Metabolic responses to acute toxicity of alkylphenols and alkylphenol polyethoxylates in Elliptio complanata measured by calorespirometry
Abstract
Alkylphenol ethoxylates (APEs) are important contaminants of world water systems with effects on aquatic life. Metabolic responses to short-term acute toxicities of alkylphenol and alkylphenolethoxylates were investigated in the freshwater bivalve mollusk Elliptio complanata using isothermal batch microcalorimetry and respirometry. Metabolic heat rates were altered following short-time exposure of the gill tissue to these compounds over the concentration range of 10−6 to 10−3 M. There was a time and concentration dependence of the effects of alkylphenol and alkylphenolethoxylates on metabolic heat rates. In general, treatment with alkylphenols and alkylphenolethoxylates at concentrations ranging from 10−6 to 10−4 M caused stimulation of metabolic heat rates, possibly due to uncoupling of oxidative metabolism. Higher concentrations subsequently caused inhibition of metabolic activity and thus decreased heat rates. Rates of oxygen consumption by the gill tissue exposed to the surfactants, as a measure of effects on electron flux through mitochondria, showed a similar pattern of respiratory rate stimulation at low concentration and inhibition at higher concentration. Therefore, the batch calorespirometric method proved to be a useful technique to assess the toxicity of a group of surfactants on the control of energy flux in gills of a freshwater bivalve mollusk.
INTRODUCTION
Alkylphenol polyethoxylates (APEs) are nonionic surfactants with an estimated worldwide consumption of 360,000 tons annually [1, 2]. Contamination of freshwater systems by degradation products, such as the alkylphenol, alkylphenol polyethoxylates, and alkylphenoxyacetic acid, present a significant environmental concern [3, 4]. These degradation products are known to be estrogenic in their biological activity [5, 6] and lipophilic in character [7]. Their lipophilic nature allows them to bioaccumulate [7, 8], and therefore these substances may have unknown long-term effects, even at low concentrations, in wildlife and humans [1, 9]. For example, evidence exists that various surfactant metabolites such as alkylphenols and alkylphenol monoethoxylates and diethoxylates act as endocrine disrupters. Endocrine disrupters decrease fertility in fish, birds, and mammals. An indirect effect of these compounds is to alter endogenous estrogen metabolism, leading to increased lifetime exposure to estrogen, which is proposed to be a major contributor of breast cancer in women [9]. The majority of APE degradation products enter the environment through wastewater treatment plants [2]. Concentrations of various degradation products found in lakes and rivers are in the range of ˜0.02 to 13.5 μg/L and up to 250 μg/L in the effluents of wastewater treatment plants in the United States [3].
Current methodology makes the lethal dose determination of a xenobiotic organic compound relatively simple. However, methods to quantify the effects of sublethal levels of exposure, particularly on energy fluxes across the cell, are more complex [10]. In a recent study, Cheney et al. [11, 12] demonstrated the advantage of differential scanning calorimetry in the study of acute and subacute metal and herbicide effects on mussels and marine algae. The method is a fast and sensitive technique to evaluate metabolic heat responses of aquatic organisms to herbicides and metal ion toxicity. Additionally, they demonstrated that this method can be used to directly quantify metabolic heat rates and generate dose-response curves [12]. Flow microcalorimetry has been used in the past to ascertain biotic responses to natural and anthropogenic stresses caused by xenobiotics [13]. However, flow microcalorimetry has a slow response and is thus more suited to research application rather than as a screening technique. In the current study, we used isothermal batch microcalorimetry and respirometry to assess the short-time acute responses of aquatic organisms exposed to alkylphenol and alkylphenol ethoxylates at the higher range of concentrations found in some lakes and rivers [3]. The freshwater bivalve mollusk Elliptio complanata was used to measure the four individual effects of selected alkylphenol and alkylphenol ethoxylates on the metabolic rates of ciliated gill tissue as indicators of metabolic changes in cells exposed to increased concentrations of nonylphenol (NP), octylphenol (OP), nonylphenol monoethoxylate and diethoxylate (NP1EO and NP2EO), and octylphenol triethoxylate (OP3EO). We focused on the freshwater mussel since APEs and their degradation products have been shown to be present in freshwater systems near industrial areas or the effluents of wastewater treatment plants [3].
