Metal accumulation by Ceratitis capitata (Diptera) and transfer to the parasitic wasp Coptera occidentalis (Hymenoptera)
Abstract
Accumulation of lead (Pb), cadmium (Cd), and copper (Cu) (from food) by the fruit fly (Ceratitis capitata Wiedemann; Diptera, Tephritidae) and the transfer of the metals to the internal solitary pupal parasitoid Coptera occidentalis Muesebeck (Hymenoptera, Proctotrupoidea, Diapriidae) were investigated experimentally by exposing host larvae to contaminated diets. Each metal was added to the diet at two concentrations: Cd, 50 and 100 μg/g; Pb and Cu, 400 and 800 μg/g diet dry weight. Whole-body concentrations of the applied metals and of zinc (Zn) in the host and parasitoid were determined by atomic absorption photospectrometry. Concentration factors (CFs) for all metals (based on initial dietary concentrations) were lower at the higher food contamination level. Cadmium (CF = 3.2–7.05) and Zn (CF = 2.79–7.05) were accumulated by fruit fly larvae more efficiently than were Pb (CF = 0.95–1.02) and Cu (CF = 0.35–0.78, except control: 37.2). Considerable quantities of the metals taken up by host larvae and retained in their pupae were eliminated via the meconium after eclosion of flies (Cd, 33%; Pb, 33–51%; Cu 24–39% of pupal metal burdens). Low proportions of the host metal content were transferred to the parasitoid (0.4–5.6% and 0.3–1.4% to pupae and adults, respectively, depending on the applied metal). The remaining amounts of the metals were detected in the host puparia that remained after wasp eclosion. Vitality and fecundity of the parasitoid were not impaired by host metal contamination. Thus, the parasitic wasp probably possesses an efficient regulatory mechanism that mediates excretion of toxic metals before pupation and that diminishes the potential hazard of high metal loads in the host.
INTRODUCTION
It is generally suggested that regulators (predators or parasitoids) of herbivorous insect populations are more sensitive to xenobiotics than are their prey or hosts; it is also suggested that some herbivorous insects even benefit from the reduced efficiency of natural enemies associated with increasing environmental pollution levels [1-3]. Representatives of top trophic levels may be exposed to relatively high concentrations of toxic metals as a result of biomagnification along food chains, as has been reported for insecticides. However, trace metal research has indicated that biomagnification at higher trophic levels cannot be regarded as a general phenomenon in food chains. More likely, only certain insect species can be classified as bioconcentrators of heavy metals.
Studies on metal transfer in arthropods that consider two trophic levels (food and primary and secondary consumers) have mainly dealt with predator-prey systems [4-6], whereas little attention has been paid to host-parasite relationships [7, 8]. This lack of knowledge on the effects of toxic metals on biocontrol agents is surprising, considering that herbivorous insects are parasitized, on average, by five to ten different parasitoids [9]. Moreover, several authors have stated that outbreaks of herbivorous insect populations in areas subjected to enhanced industrial pollution might be the consequence of high sensitivity of the corresponding antagonists [1-3, 10-12].
Life cycle studies on insects (not only on herbivorous species) revealed that both whole-body metal concentrations and contents change during development [13-15]. In addition, such bioaccumulation patterns of metals in insects were found to be metal- as well as taxon-specific. Finally, episodic events of metal elimination (e.g., molting, defecation before pupation, and voiding of meconium) during development have been reported for various species [6, 8, 16, 17]. Hence, the potential hazard of toxic metals to entomophagous insects will depend on the metal assimilation pattern of the host species and on the life stage that is parasitized. A number of studies considering two different host-parasitoid relationships with respect to metal transfer and metal effects on vitality parameters support this assumption [7, 8, 18-20].
Metal effects on various vitality parameters (such as longevity, oviposition, and fecundity) in the host-parasitoid relationship between Ceratitis capitata (Diptera: Tephritidae) and its internal pupal parasitoid, Coptera occidentalis (Hymenoptera: Diapriidae), have already been investigated [21]. Despite relatively high food contamination (lead [Pb], 400 μg/g; copper [Cu], 400 μg/g; and cadmium [Cd], 50 μg/g diet dry weight), reproductive performance of the two species was not impaired, and only larval development and pupal weight of the host were affected negatively. No appropriate explanation for the relative harmlessness of metals with relation to the parasitoid was found.
