Diffusion and degradation of atrazine in a water/sediment system
Abstract
This study determined the effects of temperature and residence time on diffusion and degradation of [14C]atrazine applied to the surface of sterilized and nonsterilized river water over a sediment. Water and sediment were placed in glass columns and incubated at 5 or 24°C. Samples were collected at 0, 7, 14, 28, 42, 56, and 112 d before and after disturbance. The warmer temperature (24°C) initially increased the rate of 14C distribution in the aqueous phase. Disturbance caused approximately 15% of the total 14C to be removed from the aqueous phase by sterile or unsterile sediment incubated at 5°C. At 24°C, the level of 14C in disturbed sediments incubated for more than 28 d gradually increased. This increased retention of 14C by sediment coincided with greater detection of the polar metabolite hydroxyatrazine (HA). More HA was recovered in nonsterilized than in sterilized sediments, indicating the contribution of biological processes to the production of this metabolite. In undisturbed sediments at 24°C, approximately 32% and 10% of the applied 14C permeated the top and bottom 1-cm segments, respectively. In columns incubated at 5°C, less than 5% of the 14C reached the surface of the submerged sediment. The potential impact of atrazine on biota associated with sediment would diminish with time because of dilution in the aqueous phase, transformation to HA, and bound residue formation.
INTRODUCTION
It is estimated that approximately 1 to 5% of field applied atrazine (6-chloro-N-ethyl-N-[1-methylethyl]-1,3,5,-triazine-2,4-diamine) is removed by surface runoff [1], with the greatest flux occurring in the spring months immediately after corn planting [2, 3]. Streams and rivers that drain agricultural watersheds can carry this atrazine to lakes, farm impoundments, and reservoirs. Ma and Spalding [4] reported that maximum atrazine inputs to Recharge Lake, Nebraska, USA, occurred in May and June runoff events, resulting in average lake concentrations of 36 and 17 μg L−1 in 1993 and 1994, respectively. Atrazine was also detected in 100% of samples collected from the Great Lakes (North America) between 1991 and 1994. Average atrazine concentrations ranged from approximately 20 to 35 ng L−1 in Lakes Huron and Michigan to 60 to 120 ng L−1 in Lakes Erie and Ontario [5]. Atrazine in such surface waters is a concern, because the herbicide could adversely affect lake biota [6] and contaminate groundwater resources by seepage from surface waters [7-9].
Atrazine can remain suspended in static surface waters or, possibly, be adsorbed by the sediment. Adsorption can occur either during a sediment disturbance event, such as waves and dredging, or after herbicide diffusion to sedimentary pore water. In shallow bodies of water such as farm impoundments, resuspension of sediment can also occur during high-velocity water flows entering the water body after high-intensity rainfall events. During such disturbance events, atrazine or its metabolites could be removed from water by adsorption to sediment and then settle to the bottom of the water body. In the absence of disturbance, atrazine can remain suspended or diffuse to the lower water depths, where most aquatic biota are located. It is important to understand the vertical distribution and fate of atrazine in both static and disturbed bodies of water to assess and predict its potential impact on aquatic biota.
Atrazine can also dissipate by degrading via biotic and abiotic processes while still in overlying water or after it is bound to sediments. This can occur by N-dealkylation and ring cleavage mediated by microorganisms or by dechlorination, which is primarily a process of chemical hydrolysis [10]. N-Dealkylation of atrazine produces deethylatrazine (6-chloro-N-[1-isopropyl]-1,3,5-triazine-2,4-diamine) and diethylatra-zine (6-chloro-N-[1-ethyl]amino-1,3,5-triazine-2,4-diamine), whereas hydroxyatrazine (HA; 2-hydroxy-4-ethylamino-6-]1-methylethyl[-1,3,5- triazine-2,4-diamine) is the first hydrolytic product. The dealkylated metabolites are further degraded to hydroxylated atrazine products such as deethylhydroxyatrazine (2-hydroxy-N-[1-ethyl]amino-1,3,5-triazine-2,4-diamine) and diethylhydroxyatrazine (2-hydroxy-N-[1-ethyl]amino-1,3,5-triazine-2,4-diamine) [11].
