charcoal (10 g/ml plasma) overnight at 4°C. The plasma-charcoal mixture was then centrifuged twice at 1,800 g for 30 min, with the plasma being drawn off after each spin. Standard curves were prepared through serial dilution of standard from 12.8 to 0.0125 ng/ml.
The sample and standard tubes containing all the assay constituents were vortexed (1 min) and allowed to incubate for 2 h at 37°C. The samples were then removed and incubated for an additional 1.5 h at room temperature. Bound-free separation was achieved by adding 1.5 ml of 60% saturated ammonium sulfate to each tube. The samples were vortexed (1 min) and then centrifuged at 1,800 g for 30 min. The supernatant containing the free label was poured off and discarded. The pellet containing the bound label was then resuspended in 2 ml of saturated ammonium sulfate:0.5% BSA in assay buffer (9:11, v/v) and vortexed for 1 min. The samples were again centrifuged for 30 min at 1,800 g. The supernatant was poured off and the pellet was dried by inverting the tubes. Tubes were counted with a Beckman gamma counter (Fullerton, CA, USA). Sample concentrations were calculated with BIO-RAD Microplate Manager® Ver 4.0 (Hercules, CA, USA). Interassay variation was 16.4%.
Statistical analyses
Significant differences (p ≤ 0.05) within and among the sites in SVL or body weight were not shown by one-way analysis of variance (ANOVA; SVL: 1999, p = 0.45, 2000, p = 0.50; body weight: 1999, p = 0.54, 2000, p = 0.53). Therefore, one-way ANOVA and Fisher's protected least significant difference post hoc analysis of log-transformed T4 concentrations were used to compare data among sites. The T4 concentrations were log transformed to achieve homogeneity of variance and normality, which were determined with an equality of variance F test and a histogram, respectively. Comparisons within sites between years were made with oneway ANOVA of the log-transformed concentrations. For comparisons of variance among sites, an equality of variances F test was used on untransformed T4 concentrations. Regression analyses, plotting SVL against log-transformed T4 concentrations, were performed for all sites. All statistical analyses were performed with StatView®, 1998 (SAS® Institute, Cary, NC, USA).
RESULTS
Sexual dimorphism in plasma thyroxine concentrations
No sexual dimorphism in mean T4 concentrations was observed at any of the sites during either field season (Table 2). Therefore, we grouped all of the animals within a site, obtained during a given year, together for purposes of statistical comparison.
Yearly comparisons of plasma thyroxine concentrations among sites
Significant differences in mean plasma T4 concentrations (log transformed) were observed among the sites for both years of the study (Fig. 2). During the 1999 season, T4 concentrations in animals from Water Conservation Area 3A were significantly elevated (p ≤ 0.001) when compared to animals from both Moonshine Bay and Belle Glade. Animals from Moonshine Bay and Belle Glade did not exhibit significant differences between mean T4 concentrations (p = 0.49).
| Locationa and year | (n) Males | (n) Females | p value |
|---|---|---|---|
| MS Bay 1999 | (28) 4.27 ± 0.30 | (5) 4.06 ± 0.98 | 0.60 |
| WCA 3A 1999 | (12) 6.31 ± 0.80 | (10) 6.19 ± 0.86 | 0.94 |
| BG 1999 | (16) 3.89 ± 0.34 | (6) 3.90 ± 0.49 | 0.86 |
| MS Bay 2000 | (24) 9.42 ± 0.85 | (16) 7.70 ± 0.56 | 0.31 |
| WCA 3A 2000 | (9) 9.20 ± 1.11 | (20)10.43 ± 1.44 | 0.92 |
| BG 2000 | (29) 8.25 ± 0.76 | (20) 6.55 ± 0.79 | 0.16 |
- a MS Bay = Moonshine Bay; WCA 3A = Water Conservation Area 3A; BG = Belle Glade (all in Florida, USA).
During the 2000 season, animals from Water Conservation Area 3A again exhibited the highest plasma T4 concentrations. Plasma T4 concentrations were significantly higher in animals collected from Water Conservation Area 3A when compared to those from Belle Glade (p = 0.02). No significant difference (p < 0.05) was found between animals from Moonshine Bay and Belle Glade (p = 0.07) or Water Conservation Area 3A (p = 0.52).
We concluded that animals from the intermediate site, Water Conservation Area 3A, exhibited the highest plasma T4 concentrations in both years. We considered Water Conservation Area 3A to be intermediately contaminated when considering organochlorine compounds, although it contains a mixture of low-level contaminants not detectable at the other sites (Table 1).
Variance associated with plasma thyroxine concentrations among sites. When variance around mean T4 concentrations was compared among sites, animals from Water Conservation Area 3A exhibited higher variance (p < 0.05) than both animals from Moonshine Bay and Belle Glade in both years (Table 3). We suggest that variance could potentially serve as a sensitive marker for contaminant exposure (or other disturbances) for wildlife species inhabiting polluted environments.