This study was initially designed to determine if the host, C. capitata, actually accumulated Pb, Cd, and Cu from its food. In the event that there was significant accumulation in the host, a subsequent objective was to find out if the parasitoid C. occidentalis was affected by such enhancement or if parasitoids efficiently regulated metal concentrations. Additionally, zinc (Zn) levels were determined in both host and parasitoid in order to reveal possible interactions between Zn and the applied metals. The present study should also broaden our knowledge regarding the transfer of trace metals through host- parasite interactions.
| Treatment | Lead (Pb) | Cadmium (Cd) | Copper (Cu) | Zinc (Zn) |
|---|---|---|---|---|
| Control (C) | 0 | 0 | 0 | 0 |
| (0.183 ± 0.029) | (0.033 ± 0.01) | (0.291 ± 0.086) | (16.32 ± 3.43) | |
| Pb 400 | 400 | 0 | 0 | 0 |
| (357.71 ± 56.41) | NM | NM | (16.32 ± 3.43) | |
| Pb 800 | 800 | 0 | 0 | 0 |
| (683.03 ± 82.8) | NM | NM | (16.32 ± 3.43) | |
| Cd 50 | 0 | 50 | 0 | 0 |
| NM | (59.54 ± 5.72) | NM | (16.32 ± 3.43) | |
| Cd 100 | 0 | 100 | 0 | 0 |
| NM | (143.55 ± 21.5) | NM | (16.32 ± 3.43) | |
| Cu 400 | 0 | 0 | 400 | 0 |
| NM | NM | (184.13 ± 19.41) | (16.32 ± 3.43) | |
| Cu 800 | 0 | 0 | 800 | 0 |
| NM | NM | (664.86 ± 34.02) | (16.32 ± 3.43) |
- aN = 10 for all treatment groups. The mean ± SD for the actual Zn concentration (background level) in food was calculated over all samples (N = 70). NM = diet samples were not analyzed for the given metal.
MATERIALS AND METHODS
Chemicals
Copper chloride (CuCl2·2H2O), cadmium chloride (CdCl22·5H2O), and lead nitrate Pb(NO3)2 were obtained from Lachema (Brno, Czech Republic); concentrated HNO3 (analytical grade) was obtained from Merck (Darmstadt, Germany).
Rearing and diets
Both species—the host, C. capitata Wiedemann (Diptera, Tephritidae), and the parasitoid, C. occidentalis Muesebeck (Hymenoptera, Diapriidae)—were derived from laboratory cultures and maintained at 23 ± 1°C and RH 60 ± 5% under a light:dark regime of 16:8 h. From hatching on, C. capitata larvae were reared on artificial diets (main components: bran, brewer's yeast, and sugar), which were separately contaminated with three metals (Table 1). Simultaneously, control specimens were reared on uncontaminated diet. Actual concentrations of the applied metals and of Zn were determined in control and contaminated diets as detailed in Table 1.
Three- to 4-d-old pupae of C. capitata were offered to adult C. occidentalis for parasitization. Reproduction parameters of both the host and parasitoid and parasitization success were investigated for the higher contamination level of each metal as described in Kazimírová et al. [21].
Whole-body concentrations of the applied metals and of Zn were determined in successive life stages of C. capitata (last instar larvae determined after gut purge; pupae determined 2 d after puparium formation; adults—males and females determined after voiding of the meconium; each N = 15) and of C. occidentalis (pupae dissected from host; adults— males and females 2 d after eclosion; each N = 15). Before sample drying, fruit fly larvae and pupae (both unparasitized and parasitized) were briefly rinsed in distilled water to eliminate food remnants. Metal concentrations were also analyzed in empty puparia of C. captitata (after eclosion of the flies; N = 15) and in the host puparia that remained after eclosion of parasitic wasps (host puparium + last larval instar exuvium and meconium of the parasitoid; N = 15), further referred to as pupa rest. Subsequently, whole-body metal contents (nanograms per specimen) were calculated for each of the successive life stages of the two species in order to draw up a balance of metal accumulation and elimination.