It is difficult to distinguish between biotic and abiotic transformations of parent herbicides to degradation products under natural conditions, because both chemical and biological processes may occur simultaneously [12]. To separate the two processes, several researchers have examined the degradation of atrazine in both sterile and nonsterile soils. Dao et al. [13] reported that degradation of atrazine during a period of 8 months was predominantly a nonbiological process in three nonsterilized soils. A greater quantity of the metabolite HA, however, was formed from atrazine in nonsterile than in autoclaved soil, indicating the contribution of biological activity to degradation of the herbicide [14]. Both biological and non-biological processes may play a role in the transformation of atrazine in water bodies.
Presently, and to our knowledge, specific information regarding the persistence and movement of atrazine in water/sediment environments that simulate static and disturbed states, and whether the degradation pathway is abiotic or biotic under anaerobic conditions, is lacking. The half-life of atrazine ranged from 15 to 20 d in an estuarine water/sediment, 2-L microcosm, and HA was the major metabolite [15]. In a similar flask-microcosm study [16], atrazine primarily degraded to HA, and a large fraction of the residue remained bound to the sediment after 252 d of incubation.
This study simulated shallow water bodies in cold (5°C) and warm (24°C) regions. The fate of atrazine in both sterile and nonsterile microcosms was also studied to evaluate the contribution of biological processes in degrading the herbicide in a water/sediment environment. The specific objective was to determine the vertical migration and transformation of atrazine in sterilized or nonsterilized water/sediment columns incubated at two temperatures (5 or 24°C). The investigation was conducted for 112 d in simulated water/sediment columns.
MATERIALS AND METHODS
Sediment/water column setup
The sediment was generated by wet sieving (53-μm sieve) Emporia soil (fine-loamy, siliceous, thermic Typic Hapludults) in 0.01 M CaCl2 solution. Only the A-horizon was used. This soil was chosen because it is an important agricultural soil in Virginia, USA. The moisture content and other physicochemical characteristics of the sediment were measured after wet sieving. The Emporia sediment consisted of 12% clay, 88% silt, and 2.7% organic matter, and it had a pH of 5.8 and a cation-exchange capacity of 2.4 cmolc kg−1 of sediment.
The sediment suspension (25 g dry wt) was then transferred to a sedimentation cylinder (diameter, 3.5 cm; length, 24 cm). Cylinders with sediment for the sterile portion of the study were autoclaved for 30 min at 121°C and 103.5 kPa for two consecutive days. River water (James River, VA, USA) was also autoclaved in 1-L beakers using the method described for sediment samples. Volumes of sterile sediment were then brought to 247 ml with autoclaved water under a laminar flow hood. The volume of cylinders containing nonsterile sediment was similarly adjusted with river water. The height of the water column was 22 cm, and the sediment beneath it was 2-cm thick. Technical-grade and [14C]atrazine (specific activity of 135 Mbq mmol−1 and radiochemical purity of 98.6%) in 3 ml of sterile or nonsterile river water were carefully pipetted on the top of the water columns to provide a total of 60 μg of atrazine in each cylinder. The cylinders were then allowed to stand for 24 h to equilibrate.
Two sets of experiments were conducted in this study. First, columns were disturbed at sampling time, and the aqueous phase was separated from the sediment by filtration. Second, the aqueous phase was separated from the sediment by siphoning the water without disturbance.
Disturbed sediment/water columns
Samples with a volume of 1 ml were first carefully taken at cylinder depths of 1, 8, and 15 cm using 1-ml volumetric pipettes. Such sequential sampling from top to bottom prevented disturbance of the water column. Radioactivity in each collected 1-ml sample was then counted with liquid scintillation spectrometry. These three cylinders for the 0-time sampling (i.e., 24 h after herbicide application) were then shaken on a wrist-action shaker for 90 min to simulate disturbance at the bottom of water bodies. The suspension was then vacuum filtered through a 0.7-μm, glass-fiber filter. Radioactivity in 1 ml of the filtered solution was counted with liquid scintillation spectrometry, and the filtrate was stored at −18°C along with the sediment. The other cylinders were placed in dark, temperature-controlled chambers at 5 or 24°C; the chambers were kept dark to prevent algal growth in cylinders. Columns were allowed to stand undisturbed for 7, 14, 28, 42, 56, and 112 d before sampling. At each sampling date, three cylinders were sacrificed from each of the 5 or 24°C chambers and processed as described for the 0-time period.