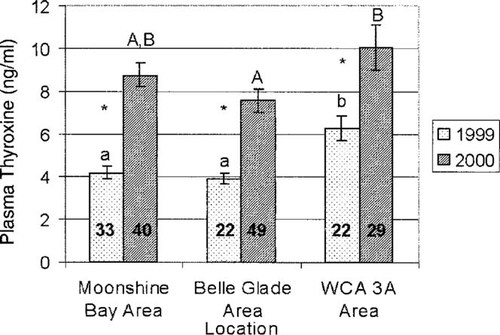
Plasma thyroxine concentrations for animals collected during May 1999 and 2000. Significant differences (p ≤ 0.05) between 1999 and 2000 values within a given site are designated by an asterisk (*). Differences among sites for the May 1999 field season are designated with a and b. Differences among sites for the May 2000 season are indicated with A and B. Sample size is included within the bar for each site (WCA 3A = Water Conservation Area 3A).
| 1999 | 2000 | |||
|---|---|---|---|---|
| Location | n | SE | n | SE |
| MS Bay | 33 | 0.29a | 40 | 0.55a |
| WCA 3A | 22 | 0.57b | 29 | 1.05b |
| BG | 22 | 0.28a | 49 | 0.56a |
Yearly differences in the relationship of plasma thyroxine concentrations to body size among sites
Regression analyses were performed on the animals within each site to determine whether T4 concentrations covaried with SVL. Perturbations in the relationship between SVL and plasma T4 concentrations could indicate abnormal thyroid function, as previously suggested [28]. We analyzed males and females both separately and combined to determine whether or not gender-specific differences existed in relation to contaminants reported at the different sites we sampled.
1999 season. Snout-vent length did not exhibit a significant relationship with plasma T4 concentrations in animals from Moonshine Bay and Belle Glade, regardless of whether males and females were considered separately or combined (Table 4). A negative relationship was observed in both male and female alligators collected from Water Conservation Area 3A. Note that the regression for females collected from Moonshine Bay is composed of six females. The low sample size is due to a predominance of males (n = 33) caught the night of collection, with few females being obtained (n = 6).
2000 season. A different regression pattern was observed among the alligators collected in the 2000 season (Table 4). A significant, although weak, relationship between SVL and plasma T4 was observed in animals from all three sites, when males and females were considered together. When the genders were analyzed separately, males from Moonshine Bay and Belle Glade exhibited a significant, although weak, relationship. No relationship was exhibited in males from Water Conservation Area 3A. Females from Water Conservation Area 3 A and Belle Glade exhibited a significant relationship (weak), whereas females from Moonshine Bay did not.
Based on the weak relationships and inconsistent patterns observed between years in the relationship between SVL and plasma T4 concentrations, we concluded that this was not a reliable marker for exposure to a mixture of contaminants in the case of the sites we investigated.
Comparison of plasma thyroxine concentrations between years
Animals from all of the sites exhibited higher plasma T4 concentrations during the 2000 season than during the 1999 season (Moonshine Bay: p < 0.0001; Belle Glade: p < 0.0001; Water Conservation Area 3A: p = 0.005; Fig. 2).
DISCUSSION
In this study, we examined spatial and temporal variations in plasma T4 concentrations within the Kissimmee River drainage basin. Our goal was to determine whether T4 serves as an effective marker for exposure to a mixture of contaminants in wildlife species. We examined mean T4 concentrations, the relationship between body size and T4 concentrations, and variance among sites in T4 concentrations. Spatial variation was observed, with animals from Water Conservation Area 3A exhibiting the highest mean plasma T4 concentrations in both years. Animals collected during the 2000 season exhibited higher mean plasma T4 concentrations than animals collected in 1999 within each of the sites. The same general patterns were present despite the higher concentrations observed, with animals from Water Conservation Area 3 A exhibiting the highest concentrations in both years relative to the other sites. No obvious patterns emerged in the relationship between body size and plasma T4 concentration. The results varied among sites and between years. Variance in T4 concentrations seemed to be the most sensitive marker, with animals from Water Conservation Area 3 A exhibiting statistically higher variance than those from Moonshine Bay and Belle Glade in both years.