Metal analyses
All material was dried to constant weight at 65°C and was maintained in a dessicator until sample preparation. Single specimens (control: two specimens) were digested with 0.3 ml of concentrated nitric acid for 4 h at 70°C. Afterwards, samples were diluted with redistilled water (1:1, v/v), yielding a final volume of 0.6 ml. Blanks and biological standard tissue material (TORT-1, GBW 08571) obtained from the Laboratory of the Government Chemist (Middlesex, UK) were treated in the same way; they were measured in parallel to ensure accuracy of measurements.
Metal analyses were carried out on an atomic absorption photospectrometer Varian Spectr AA-300 Plus, GTA-96 (Varian Techtron, Victoria, Australia). Copper, Cd, and Pb were determined by graphite tube; Zn was measured either by graphite tube or by flame, depending on the sample concentrations. In the case of Pb and Cd, control sample concentrations lay partly beyond the detection limit of 0.01 ng/g.
Statistics
Statistical analyses were performed on a personal computer using Statgraphics® (Manugistics, Rockville, MD, USA) [22]. Distribution of data was checked using the Kolmogorov-Smirnov test. Host and parasitoid fecundity, parasitization rate, metal concentrations, and contents of the successive life stages of host and parasitoid from the control and metal-exposed groups were statistically compared using one-way analysis of variance followed by Bonferroni's test. Sex-specific differences in weights and metal levels of host and parasitoid were evaluated by Student's t test. For each life stage, Spearman's rank correlation coefficients (r) were calculated for correlation between whole-body metal contents and corresponding dry weights and between contents of each applied metal and Zn. The level of significance for all analyses was p < 0.05. Since no sex-specific differences in metal levels occurred in most of the experimental groups, values for males and females were pooled for statistical analyses.
RESULTS
Metal accumulation and transfer within the host
Ceratitis capitata significantly accumulated heavy metals from food. Whole-body metal concentrations and contents depended on the life stage, the metal, and its applied concentration (Fig. 1).
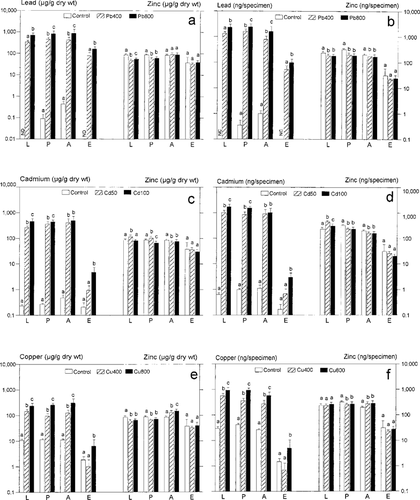
Whole-body metal concentrations (μg/g dry weight) and contents (ng/specimen) of successive developmental stages of Ceratitis capitata exposed to lead (Pb), cadmium (Cd), and copper (Cu). (a, b) Exposure to Pb (400 and 800 μg/g diet dry weight); (c, d) Exposure to Cd (50 and 100 μg/g diet dry weight); (e, f) Exposure to Cu (400 and 800 μg/g diet dry weight). Means + standard deviation (SD) are shown. Note logarithmic scaling. L = larvae; P = pupae; A = adults; E = empty puparia. “Not detected” (ND) indicates that metal concentration lie partly beyond the detection limit of 0.01 ng/g; “not calculated” (NC) indicates that metal content was not calculated. Means within a developmental stage indicated by the same letters are not significantly different (p < 0.05; analysis of variance followed by Bonferroni's test). N = 14 to 15 (28–30 for adults) per group.
Lead concentrations in fruit fly larvae (except control) were related to those in the ingested diet (concentration factor [CF] = 0.92–1.32; Table 2) and remained more or less at the same levels in successive life stages within each experimental Pb group (Fig. 1a). On the other hand, Pb content decreased significantly from pupae to adults (Fig. 1b): 51% (Pb 400) and 33% (Pb 800) of pupal metal content were voided via the meconium. Most of the remaining Pb amount was found in adults (Pb 400: 783 ± 257 ng/fly; Pb 800: 1,605 ± 874 ng/fly; mean ± standard deviation [SD]), whereas only small amounts were detected in puparia of the contaminated fruit flies (53 ± 21 and 99 ± 33 ng/puparium, respectively).