Undisturbed sediment/water columns
Diffusion of 14C from water to the bottom sediment was investigated in another set of columns treated and incubated as described previously. At each sampling time (0, 7, 14, 28, 42, 56, and 112 d), the water was siphoned to the 90-ml level and saved for analysis. The glass cylinders containing the sediment were then frozen for 3 d at −22°C. After freezing, the glass was broken and the sediment cut with a hacksaw into two equal parts. The thicknesses of both the top and bottom sediment pieces were approximately 1 cm. Water and sediment samples were processed and stored as described for disturbed columns. Another set of columns incubated at 5 and 24°C was used to determine redox potential (Eh) of the sediments and water. Redox potentials were measured at each sampling time using a pH/millivolt meter (Orion 290A; Orion Research, Boston, MA, USA) with a combination of a redox electrode and a reference probe buried in the sediment or suspended in the water column of the cylinders.
Chemicals
Purity of reference standard atrazine and its metabolite HA were 98% and 97%, respectively. Terbuthylazine (2-tert-butylamino-4-chlor-6-ethylamino-1,3,5,-triazine; purity, 99%) was the internal standard for atrazine, whereas hydroxypropazine (2-hydroxy-4,6-di[isopropylamino]-1,3,5-triazine; purity, 98%) was the internal standard for HA. Atrazine and hydroxypropazine were purchased from Riedel-deHaën (Seelze, Germany); the HA was obtained from Novartis Crop Protection (Greensboro, NC, USA). Other reagents used in the experiment were either high-performance liquid chromatographic or analytical grade.
Atrazine and metabolite extraction
Radioactivity (14C) in sediment before herbicide extraction was determined by combusting 300 to 500 mg (dry wt) of sediment mixed with an equivalent amount of cellulose in a biological material oxidizer (OX500; R. J. Harvey Instrument, NJ, USA) and by trapping the released [14]CO2 in a cocktail (Carbon-14 Cocktail; R. J. Harvey). The combustion efficiency of the oxidizer was 97%. The radioactivity content of the trap was then measured by liquid scintillation spectrometry, corrected for quenching, and expressed as an atrazine or metabolite concentration of the sediment sample.
Sediment samples of 15 to 20 g (dry wt) were sequentially extracted twice with 50 ml of a methanol/water mixture (4:1, v/v) and then combined. At each of the two steps, the mixture was shaken for 30 min at 23°C and then centrifuged at 3,000 g for 15 min. On completion of the extraction procedure, each sediment sample was combusted, and the trapped [14]CO2 was assayed by liquid scintillation spectrometry. Soil extracts along with aqueous solution (100 ml) from each column were then evaporated and reconstituted in 90% aqueous solution, containing 10 mM of ammonium acetate (pH, 5.7) and 10% methanol.
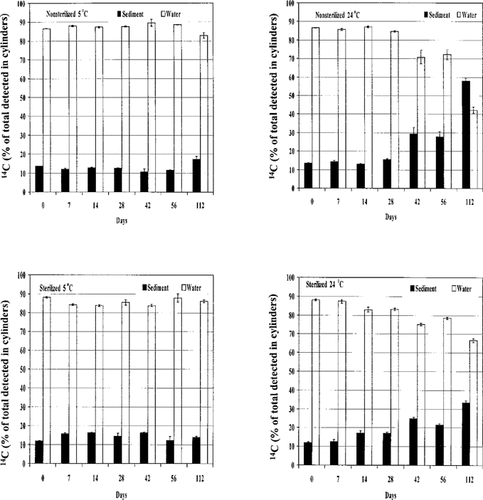
Distribution of 14C residue in water and sediment after disturbance at different sampling times. Mean ± standard error of three observations.