When aquatic organisms are exposed to environmental contaminants such as the ones studied here, their regulatory mechanism involved in energy metabolism is compromised, affecting their ability to compete and survive in the environment.
MATERIALS AND METHODS
The freshwater mussels E. complanata used in these studies were purchased from Connecticut Valley Biological, South Hampton, Massachusetts, USA, and were maintained in an aerated tank at a temperature of 18°C until just prior to use. They were then acclimated to room temperature (25 ± 1°C) for 1 h before removing the gill tissue samples. Inner gills were removed from the mussel and incubated for 15 min with shaking in distilled water to remove the trauma.
Approximately 2 cm2 (20–30 mg dry wt) sections of tissue were remove, drained, blotted lightly on a paper towel and placed in a test solution containing one of the four compounds of interest.
Mixtures of p-isomers of nonylphenol and octylphenol were purchased from Sigma-Aldrich in Allentown, Pennsylvania, USA. Nonylphenol monoethoxylate and diethoxylate (NP1EO and NP2EO) and octylphenol triethoxylate (OP3EO) were purchased from Chem Service, West Chester, Pennsylvania, USA. Stock solutions of APEs were prepared by using dimethyl formamide as a solvent. An aliquot (1.0 ml) of APE solution was further diluted into 99 ml of distilled water containing Tween 80 (200 mg/L) to limit loss by volatilization and sorption to the container. The net solution by volume contained approximately 1% dimethylformamide, approximately 0.02% Tween 80 surfactant, and more than 98% distilled water.
At predetermined times, gill tissues were removed from the APE-containing solution, placed on a wet (distilled water) filter paper disk, and sealed in a 1-cm3 hastalloy calorimeter ampule with ample head space O2 to maintain metabolism. Inside the ampules, gill tissue samples were exposed to head-space gases at all times during the run. Following measurements of metabolic heat rate, tissue samples were dried to constant weight at 60°C for 24 h. The calorimeter employed made it possible to examine three samples simultaneously. Thus, each set of experiments included parallel measurements of heat rates from untreated control tissue samples and two similarly sized test samples from the same test animal. A fresh animal (three samples of gill tissue) was used for each analysis at each exposure time and concentration.
Respiratory heat production by differential scanning calorimetry
Metabolic heat rates were quantified with a Hart model 7707 (Hart Scientific, American Fork, UT, USA) differential scanning calorimeter with four removable, 1-ml hastalloy ampules with screw lids in isothermal mode at 30°C. This type of calorimeter measures heat rates directly in microwatts. Normalizing calorimetric measurements to dry weight of the tissue tested yields specific heat rates (microwatts/mg).
Respiratory oxygen consumption
A Clark oxygen electrode (Hansatech, UK) was used for gill tissue respiration assays, which were conducted at room temperature (25 ± 1°C). For all measurements, the general gill tissue preparation procedure described before was used. Briefly, approximately 2 cm2 of tissue sections were placed in the reaction vessel. An oxygen consumption rate of about 0.08 to 0.1 ± 0.01 nmol/min was typical for a healthy organism. After establishing initial oxidation rates, the surfactants were injected into the reaction vessel with a microsyringe and the rates of O2 consumption monitored on a Soltec chart recorder (Lehman Scientific, Wrightsville, PA, USA). Activity in our assay was serotonin and sodium azide sensitive, indicating tissue oxidation of substrate possibly through cytochrome oxidase.
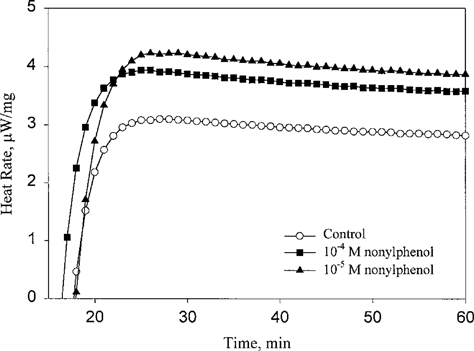
Typical isothermal calorimetric output showing heat rate data versus time for gill tissue sections of Elliptio complanata exposed to nonylphenol for 50 min.