Cadmium was accumulated well above dietary levels by C. capitata larvae (CF = 3.2–7.05; Table 2). In subsequent life stages within each contaminated group, Cd concentrations and contents remained at similar levels (Table 2 and Fig. 1c and d). Although Cd 100 larvae accumulated significantly more Cd than did those of the Cd 50 group, the Cd content of the corresponding adults did not differ (926 ± 410 ng/fly; 1,028 ± 490 ng/fly, respectively; Fig. 1c and d). Again, Cd contents were low in puparia (control: 0.2 ±0.1 ng; Cd groups: 0.7 ± 0.4, 3.0 ±1.4 ng/puparium each; Fig. 1d), and up to 33% (Cd 100) of pupal metal content was excreted via the meconium.
| Cu | Cd | Pb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| System | Control | Cu 400 | Cu 800 | Control | Cd 50 | Cd 100 | Control | Pb 400 | Pb 800 |
| Ceratitis larva/food | 37.17 (5.25) | 0.78 (3.27) | 0.35 (3.96) | 7.05 | 4.39 (7.05) | 3.20 (5.0) | NE | 0.95 (2.79) | 1.02 (3.4) |
| Ceratitis pupa/larva | 1.06 (1.02) | 0.66 (1.13) | 1.10 (1.10) | 1.14 | 1.39 (0.88) | 0.96 (0.82) | NE | 1.32 (1.22) | 1.15 (1.10) |
| Ceratitis adult/pupa | 0.98 (0.96) | 1.32 (1.94) | 1.19 (2.05) | 1.74 | 1.12 (0.68) | 1.13 (1.14) | 4.78 | 0.92 (1.64) | 1.07 (1.46) |
| Coptera pupa/Ceratitis pupa | 1.65 (1.37) | 0.30 (0.95) | 0.11 (0.85) | NE | 0.07 (1.12) | 0.07 (1.11) | NE | 0.05 (1.33) | 0.02 (1.37) |
| Coptera adult/pupa | 0.69 (0.32) | 0.41 (2.16) | 0.51 (1.83) | NE | 0.35 (0.99) | 0.33 (1.78) | NE | 1.10 (1.27) | 0.99 (1.12) |
| Coptera adult/Ceratitis pupa | 1.13 (0.44) | 0.12 (2.04) | 0.06 (1.56) | NE | 0.02 (1.11) | 0.02 (1.97) | NE | 0.03 (1.70) | 0.02 (1.54) |
- Concentration factors are presented only for actual food concentrations of the applied metals (see Table 1). NE = not evaluated, because in most of the samples, Cd and Pb concentrations were under the detection limit of 0.01 ng/g.
The trophic transfer of Cu yielded a CF of 37.2 in control larvae, whereas CFs for the two Cu treatments were below 1 (Table 2). The accumulation patterns of Cu in successive life stages differed among the treatments. Copper concentrations in adults were either similar (control) or higher (Cu 400, Cu 800) than in pupae (Table 2 and Fig. 1e), but Cu contents decreased from pupae to adults (Fig. 1f), indicating elimination via the meconium (Cu 400: 24%; Cu 800: 39% of pupal metal content). Small amounts of Cu were detected in puparia (control: 1.5 ± 0.5 ng; Cu groups: 0.7 ± 0.6, 5.0 ± 5.3 ng/puparium, respectively).
Generally, enhanced metal ingestion affected Zn accumulation by the fruit fly in a metal-specific manner (Fig. 1). Despite the same dietary background level of Zn for all treatments, Ceratitis larva/food CFs ranged between 2.79 and 7.05 (Table 2). Exposure to the nonessential metals Pb and Cd resulted in lower Zn contents of pupae and adults than in corresponding control specimens (Fig. 1b and d), whereas in the presence of enhanced Cu content, Zn content was lower in pupae but higher in adults (each compared to control; Fig. 1f). Zinc deposition in puparia was independent of heavy metal administration and varied between 20.1 ±4.1 (Cd 100) and 31.3 ± 27.1 ng/puparium (control).
Significant sex-specific differences in metal contents were restricted to group Cd 100; in this group, fruit fly females showed only 60.60% of the metal content of the corresponding males (775.6 ± 380.4 ng/female; 1,280 ± 464.1 ng/male; N = 15).