Based on previous findings in our laboratory, we expected that elevated plasma concentrations of T4 would be observed in animals collected from the more contaminated sites (Belle Glade and Water Conservation Area 3A) when compared to animals collected from an area with lower reported pesticide sediment concentrations (Moonshine Bay) [28]. We found that animals from Water Conservation Area 3A exhibited the highest T4 concentrations in both years, whereas those from Belle Glade exhibited the lowest T4 concentrations in both years. These findings are counter to what was expected. The two sites exhibiting higher organochlorine contamination, Water Conservation Area 3 A (intermediate) and Belle Glade (higher contamination relative to the other two sites), had the highest and lowest T4 concentrations, with the mean concentrations for animals from Moonshine Bay (lowest contamination) falling between the means of the contaminated sites. This could in part be explained by the fact that different mixtures of compounds are present in Belle Glade and Water Conservation Area 3A, which could influence thyroid function in different ways. The area in and around Belle Glade has the highest reported sediment concentrations of organochlorine pesticides of the sites we sampled, whereas the areas in and around Water Conservation Area 3A have low levels of a wider array of contaminants (Table 1). Little or no work has been done to examine the impacts of mixtures of these compounds on thyroid activity in alligators or other wildlife. Further contaminant analysis of alligator serum, fat, and muscle would be helpful in determining whether associations exist between specific compounds and plasma T4 concentrations, given the fact that significant bioaccumulation in animal tissues takes place in the case of several of the compounds reported to be present at the sites from which we collected animals (Table 1).
| Year | Sex | (n) | Moonshine Bay | (n) | Belle Glade | (n) | WCA 3A | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | M | (28) | p = 0.13, | R2 = 0.08 | (16) | p = 0.07, | R2 = 0.21 | (12) | p = 0.02, | R2 = 0.44 |
| y = 3.96 - 0.006x | y = 4.19–0.01x | y = 4.56–0.012x | ||||||||
| F | (5) | p = 0.62. | R2 = 0.092 | (6) | p = 0.46. | R2 = 0.14 | (10) | p = 0.005. | R2 = 0.65 | |
| y = 3.96–0.006x | y = 3.88–0.005x | y = 4.5–0.013x | ||||||||
| M & F | (33) | p = 0.11, | R2 = 0.08 | (22) | p = 0.06, | R2 = 0.17 | (22) | p = 0.0002, | R2 = 0.5 | |
| y = 3.96–0.006x | y = 4.05–0.008x | y = 4.52–0.012x | ||||||||
| 2000 | M | (24) | p = 0.05. | R2 = 0.31 | (29) | p = 0.002. | R2 = 0.41 | (9) | p = 0.23. | R2 = 0.20 |
| y = 4.48–0.01x | y = 4.72–0.016x | y = 4.26–0.005x | ||||||||
| F | (16) | p = 0.22, | R2 = 0.10 | (20) | p = 0.005, | R2 = 0.37 | (20) | p = 0.003, | R2 = 0.39 | |
| y = 4.07–0.004x | y = 4.48–0.013x | y = 4.5–0.01x | ||||||||
| M & F | (40) | p = 0.002. | R2 = 0.22 | (49) | p = 0.0001. | R2 = 0.39 | (29) | p = 0.0009. | R2 = 0.34 | |
| y = 4.3–0.007x | y = 4.62–0.015x | y = 4.44–0.009x | ||||||||
- a WCA 3A = Water Conservation Area 3A; M = male; F = female.
The observation that animals from Belle Glade exhibit the lowest T4 concentrations fits those patterns reported in other laboratory and field studies that describe decreases in T4 concentrations through exposure to PCBs and organochlorine pesticides [23, 26, 27, 37]. One proposed mechanism for the lowering of T4 concentrations by xenobiotics is that the compounds compete with the endogenous hormone for serum-binding proteins that normally serve to protect the hormone from biotransformation and excretion [23]. Excess free T4 results from the xenobiotic compounds competing for serum-binding proteins, with the final result of more T4 being removed from circulation through hepatic biotransformation [22, 23]. In humans, hydroxylated PCBs recently have been demonstrated to have the same binding affinity for transthyretin as T4 [27]. Based on sediment information from the Lake Okeechobee watershed, no indication was found that abnormal levels of PCBs are acting in this ecosystem, although PCB 1260 was detected in the area around Water Conservation Area 3 A (Table 1). Other compounds that were present could exhibit affinities for thyroid hormone binding protein in alligators, although this has yet to be demonstrated.
Animals from Water Conservation Area 3A exhibited the highest T4 concentrations in both years of this study. Mercury has been demonstrated to elevate T4 concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Interestingly, mercury is found to be elevated in alligator tissues collected from the Everglades [24, 34, 35]. Further, mercury concentrations in alligator tissues collected from lakes that we commonly sample in northern Florida were lower than in animals from sites in the Everglades [38]. This pattern fits the data presented in this study as well as in previous studies that demonstrate that alligators from Lake Woodruff exhibit lower T4 concentrations than those from Lake Okeechobee, which in turn exhibit lower T4 concentrations than alligators from Water Conservation Area 3A [28].
