Use of a bench-top photochemical reactor and solid-phase microextraction to measure semivolatile organic compound-hydroxyl radical rate constants
Abstract
It is increasingly important to be able to measure semivolatile organic compound-hydroxyl (SOC-OH) radical rate constants and estimate semivolatile organic compounds' (SOCs) atmospheric half-lives because of potential for atmospheric long-range transport. We have used a bench-top photochemical reactor, along with solid-phase microextraction (SPME) and ethyl nitrite, to successfully measure the rate constants of naphthalene, linalool, biphenyl, and phenanthrene with hydroxyl (OH) radical. Biphenyl and phenanthrene underwent wall loss in the reactor. The wall loss rates were determined and were used to correct the measured gas-phase rate constants. The reaction rate constants for naphthalene, linalool, biphenyl, and phenanthrene with OH radical, in our bench-top system at 295 ± 3 K, were determined to be 2.73 ± 0.37 × 10−11, 1.93 ± 0.24 × 10−10, 7.44 ± 1.9 × 10−12, 1.73 ± 0.21 × 10−11 (cm3/molecule/s), respectively, and were in excellent agreement with previous studies and model predictions. Based on the range of experimental and predicted rate constants for these reactants and an estimated average OH concentration in the atmosphere, the atmospheric half-lives of these SOCs are significantly less than 2 d. This indicates that the global presence of these compounds in the atmosphere is primarily due to regional sources and not to atmospheric long-range transport. This study shows that bench-top reactors, combined with corrections for reactant wall loss and simplified analytical tools (such as solid-phase microextraction), can be used to measure SOC-OH rate constants.
INTRODUCTION
Transport in the atmosphere is the major route by which semivolatile organic compounds (SOCs), such as organochlorine pesticides, polychlorinated biphenyls, and polychlorinated dibenzo-p-dioxins and furans, become globally distributed [1, 2]. Reaction with hydroxyl radicals in the atmosphere has been shown to be the most significant permanent removal mechanism for some SOCs [3].
There is general consensus that, for SOCs that partition appreciably to the atmosphere, an atmospheric half-life greater than 2 d can result in the SOC undergoing long-range transport. This 2-d atmospheric half-life cutoff has become one of the criteria by which regulators define persistent, bioaccumulative, and toxic substances [4-6]. For this reason, it is becoming increasingly important to be able to generate atmospheric half-life data and/or predict atmospheric half-lives for SOCs. Although SOCs may be removed from the atmosphere by reaction with O3 and NO3 in the atmosphere, the SOC-OH rate constant (along with the average OH concentration in the atmosphere) can be used to conservatively estimate atmospheric half-lives.
Historically, large (6,500 L) photochemical reactors have been used, at room temperature, to measure SOC-OH rate constants with good success because of the decreased surface-to-volume ratio and the minimization of wall effects [7-12]. Other researchers have used small (195 ml), heated reactors to minimize wall effects and the Arrhenius relationship to estimate the SOC-OH rate constant at 25°C [3, 13, 14]. However, the majority of atmospheric chemistry laboratories have bench-top size reactors (75–150 L) that have been used for volatile organic compound studies. The objective of our work was to show that bench-top size reactors, combined with corrections for reactant wall loss and simplified analytical tools, could be used for SOC-OH studies.
Our bench-top reactor has been used previously to generate volatile organic compound-Cl atom kinetic data [15]. To show that this type of system can be used to measure SOC-OH rate constants, we chose naphthalene, linalool (3,7-dimethyl-1,6-octadien-3-ol), biphenyl, and phenanthrene as reactants because reaction rates have been previously generated for these compounds by other workers [3, 7-12] and it allowed us to test SOC vapor pressures as low as 0.02 Pa. Photolysis of ethyl nitrite was chosen as the source of OH radicals over methyl nitrite [7, 8, 10-12, 16, 17], O3 [3, 13, 14], and H2O2 [14, 17] because of the ease of distillation of ethyl nitrite from the commercially available ethanol mixture, its relative stability, and improved occupational safety profile. Ethyl nitrite has been previously used for OH radical generation [18]. In addition, solid-phase microextraction (SPME), combined with gas chromatographic mass spectrometry, was used to measure the relative disappearance of reactant and reference compounds from the reactor as well as measuring the appearance of some reaction products.
On-line measurement of the reactants and reference compounds in the reactor by mass spectrometry is ideal [3, 13, 14]. However, this is not an option for most researchers, and adsorption onto Tenax® (Tenax, Wuppertal, Germany) is commonly used for gas sampling [7, 8, 10-12]. In addition, positive identification of reaction products is complex without chromatographic separation prior to detection by mass spectrometry (at least when using a single quadrupole instrument).