Correlation analyses of whole-body metal contents and body mass (dry weights) of fruit flies did not reveal any significant relationships for the nonessential metals, but in the Cu 800 group, weight and Zn content were positively correlated in each of the life stages (r = 0.618–0.798). In all but the Cd 50 group, metal contamination led to reduced weights (dry weights) of C. capitata adults.
Metal transfer to and within the parasitoid
Metal determinations in C. occidentalis revealed trophic transfer of metals from the contaminated host to the parasitoid. However, the metal concentrations and contents of parasitoid pupae and adults were one (Cu: CF = 0.11–0.3) to two (Cd and Pb: CF = 0.02–0.07) orders of magnitude lower than those of the corresponding host pupae (Table 2 and Figs. Fig. 1., Fig. 2.). Depending on the metal applied, 0.4 to 5.6% of the host pupae metal contents were detected in parasitoid pupae. Hence, most of the metal was voided with pupae remnants after parasitoid emergence (94.4–99.6%). Additionally, metal contents diminished from Coptera pupae to adults within each experimental group (Fig. 2b, d, and f).
Despite different host contamination, Pb concentrations in the parasitoids were similar in both experimental Pb groups and in pupae and adults within one group (CF = 1.10, 0.99, respectively; Fig. 2a).
Cadmium concentrations and contents were higher in parasitoids emerged from Cd 50 hosts than in those from Cd 100 hosts. In addition, they were higher in pupae than in adults (CF = 0.33–0.35; Table 2 and Fig. 2c).
As was the case in Pb-contaminated groups, Cu concentrations and contents in Coptera pupae were enhanced compared with similar concentrations and contents in controls, but they were independent of the host contamination level. This phenomenon vanished in adults, in which Cu levels did not differ significantly between the control and Cu treatments (Table 2 and Fig. 2e and f).
Zinc was accumulated by the parasitoid to a greater extent than were the applied metals (CFs >1; Table 2). Depending on the metal, 14 to 31% of the host Zn content was transferred to parasitoid pupae. Compared with controls, each of the added metals resulted in lower Zn concentrations and contents in parasitoid pupae and higher ones in corresponding adults, but these changes did not depend on initial contamination levels (see Fig. 2).
Significant differences in the metal contents of male and female parasitoids, accompanied by weight differences in sexes (males > females), were found in group Cd 50 (3.3 ±1.0 ng/female; 2.1 ± 0.7 ng/male; N = 15).
Correlation analyses of parasitoid metal contents with the corresponding body mass (dry weight) or with Zn contents were inconsistent. Significant correlations of whole-body metal contents with weights were restricted to Cu in the control (pupae: r = 0.750; adults: r = 0.645) and to Zn in the Cu 800 group (pupae: r = 0.781; adults: r = 0.694). Zinc and Cu content correlated positively in control pupae (r = 0.723).
Reproduction and parasitization success
Metal contamination did not affect reproduction and vitality of the investigated species. Moreover, no negative effects of host metal contamination were shown with respect to parasitization success or sex ratio of C. occidentalis. Fecundity (number of offspring per female) of C. occidentalis was 27 ± 1.3 (mean ± SE, N = 10 cages with five females) for the control and 25.4 ± 1.3 to 26.6 ±1.6 for the metal groups. The percentages of female offspring in the F1 generation were 54.8 ± 3.8% (control), 53.3 ± 3.3% (Pb 800), 44.5 ± 2.6% (Cd 100), and 55.6 ± 2.3% (Cu 800).