RESULTS
Data from isothermal heat-conduction calorimetry consists of two phases. The first, lasting for approximately 25 min, reflects thermal equilibration of the ampules and sample (Fig. 1). This is followed by a stable heat signal that is a measure of the metabolic heat rate of the tissue sections. Metabolic heat rates of the tissue samples decreased slowly over the 1-h measurement intervals. Average heat rates between 3.3 and 4.5 μW/mg were commonly obtained for the control samples due to differences between individuals. For all exposure times, the heat rates for the control organisms were normalized to a value of 5 μW/mg. Heat rates per milligram of the paired test samples were also normalized by the same factor. This enables direct focus on the metabolic effects of the compounds while minimizing metabolic heat differences among gill tissues from different individuals. All the data (this and subsequent figures) have been corrected for the baseline and for dry weight of the sample. An empty ampule gives heat rates of 0.0 μW/mg.
Nonylphenol at 10−5 M caused a significant initial increase in metabolic heat rate (Fig. 1) compared to the untreated control tissue sample. The extent of the increase was 36% above the control and remained at this level for the duration of the experiment. At 10−4 M, NP also increased the heat rate significantly (about 21% above the control) but less than at 10−5 M. The stimulation at both concentrations of NP indicates only one metabolic response to NP at this 50-min exposure time.
Figure 2a shows heat rate responses as a function of nonylphenol concentration and duration of exposure to the surfactant metabolite. At 10−6 M NP, metabolic heat rate was increased to 6.0 μW/mg following 40 min of exposure and remained near 6.0 μW/mg at 50 min. The NP at concentrations of 10−5 and 10−4 M caused no significant effect in the first 20 min of exposure and increased rapidly, staying below the 10−6 M level, at subsequent exposure time and reached a value near 6.0 μW/mg at 50 min. At 10−3 M NP, measured heat rate decreased to near 4.5 μW/mg within 20 min of exposure and remained low with longer exposure duration.
Figure 2b shows metabolic responses to octylphenol at different concentrations and time of exposure to the compound. Octylphenol at 10−6 M caused an increase in metabolic heat rate to 5.5 μW/mg following 40 min of exposure and increased again to a value near 6.0 μW/mg at 50 min. At 10−5 and 10−4 M OP, there was an increase in metabolic heat rate after 20 min, and rates remained above the control for the rest of the treatment times. At 10−3 M OP, there was an initial increase in metabolic heat rate followed by a decrease to a maximum near 4.6 μW/mg at 30 min of exposure, and rates remained below the control for the subsequent exposure times.
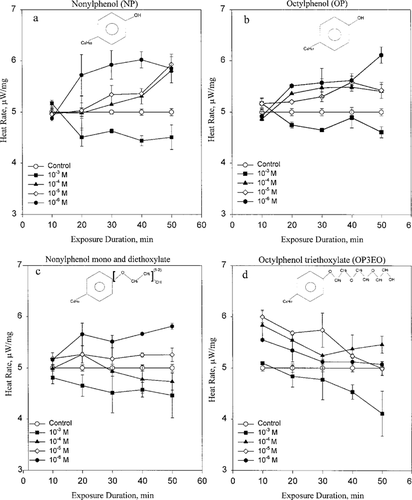
Heat rate versus exposure duration for gill tissue samples of Elliptio complanata to (a) nonylphenol, (b) octylphenol, (c) nonylphenol monoethoxylate and diethoxylate, and (d) octylphenol triethoxylate.