High-performance liquid chromatography
A reverse-phase high-performance liquid chromatographic system (Waters, Milford, MA, USA) was used to analyze atrazine and its metabolites from the sediment extracts. The system consisted of an LC-8, 4.6 i.d. × 150 mm deactivated C8 (3-μm spherical silica) column (Supelco, Bellefonte, PA, USA) that was deactivated for analysis of organic bases, dual Waters 510 pumps with a control module, a Waters 717+ Autosampler, and a Waters 996 Photodiode Array Detector. The Waters Millennium 2010 Chromatography Manager was used for system control, data acquisition, and data analysis. The organic portion (solvent A) of the mobile phase was methanol. The buffer was 10 mM ammonium acetate (pH, 5.7) aqueous solution, and the aqueous portion (solvent B) of the mobile phase was buffer/methanol (20:1, v/v). The atrazine metabolites were baseline separated with a gradient elution. The first gradient was with solvent B for 1 min and then linearly increased to 100% solvent A in 20 min, then decreased to 100% solvent B in 5 min, and then held at this point for another 5 min. The retention times of the analytes were 16.3 min for HA, 17.92 min for hydroxypropazine, 18.92 min for atrazine, and 20.28 min for terbuthylazine. The method detection limits for atrazine and HA were 0.12 and 3.1 μg ml−1, respectively. Based on the combustion results of 0-time sediments both before and after extraction, approximately 85% and 78% of the 14C was extracted from sediments incubated at 5 and 24°C, respectively. Only 30% of the 14C, however, was extracted from samples incubated for 112 d at 24°C.
Data analysis
All treatments were replicated three times and arranged in a completely randomized design. Data were subjected to analysis of variance using SAS (Statistical Analysis System, Cary, NC, USA). Treatment means were compared using standard errors.
RESULTS AND DISCUSSION
The average redox potentials at 112 d of sampling for the aqueous and sediment phases incubated at 24°C were 117 ± 16 and -143 ± 14 mV, respectively (data not presented). The aqueous phase was mildly anaerobic, whereas the submerged sediment was in a highly reduced state. Such conditions would be expected in natural water/sediment systems, in which the environment is dominated by lack of O2 as an electron acceptor.
Disturbed water/sediment columns
14C in the aqueous phase. Columns were disturbed for 90 min on a wrist-action shaker to simulate resuspension of sediment because of waves, dredging, or high-intensity incoming flows of water. At 5°C, disturbance caused approximately 15% of the total 14C in the water column to be adsorbed by the disturbed sediment (Fig. 1). No difference was found in the amount of 14C removed by the disturbed sediment between autoclaved and nonautoclaved treatments. These results of atrazine partitioning at 5°C would simulate conditions in shallow bodies of water in cold climates. In such regions, sediments would adsorb the same amount of atrazine after disturbance during the 112 d of incubation regardless of the time at which the herbicide entered the water body.
| Aqueous | Sediment | ||||
|---|---|---|---|---|---|
| Sediment | Sample (d) | AT | HA | AT | HA |
| μg L−1 | μg kg−1 | ||||
| 0 | 181 ± 20 | 4.4 ±1.6 | 811 ± 16 | 5 ± 0.4 | |
| 7 | 186 ± 36 | 3.9 ± 2.5 | 693 ±81 | 10 ± 0.1 | |
| 14 | 173 ± 19 | 3.7 ± 1.2 | 533 ± 43 | 13 ± 2 | |
| 28 | 171 ± 6 | 5.2 ± 1.3 | 634 ±13 | 32 ± 4 | |
| 42 | 135 ± 7 | 7.1 ± 0.3 | 675 ± 172 | 41 ± 11 | |
| 56 | 128 ± 17 19.5 ± 0.6 | 707 ± 58 | 99 ± 5 | ||
| Nonsterile | 112 | 58 ± 5 | 8.1 ± 0.9 | 409 ± 43 | 191 ± 18 |
| 0 | 181 ±20 | 4.4 ±1.6 | 811 ± 16 | 4.7 ± 0.4 | |
| 7 | 185 ± 6 | 1.3 ± 0.3 | 867 ± 14 | 6.1 ± 0.3 | |
| 14 | 161 ± 15 | 3.8 ± 2.0 | 646 ± 12 | 5.8 ± 3 | |
| 28 | 174 ± 3 | 6.9 ± 1.7 | 997 ±16 15.2 ±1 | ||
| 42 | 186 ± 41 | 6.7 ± 1.8 | 962 ±10 | 23 ± 4 | |
| 56 | 168 ± 12 | 8.5 ± 0.2 | 953 ± 13 | 49 ± 3 | |
| Sterile | 112 | 160 ± 5 | 16.2 ± 8.2 | 782 ±15 | 127 ± 9 |
- a All values are mean ± standard error of three observations.