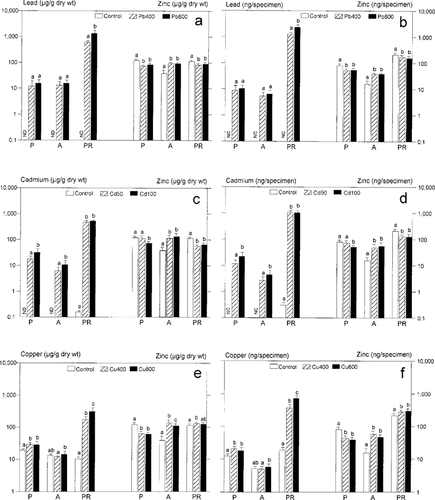
Whole-body metal concentrations (μg/g dry weight) and contents (ng/specimen) of Coptera occidentalis parasitizing Ceratitis capitata pupae exposed to lead (Pb), cadmium (Cd), and copper (Cu). (a, b) Host exposure to Pb (400 and 800 μg/g diet dry weight); (c, d) Host exposure to Cd (50 and 100 μg/g diet dry weight); (e, f) Host exposure to Cu (400 and 800 μg/g diet dry weight). Means + standard deviation (SD) are shown. Note logarithmic scaling. P = pupae; A = adults; PR = pupae rest (host puparia + last larval instar exuvium + meconium of the parasitoid). “Not detected” (ND) indicates that metal concentration lie partly beyond the detection limit of 0.01 ng/g; “not calculated” (NC) indicates that metal content was not calculated. Means within a developmental stage indicated by the same letters are not significantly different (p < 0.05; analysis of variance followed by Bonferroni's test). N = 14 to 15 (28–30 for adults) per group.
DISCUSSION
Negative effects of anthropogenic toxicants on different life parameters of arthropods have been observed both in polluted areas and in laboratory experiments [2, 12, 15, 20, 23, 24]. However, comprehensive studies on the trophic transfer of metals along food chains in aquatic or terrestrial ecosystems are still lacking [2, 24], and much more information is required on the consequences of metal pollution on predators and parasites [3, 6, 25].
Metal assimilation strategies in invertebrates have been shown to differ according to the trophic position, taxon, and/or physiology of the invertebrate [24]. Concentrations of trace metals in individuals of species living under identical conditions were also found to change with developmental stage, size, sex, or age [8, 15, 16, 24, 26]. Essential metals (Cu and Zn) are involved in many physiological processes, and, in contrast to nonessential metals, their homeostatic control has been confirmed in insects. Moreover, both metals seem to be important in avoiding detrimental effects of toxic metals [2, 14, 24, 25, 27].
The metal- and stage-specific accumulation of heavy metals in C. capitata proved to be similar to that of other primary consumers [8, 14, 15]. Although the nonessential Cd was accumulated by fruit fly larvae well above dietary levels (larva/food CFs >3), Pb concentrations corresponded more or less to dietary levels (CF = 0.95–1.02). These findings agree well with earlier observations on insects from different orders in that Cd is biomagnified as a result of the absence of homeostatic control [14, 15, 25, 28] and species-specific accumulation of Pb [8, 15, 23], which were reported. In contrast to the nonessential metals, Ceratitis larvae/food CFs for Cu decreased with increasing contamination: CF = 37.2 for control and CF = 0.78 to 0.35 for Cu treatments. Requirements of fruit fly larvae for Cu probably lie above the control diet level (hence, Cu is accumulated), whereas Cu support in excess leads to CFs of below 1. In general, CFs for Cu in control fruit flies were 48 to 106 times higher (depending on the life stage) than those found in the metal treatments, which suggests that uptake/loss of Cu by C. capitata is well regulated. These results are consistent with findings obtained for other insects [24, 29].
Most of the Cd, Pb, or Cu accumulated by larvae was retained in pupae (Table 2), suggesting that C. capitata does not eliminate excessive metal burdens before pupation through defecation, as do other Diptera [28, 30] or Lepidoptera [8, 15]. Deposition of metals in pupal integument and their elimination during metamorphosis is another efficient strategy to get rid of excessive metals and is well known in a number of holometabolous insects [13, 24, 31]. This does not seem to apply to C. capitata, since only small proportions of pupal metal contents were detected in puparia (0.1–0.2% Cd, 2–4% Pb, 0.2–3.5% Cu). Moreover, metal concentrations of adult fruit flies were found to be equal to or even higher than the corresponding values in pupae (adult/pupa CFs = 0.92–1.32), probably because of the concentration effect connected with weight loss during metamorphosis. The fact that metal contents were lower in adults (Cd: 33%; Pb: 33–51%; Cu: 24–39%) than in corresponding pupae indicates that fruit flies excrete excessive metals, mainly through the meconium, immediately after emergence. The results are in agreement with those of studies conducted on, for example, Neuroptera [6], Lepidoptera [8, 15], or Diptera [16]. Another important finding was that the proportion of Pb lost by Ceratitis adults via meconium decreased with increasing metal contamination, whereas the opposite was true for Cd and Cu. Moreover, the concentrations/contents of Pb and Cu in adults increased with increasing dietary contamination, whereas there were no significant differences between the two contamination levels in the Cd treatments.