Another possible mechanism to explain altered plasma T4 concentrations could be alterations in enzymes associated with biotransformation in the liver and peripheral tissues. The PCB, polybrominated biphenyl, and dioxin families all have been demonstrated to lower plasma and serum T4 levels, increase thyroid gland size, and increase glucuronidation and clearance of T4 [23]. If this is in fact occurring, multiple points in the thyroid hormone synthesis and biotransformation pathway would need to be simultaneously disrupted so that the homeostatic mechanisms normally in place could not compensate for the increased removal of hormone from circulation. In a recent study involving the histological analysis of thyroids collected from animals from the same three sites examined in this study, we found that the percent colloid observed within the follicle did not match the pattern expected for the observed thyroid hormone concentrations circulating in the plasma (E. A. Hewitt et al., unpublished data). Alterations in thyroid hormone biotransformation could offer a plausible explanation for these differences. Differences in hepatic testosterone biotransformation have been found in animals collected from these sites [39]. Thus, it would be interesting to examine thyroid hormone biotransformation in alligators collected from these sites to determine whether differences exist.
The observed differences possibly are due to genetic differences among the populations. In part, this was one of the reasons we chose a relatively contiguous watershed—Lake Okeechobee and the Everglades. Adult and juvenile alligators easily are capable of moving to various locations within the watershed. Recent genetic evidence suggests that the alligator populations in Florida are relatively homogeneous, because no genetic marker studied to date can distinguish populations from various lakes, but a genetic marker can separate Georgia populations from those in Florida (L. Davis et al., unpublished data).
Nutrition can influence thyroid hormone concentrations in different ways, depending on the nature of nutritional stress and species studied [18-20]. Statistical analysis of body weights among the sites indicated that no significant differences in animal body length or weight existed among the sites. Although this is by no means a comprehensive indicator of the nutritional status of the animals, it suggests that the animals from any one particular site are not nutritionally restricted relative to the others.
Temperature is known to influence thyroid activity in reptiles [12]. The average water temperatures among sites on the nights we sampled were 27.0 ± 0.68°C for the May 1999 season and 27.3 ± 2.31°C for the May 2000 season. We do not believe that differences in temperature provide a plausible explanation for the observed differences.
The relationship between body size and plasma T4 concentrations also was examined in this study. Alterations in this relationship could suggest a perturbation of normal thyroid activity given the fact that a strong relationship has been reported in alligators from Lake Woodruff, an established reference site used by our laboratory [28]. Previously, no relationship between body size and plasma T4 was reported to exist in alligators collected from Lake Okeechobee [28]. In this study, we expected to see a relationship in animals from Moonshine Bay but not in those from the more contaminated sites (Water Conservation Area 3 A and Belle Glade Area). Our results did not reveal a consistent pattern among sites or between years with relation to contaminants.
Variance in T4 concentrations among sites also was examined in this study. Recently, variance has been proposed as a more sensitive biomarker for various environmental disturbances than simply considering mean values for physiological or morphological traits [40]. An increase in the variance of different traits has been associated with organisms living at the perimeter of a population's range, those introduced into new environments, and organisms exposed to pollution [40]. Organisms in environments that are heterogeneous, because of pollution or other factors, exhibit greater genetic or physiological variance because they are struggling to adapt or maintain homeostasis (reviewed by Orlando and Guillette [40]). In this study, we found the greatest variance in T4 concentrations in animals from Water Conservation Area 3A. This is interesting in light of the fact that Water Conservation Area 3A is an intermediate site (transition zone) in terms of organochlorine contamination. This site also contains a wider variety of low levels of contaminants that include PCBs, ametryn, atrazine, simazine, metalochlor, hexazinone, atrazine desisopropyl, and atrazine desethyl (Table 1). Thus, Moonshine Bay and Belle Glade could be considered to be more homogeneous environments than Water Conservation Area 3A.
In conclusion, differences were found among the sites, with animals from Water Conservation Area 3A consistently exhibiting the highest T4 concentrations when compared to those from sites on Lake Okeechobee. Differences in T4 were observed between years within the same sites, stressing the importance of focusing on overall patterns of hormone concentrations among sites during a given time period rather than comparing absolute hormone concentrations among studies. The observed differences do not seem to be related to differences in genetics, nutrition, or temperature among animals from the three sites. A consistent pattern did not exist among sites regarding the relationship between body size and plasma T4 concentrations. Variance in T4 concentrations was consistently higher in animals from Water Conservation Area 3A in both years, suggesting that this parameter could serve as a useful biomarker for contaminant exposure in wild populations.
Acknowledgements
We thank A. Woodward, L. Hord, C. Tucker, K. Hord, T. Hord, and the Florida Fish and Wildlife Conservation Commission for the invaluable help they offered in the collection of the animals. This project was funded in part by U.S. Environmental Protection Agency grants (CR 826357–01–1 and CR 162460–91–6).