At 24°C, the amount of 14C removed by the disturbed sediments gradually increased over time (Fig. 1). At 28 d, only 15% of the 14C was adsorbed by the sediments in both sterile and nonsterile columns. At 42 d, the proportion of 14C in sediment relative to the aqueous phase increased in both sterile and nonsterile columns. At the same sampling time, more 14C residue was found in nonsterile than in sterile sediment. At the last day of sampling (day 112), the unsterilized sediment contained more 14C (58%) than the aqueous phase (42%), which contrasts with the sterile columns, which had 33% in sediment and 66% in water. This suggests biological degradation of atrazine to metabolites that are more highly adsorbed than atrazine.
Atrazine and HA concentrations. Sediment and water samples from the columns incubated at 5°C contained similar amounts of atrazine at all sampling times except day 112, when the amount of atrazine declined (data not presented). At 24°C, the concentration of atrazine in nonsterilized aqueous phase declined after 28 d of incubation and was only 58 μg L-1 at the last sampling day (Table 1). This decrease was not evident until day 112 in sterile water phase. The decline in atrazine level in the aqueous phase corresponded with the reduced detection of 14C by time observed, as shown in Figure 1. Some HA remained in both sterilized and nonsterilized water phases after disturbance. Maximum amounts were detected in samples obtained at 56 and 112 d (Table 1).
In nonsterile columns, atrazine concentrations in sediment were reduced by 50% at the last day of sampling (Table 1). Again, the transformation of atrazine in sterile sediment was smaller than that in nonsterile samples. In both sediment treatments, however, the level of HA started to increase beginning at day 28 and reached 191 μg kg−1 in nonsterile sediment. The HA was produced in the sediment and/or adsorbed from the aqueous solution during disturbance. These results suggest that at 24°C, atrazine was degraded to its polar metabolite HA, which was selectively retained by sediment because of its greater adsorption coefficient (Kf) compared with atrazine [17]. Dao et al. [13] reported that the ratio of polar to nonpolar compounds steadily increased with the incubation time of atrazine. Therefore, in addition to HA, the disturbed sediment sampled after 28 d might have contained other polar hydroxylated metabolites such as deethylhdroxyatrazine and diethyl-hydroxyatrazine, which are also more tightly bound to sediment than atrazine.
The total HA produced in nonsterile sediment was approximately twice that of sterile samples at all sampling times (Table 1), indicating the contribution of biological processes in the degradation of atrazine to HA. This difference in the production of HA at 24°C would explain the greater retention of 14C in nonsterile than in sterile sediments at 56 and 112 d, as shown in Figure 1. Previously, the transformation of atrazine to HA was primarily considered to result from chemical hydrolysis [18, 19]. Recent studies, however, have shown that atrazine can be converted to HA by microbial isolates [20-22].
Static water/sediment columns
14C in the aqueous phase. No significant difference was found in the diffusion of 14C in sterile and nonsterile water columns at 5 or 24°C. Therefore, sterile and nonsterile data were combined (Fig. 2). The vertical movement of 14C was affected by temperature. At 5°C, the applied atrazine remained on the surface for 2 weeks after application. After 42 d, approximately 35% of the 14C moved to the 8-cm depth, whereas 55% of it remained at the point of application. In contrast, columns incubated at 24°C contained nearly equal amounts of 14C at 1- and 8-cm depths within 7 d, whereas at 5°C, the 14C needed 56 days to diffuse throughout the 8-cm depth. Schottler et al. [5] found no difference between atrazine concentrations at different depths in each of the five Great Lakes. They attributed this lack of variation by depth to a water-column residence time that was long enough to allow for thorough vertical mixing. Similarly, in this study, the vertical migration of atrazine was initially slow at 5°C, but by day 112, radioactivity was equally distributed throughout the 22-cm water column. Such rapid vertical distribution of atrazine, especially in the warm water column, would mean that the herbicide could reach benthic organisms associated with sediment in a short period of time. The rapid vertical mixing would also indicate, however, that the herbicide would become distributed throughout the water body (i.e., diluted), thereby reducing its overall concentration.