Although antagonistic effects of essential and nonessential metals are known in invertebrates [2, 32-34], exact measurements of changes in Zn levels in insects under the effect of other heavy metals are scarce. Accordingly, enhanced Zn uptake might be one of the mechanisms that reduces toxic metal accumulation and supports their excretion [33, 35]. On the other hand, our data on C. capitata suggest that metal impact interferes with Zn uptake/loss in a metal-specific manner (Table 2 and Fig. 1). In all treatments, Ceratitis larvae accumulated Zn above dietary levels (larva/food CFs >2.5), and Zn concentrations remained relatively stable during subsequent life stages (except Cd 100 group). Zinc concentrations in C. capitata exceeded those of Cu by a factor of 7.4 (adults) to 8.1 (larvae), whereas Zn concentrations in other insects were found to be one order of magnitude higher than those of Cu [16, 24, 29]. Compared with the flesh fly [16], more Zn was deposited in fruit fly puparia (8.5–12.9% of pupal load), which may indicate that to some extent, Zn is bound to cuticular matter, as was reported, for example, for Collembola [32]. In Ceratitis, Zn discharge via the meconium amounted to as much as 29% (control) or 23 to 25% (Cd treatments) of the pupal contents, whereas Zn discharge was negligible in the other treatments. These results are in contrast with those related to Cd-exposed flesh fly adults, which excreted less Zn than control flies [16]. Nevertheless, the quantity of Zn retained in Ceratitis adults decreased after exposure to nonessential metals and increased under the impact of Cu. Our findings indicate that Zn uptake/loss in Ceratitis is regulated in a manner similar to that associated with other insects: e.g., the gypsy moth [15], the flesh fly [16], or the mealworm [33].
Sex-specific differences in metal concentrations reported for some insects [15, 24, 25] were restricted in Ceratitis to Cd treatments: higher Cd levels were seen in males than in females (adult/pupa CF >1 for males, CF <1 for females).
In contrast to the relatively large body of knowledge about metal accumulation and its impact on primary consumers, data on metal transfer to secondary consumers are scarce. Most information on the effects of toxic metals on entomophagous insects was derived from field observations [1, 3, 36], whereas laboratory investigations are limited to three species [7, 8, 18-21].
Laboratory studies involving two different host/parasitoid systems, namely Pimpla turionellae (a solitary pupal parasitoid consuming all tissues of the host pupa)/Galleria mellonella (Lepidoptera) [8] and Glyptapanteles liparidis (a larval parasitoid feeding exclusively on host hemolymph)/Lymantria dispar (Lepidoptera) [7, 20], revealed that the stage of the host that is parasitized and the feeding physiology of the parasitoid are key factors determining the potential hazard of elevated host metal levels to the parasitoid. Since most of the Cd, Pb, or Cu ingested by C. capitata larvae was retained in their pupae, larvae of the pupal parasitoid Coptera occidentalis were also exposed to elevated metal levels. Like other internal pupal parasitoids, diapriid larvae consume the whole content of the host pupa during development, and the gut of a full-grown last instar larva contains most of the ingested host tissues [37]: selective feeding on only particular host tissues can be excluded.
After defecation prior to pupation, both the concentrations and contents of the applied metals in Coptera pupae were one to two orders of magnitude lower than they were in their hosts (CFs = 0.02–0.3). Nevertheless, metal levels in parasitoids from contaminated Ceratitis pupae were elevated compared with controls. Depending on the metal, 0.4 to 5.6% of the host metal contents were transferred to C. occidentalis pupae. In contrast, in the comparable system P. turionellae/G. mellonella, host pupa/wasp CFs for Cd and Pb ranged between 3 and 14 and between 0.6 and 2.5, respectively [8]. Hence, our results suggest that Coptera larvae are either able to prevent assimilation of excessive metals from the digestive tract or that they efficiently excrete assimilated metals before pupation. This issue, however, cannot be sufficiently resolved based on the data obtained in the present study and will require further investigation of the bioavailability of metals associated with host tissues and of parasitoid physiology.