Metabolic responses to nonylphenol monoethoxylate and diethoxylate (NPEO) at different concentrations and exposure times are shown in Figure 2c. At 10−6 and 10−5 M, nonylphenol monoethoxylate and diethoxylate all stimulated metabolic heat rate early and remained above the control with longer exposure times. At 10−4 M, NPEO heat rate was increased to a value near 5.2 μW/mg above the control following 20 min of exposure and then decreased to a value near 4.4 μW/mg below the control with longer treatment. At −3 M NPEO, measured heat rate decreased to near 4.7 μW/mg within 10 min of exposure and remained low with longer treatment.
Octylphenol triethoxylate (OP3EO) effects on metabolic heat rates are qualitatively different from those of NP, OP, and NP1EO + NP2EO. A concentration-dependent increase in metabolic heat rate at short times is followed by inhibition at longer times of exposure (Fig. 2d). The stimulation at low concentrations and inhibition at high concentrations of octylphenol ethoxylate (OPE) indicate more than one metabolic response to OPE.
Figure 3a through d shows the relationship between the specific heat rate of gill tissue and the logarithm of the alkylphenol ethoxylates concentration. The trend that becomes apparent is a concentration-dependent change in metabolism. The concentrations where the effect on metabolism changes from stimulatory to inhibitory are near 10−3.5 M for NP and OP and 10−4.5 M for NPE. For OP3EO, these metabolic changes are less defined. The time course and level of responses differ for OPE, but major similarities exist in response to NP, OP, and NPE.
Table 1 shows the rates of oxygen consumption by the gill tissue when exposed to a surfactant. Nonylphenol accelerates respiration at 10−4 M but inhibits it at 10−3 M, which is analogous to the pattern of effects on heat rates of tissue samples in Figure 1. Sodium azide at 10 mM inhibited oxygen consumption by 90%.
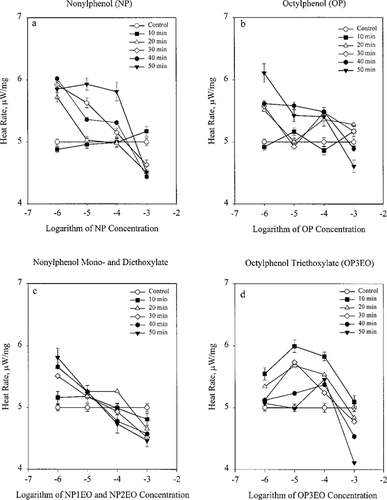
Dose response curves (heat rate versus logarithm of concentration) for (a) nonylphenol, (b) octylphenol, (c) nonylphenol monoethoxylate and diethoxylate, and (d) octylphenol triethoxylate.
DISCUSSION
The nonionic surfactant metabolites nonylphenol, octylphenol, nonylphenol monoethoxylate and diethoxylate, and octylphenol triethoxylate generally increased the metabolic heat rate of gill tissues from the freshwater mussel E. complanata at low concentrations and decreased heat rates at higher concentrations (Figs. Fig. 1., Fig. 2.). There was a concentration and time dependence of the increase to a specific compound.
| Rate of oxygen consumption (% change relative to control; n = 3) | |
|---|---|
| Nonylphenol (M) | |
| 10−5 | +30 ± 1.5 |
| 10−4 | +20 ± 1.3 |
| 10−3 | −30 ± 1.6 |
| Sodium azide (M) | |
| 10−4 | −90 ± 2.0 |
Within experimental error, the order of effect on the increase in metabolic heat rate of E. complanata of the compounds investigated is nonylphenol ≈︁ octylphenol and nonylphenol monoethoxylate and diethoxylate > octylphenol triethoxylate.
The increase in heat rate was greater at low concentration for all exposure times except for octylphenol triethoxylate. All compounds exhibited a decrease in heat rate at high concentration and exposure times. The maximum increase observed was 20% above the control at 10−6 M nonylphenol. The maximum inhibition was 20% of the control at 10−3 M octylphenol triethoxylate. Complete inhibition of gill tissue metabolism was not observed under any of our experimental conditions for the compounds investigated. Sodium azide is known to completely block oxidative metabolism. Control experiments in which the gill tissue was exposed to this compound at a concentration of 10−4 M showed that the metabolic heat rate was inhibited by about 90%. This observation led us to conclude that all compounds at high concentrations block most oxidative metabolism of the gill tissue as evidenced by a decrease in metabolic heat rate. This conclusion is consistent with earlier work [13] showing that mussel gill tissue is highly aerobic.