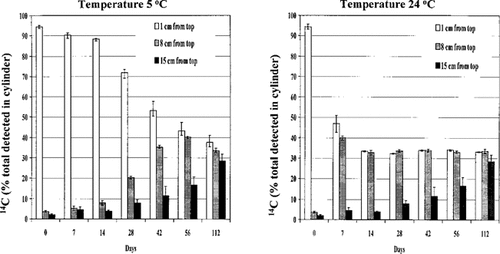
Diffusion of 14C from top of the water surface after different incubation periods at 5 and 24°C. Mean ± standard error of six observations.
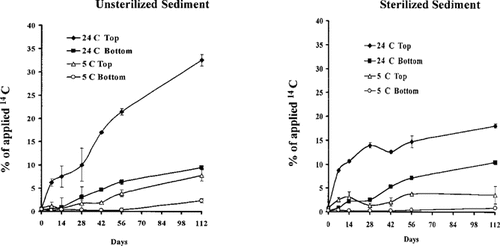
Permeation of [14C]atrazine from surface of the aqueous phase to unsterilized and sterilized sediment sections. Mean ± standard error of three observations.
14C in the sediment phase. Greater amounts of 14C were detected in the top 1 cm of sediment than in the lower segment in all cases (Fig. 3). The maximum amount detected in the top segment of sterile sediment incubated at 5°C was 4% of the applied 14C, whereas in the bottom part, it was less than 1%. Similarly, nonsterile sediment at 5°C contained a maximum of 7.8% and 2.4% of the applied 14C in the top and bottom segments, respectively. These results indicate that 14C residue was permeating or diffusing into the sediment and/or adsorbing onto its surface from static waters.
Permeation of 14C was greater at 24 than at 5°C in both sediment treatments (Fig. 3), which is similar to the faster diffusion of 14C observed in the warmer than in the colder aqueous phase (Fig. 2). A greater amount of 14C residue was also found in nonsterile than in sterile sediment at each sampling time. At 24°C, 40% of the applied 14C was detected in the top, nonsterile sediment, whereas in the sterile columns, it was approximately 20%. These results indicate that 14C residue applied to the surface of static waters diffuses to the lower depths and permeates the sediment. The process was faster at 24 than at 5°C, and it was more extensive in nonsterile than in sterile columns.
Atrazine and HA concentrations. The atrazine concentration declined rapidly in the aqueous phase of undisturbed columns incubated at 24°C (Table 2). Atrazine was degrading in the aqueous phase, with concomitant diffusion into the sediment. At 0-time sampling, the top 1-cm segment of sediment contained atrazine, indicating rapid movement of the herbicide at 24°C. Subsequently, the atrazine concentration remained the same between 7 and 56 d and then significantly declined by 112 d. Between 0 and 56 d, an atrazine concentration gradient from the aqueous to the sediment phase facilitated downward movement of the herbicide. At 112 d, the amount of atrazine in sediment started to decline because of the transformation of atrazine to HA, and less atrazine was permeating from the overlying water. Relatively more HA was detected in sediment than in the overlying aqueous phase at all sampling times. This indicates that HA diffused from the above water column and adsorbed to the sediment, the portion produced on the sediment (from degradation) remained tightly bound without partitioning back to the water phase, or both.
Hydroxyatrazine was the major metabolite of atrazine detected in both water and sediment. Other hydroxylated atrazine metabolites, such as deethylhdroxyatrazine and diethylhydroxyatrazine, were not targets of this study, because their detection frequency in surface water is low compared with that of HA [23]. Concentrations of the dealkylated atrazine metabolites deethylatrazine and diethylatrazine were negligible at all sampling times. In a similar study [16], no diethylatrazine was detected and only very low amounts of deethylatrazine were found in a sediment/water microcosm treated with atrazine. Ma and Spalding [4] also reported an insignificant change in the adjustable deethylatrazine concentration with decreased adjustable atrazine concentration in Recharge Lake, Nebraska, USA. They reasoned that atrazine was primarily degrading to HA in the lake water. Similar to our results, HA was the major atrazine transformation product in an estuarine water/sediment microcosm [15], in anoxic sediment slurries [24], in a river water/sediment microcosm [16], in flooded soils [18], and in surface water [23].