Moreover, Coptera adults discharge further amounts of toxic metals (Cd: 77–80%; Cu: 68–76%; Pb: 38% of pupal contents) during emergence. Nevertheless, whole-body concentrations of wasps ranged between 4 and 11 μg/g Cd and between 12 and 18 μg/g Pb, which caused physiological impairment in P. turionellae [18].
Transfer of Zn to Coptera appears to be related to host metal levels. Generally, parasitoid pupa/host CFs for Zn were 2.1 to 6.3 times lower than those for host larva/food. Twenty-five percent of the host Zn load was detected in control parasitoid pupae, whereas this percentage varied in the treatment groups according to the applied metal (Pb: 26–29%; Cd: 22–31%; Cu: 15–18%). The amount of Zn eliminated during wasp eclosion also seems to depend on the second metal involved. Accordingly, the proportion of Zn excreted by control wasps was highest (81%), yielding the lowest adult Zn burdens of all groups. A range of families of parasitic wasps is known to utilize Zn not only for their basic metabolic needs but also for hardening their mandibles and their ovipositor, which may explain the CFs of >1 in all experimental groups [38]. Retention of more Zn in Coptera adults under toxic metal impact may be evidence of a strategy to protect the parasitoid against the detrimental effects of toxic metals, as has been suggested for other insects [26]. However, the present results allow only preliminary conclusions concerning the interaction of Zn with the applied metals and its implications with regard to the parasitoid.
Although P. turionellae and C. occidentalis represent similar life-form types, metal accumulation and transfer differ. Whereas Coptera larvae assimilate low amounts of toxic metals and excrete relatively high proportions of the ingested metals before pupation, Pimpla apparently lack this ability [8]. Hence, in conformity with Dallinger's classification [34], C. occidentalis could be classified as a deconcentrator of metals, whereas P. turionellae could be classified as macroconcentrator of metals [8].
Heavy metals frequently impair both reproductive performance and vitality of insects [2, 12, 23, 39, 40]. On the other hand, sublethal doses of Cd or Pb did not significantly affect the fecundity of the fruit fly Drosophila melanogaster [23], and moreover, Drosophila can achieve metal adaptation under long-term metal stress [41]. Earlier findings [21], which are supported by the present study, show that C. capitata can be ranked among the insects that are quite resistant to metal stress: reproductive performance was not significantly impaired after exposure to 800 μg/g Pb, 800 μg/g Cu, or 100 μg/g Cd. Whether Ceratitis exhibits metal adaptation remains to be clarified (through the rearing of more generations under toxic metal exposure).
The field-derived data related to toxicant effects on parasitoid reproductive performance suggest a decline of parasitoid populations in polluted areas [3, 36]. In the laboratory, host heavy metal exposure has been proved to be relatively harmless to the solitary pupal parasitoid C. occidentalis [21]. These findings were confirmed in the present study by administering twice the Pb, Cd, or Cu concentrations given to the food of C. capitata. As in the case of the host, however, the impact of heavy metals might affect the next generations of the parasitoid. Investigations of long-term host metal stress on C. occidentalis are therefore necessary.
In conclusion, only low proportions of host metal contents were transferred from the fruit fly C. capitata to the solitary pupal parasitoid C. occidentalis. Moreover, metal concentrations in wasps eclosed from contaminated hosts were one order of magnitude lower (Pb: 34–52 times; Cd: 41–59 times; Cu: 8–18 times) than host contamination levels. This suggests that an efficient regulatory mechanism of metal uptake and/or excretion is present in the parasitoid and might explain the negligible effect of host metal stress on parasitoid life expectancy and reproductive performance.
The results of this and two other host-parasitoid relationships [7, 8, 19] indicate that bioaccumulation is species-specific in parasitoids, as in species within other trophic levels. Hence, a general assumption that pollutants are more hazardous to parasitoids than to their hosts should be treated with utmost caution and remains to be proved for other host-parasitoid systems.
Acknowledgements
This study received financial support from the Hochschuljubiläumsstiftung der Stadt Wien Project H-00051/97. We gratefully acknowledge the support of H.J. Nopp, who provided laboratory facilities and working materials. Thanks also to M. Komadová and two anonymous reviewers.