The heat evolved from the gill tissue is produced largely by oxidative reactions that occur predominantly in the mitochondria [14]. Thus, the increased heat evolution at short exposure times, even at low concentrations of surfactant metabolites, suggests a possible uncoupling of mitochondrial oxidative reactions. The fact that the increase at low concentrations remained above the control for the duration of the experiment also suggests that the uncoupling is continuous. Commonly, an observed reaction to uncouplers is a short-term stimulation of rate of mitochondrial oxidation [15]. The adverse effect of a continuous uncoupling of such metabolism as the one observed here for E. complanata is that its regulatory mechanism involved in energy metabolism is compromised. More substrate must be respired to obtain the necessary energy for the cell to grow and maintain itself. This ultimately decreases the organism's ability to compete and survive in the environment. In this study, low levels of surfactant metabolites enhanced tissue respiration rates; higher concentrations caused inhibition, possibly due to uncoupling activity (Table 1 and Fig. 2). The enhancement and inhibition of metabolic activity as a function of time by the surfactant metabolites thus appears to be a rapid stimulation of metabolic rate, possibly by uncoupling oxidative phosphorylation. This in turn is followed by a general inactivation of oxidative metabolism.
The change in metabolism in response to the surfactant metabolites was usually more defined at the longer exposure times. This effect is best represented by the dose-response graphs of NP and NPEO shown in Figure 3a, b, and c. The above-mentioned effect is also apparent in the OP graph (Fig. 3d); however, it is not as well defined. The only compound that did not follow this trend was OPE. The greatest increase by the lower concentrations occurred at the 10-min exposure; however, for 10−3 M, OPE caused the same inhibition as the other compounds. What is clear from the dose-response curves is that the surfactant metabolites' effects on energy metabolism parallel their toxicity and correlate with the size of the molecules. Less toxic larger molecules such as OP3EO cannot cross the tissue membrane as freely as do the smaller more toxic molecules NP, NP1EO, and NP2EO. This may help explain why NP, NP1EO, and NP2EO are the only NPEOs that show significant toxicity and are reported to produce estrogenic effects.
The greatest inhibition was caused by 10−3 M OPE at 50 min of exposure, which reached a rate near 4 μW/mg. The largest increase was caused by 10−6 M OP at the 50-min exposure, which peaked at a little over 6 μW/mg.
The lower surfactant metabolite concentrations studied here are at levels that commonly occur in the effluents of wastewater treatment plants in the United States and in some rivers around the world [15, 16]. The higher concentrations used occur only in extreme situations such as spills or in highly contaminated areas [3]. Even at 10−6 M, the lowest concentration of surfactant metabolites used in these experiments, the effects on the metabolism of E. complanata were not excessively large. However, some of these compounds have been shown to bioaccumulate, particularly NP and NPEO [1]. Since the log Kow of OP and OPE are similar to those for NP and NPE (4.0–4.6), it follows that OP and OPE probably bioaccumulate in aquaticorganisms as well [8, 17]. Thus, chronic exposures at concentrations too low to cause measurable effects in this study may cause results similar to those reported here if the surfactant metabolites accumulate in tissues of aquatic organisms. Batch calorimetric methods are useful screening techniques for the study of surfactant metabolite effects on energy metabolism and permit the analysis of dose responses and time course of metabolic changes, which can shed light into possible mechanisms of action of environmental pollutants.
Acknowledgements
The authors wish to thank all who have contributed directly and/or indirectly through insightful discussions and reviews. Thanks to Jordi Dachs and Robert Tate for suggestions on how to improve the manuscript. This research was supported in part by Hatch/McIntyre-Stennis grant 07139 and U.S. Department of Energy grants DE-AC03–76SF00098 and DE-FG 0297-ER 14755 to M. Cheney.