| Aqueous | Sediment | ||||
|---|---|---|---|---|---|
| Sediment | Sample (d) | AT | HA | AT | HA |
| μg L−1 | μg kg−1 | ||||
| Nonsterile | 0 | 228 ±15 | 0 ± 0 | 283 ± 22 | 4 ± 1 |
| 7 | 138 ± 4 | 5 ± 1 | 374 ±91 | 17 ± 10 | |
| 14 | 131 ± 12 | 4 ± 1 | 381 ± 38 | 41 ± 7 | |
| 28 | 122 ± 10 | 16 ± 3 | 347 ± 83 | 60 ± 2 | |
| 42 | 98 ± 22 | 19 ± 2 | 340 ± 56 | 83 ± 8 | |
| 56 | 71 ± 5 | 20 ± 1 | 303 ± 29 | 97 ± 13 | |
| 112 | 76 ± 5 | 33 ± 3 | 184 ±42 | 122 ± 57 | |
| Sterile | 0 | 228 ±15 | 0 ± 0 | 283 ± 22 | 5 ± 2 |
| 7 | 173 ± 14 | 36 ± 12 | 266 ± 54 | 32 ± 13 | |
| 14 | 133 ± 6 | 28 ± 6 | 342 ± 77 | 26 ± 13 | |
| 28 | 101 ±11 | 19 ± 1 | 419 ± 172 | 59 ± 26 | |
| 42 | 84 ± 23 | 10 ± 3 | 378 ± 48 | 49 ± 15 | |
| 56 | 87 ± 7 | 25 ± 6 | 333 ± 37 | 68 ± 28 | |
| 112 | 96 ± 16 | 22 ± 1 | 244 ± 22 | 83 ± 42 | |
- a All values are mean ± standard error of three observations.
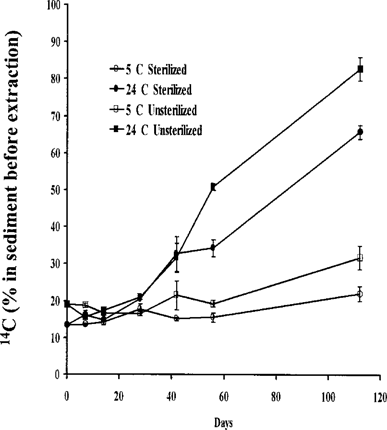
Amount of 14C present in sediment after extraction as percentage of activity before extraction. Mean ± standard error of three observations.
14C extractability from sediment. Similar amounts of non-extractable 14C were detected in all sediments maintained for 0, 7, 14, and 28 d (Fig. 4). During this period (0–28 d), approximately 15% of the 14C before extraction remained in the sediment. After 28 d, greater amounts of 14C were bound in sediments placed at 24 than at 5°C. Also, at 56 and 112 d of sampling, more 14C was bound to nonsterilized than to sterilized sediment. The proportion of nonextractable 14C increased with exposure time, as reported previously [16, 25, 26], and was greater at 24 than at 5°C. The extensive breakdown of atrazine to HA in nonsterilized sediment at 24°C would account for the greater quantity of this nonextractable residue. Increasing amounts of atrazine would also be sequestered and trapped in sediment with time, thereby reducing its availability for extraction. Therefore, in addition to degradation, the sharp decline in the atrazine concentration in sediments at day 112 (Tables 1 and 2) could result from nonextractability of the residues.
CONCLUSION
Data on the vertical distribution and degradation of atrazine in water bodies is key to assessing its potential impact on aquatic ecosystems. In static water columns, atrazine diffuses, and its concentration is equilibrated throughout the column. This process is faster at 24 than at 5°C, but given enough time, the herbicide can mix vertically regardless of the ambient temperature. At 5°C, sterilization and incubation time did not affect the removal of [14C]atrazine from water by disturbing the sediment. At 24°C, however, more herbicide residue was found in sediment after disturbance, and this coincided with the production of greater quantities of HA. Sterilized water/sediment columns retained their capacity to form HA, indicating the role of abiotic processes in the transformation of atrazine. Data also showed, however, that biological activity in nonsterile columns contributed to the enhanced transformation of atrazine to HA. These results indicate that while atrazine could potentially reach benthic organisms associated with sediment, its impact would be greatly diminished with time because of its degradation to HA.