The SPME method offers several advantages over Tenax gas sampling techniques. Because air is not removed from the reactor with SPME, as it is with Tenax gas sampling, the pressure in the reactor does not decrease over time (this could be a problem for small-volume reactors). Solid-phase microex-traction is relatively inexpensive, selective, sensitive, and fast, and SPME fiber coatings are available in a variety of thicknesses and polarities so that a wide range of analytes can be sampled. An extensive desorption step is not needed, and the only limiting time factor for sampling the reactor is the length of the gas chromatography (GC) run. Although SPME can be used quantitatively by developing calibration curves, we have used SPME in these studies to measure the relative disappearance of reactant and reference compounds.
EXPERIMENTAL SECTION
Kinetic and wall loss experiments
All experiments were conducted in a 135-L, 2-m-long Pyrex® (Corning Glass, Corning, NY, USA) reaction chamber at 295 ± 3 K and 742 ± 2 torr total pressure. This system has been previously described [15]. The photochemical reactor is surrounded with two rows of twelve 40-watt fluorescent black-light lamps (Sylvania F30T8/350BL; GTE, Waltham, MA, USA) to provide ultraviolet light. The lights and reactor are encased in a reflective aluminum shell to increase light intensity in the reactor and keep out extraneous light. The reactor is interfaced with a Mattson (Madison, WI, USA) Research Series-1000 high resolution Fourier-transform infrared spectrometer and contains a White-type multipass mirror system. Modifications to the reactor for testing SOCs include installation and use of a three-way valve heated to ˜80°C to facilitate SOC introduction to the reactor and a septum interface was used for gas sampling from the reactor.
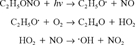
The initial concentrations of reactants, references, and ethyl nitrite are given in Table 1 for the various experiments. Concentrations were targeted to previous studies and were adjusted to allow for some variation in the ratio of reactant to reference compound concentrations. The introduction of high concentrations of the lower vapor pressure SOCs was difficult, and this was reflected in the lower reactant to reference ratios in Table 1.
Because of the size and surface of our reactor, it was critical that we conducted wall loss studies, in addition to our kinetic studies, for the various SOCs. For kinetic and wall loss experiments, the reactor was filled in the following sequence: first ethyl nitrite was flushed into the reactor with nitrogen to a reactor pressure of approximately 50 torr, known volumes and pressures of volatile references were then flushed into the reactor with air, and finally, the SOC reactant was introduced. Volatile reference compounds and ethyl nitrite (at known pressures and volume) were flushed into the reactor with a stream of diluent air. Gas pressure in the reactor was measured with a MKS (Andover, MA, USA) Baratron type 122A Absolute Pressure Gauge, 1 to 10 torr and 10 to 1,000 torr. The SOC reactants were introduced into the reactor by flowing air through a U tube and into the reactor. One to 5 g of the solid SOC compound were packed into the U tube between glass wool plugs. Naphthalene and biphenyl were introduced at room temperature and an air flow of ˜900 ml/min. Phenanthrene introduction required the U tube to be heated (˜45°C) and an air flow rate of ˜1,800 ml/min used. Linalool was wetted onto a glass fiber filter, inserted into the U tube at room temperature, and an air flow rate of ˜ 50 ml/min was used.
| Reactant | Reference | Ethyl nitrite | Reactant/reference |
|---|---|---|---|
| Naphthalene | Nonane | ||
| 1.98 × 1013 | 2.6 × 1013 | 3.1 × 1014 | 0.76 |
| 1.98 × 1013 | 6.0 × 1013 | 2.6 × 1014 | 0.33 |
| Linalool | Styrene | ||
| 3.28 × 1012 | 5.4 × 1013 | 4.1 × 1014 | 0.061 |
| 3.36 × 1012 | 5.1 × 1013 | 4.1 × 1014 | 0.066 |
| Biphenyl | Nonane | ||
| 1.88 × 1013 | 2.4 × 1013 | 3.1 × 1014 | 0.78 |
| 0.7 × 1013 | 4.8 × 1013 | 3.4 × 1014 | 0.15 |
| Phenanthrene | Propene | ||
| 13.8 × 1011 | 9.6 × 1013 | 4.7 × 1014 | 0.014 |
| 4.77 × 1011 | 13 × 1013 | 4.3 × 1014 | 0.0037 |
| Phenanthrene | Propene | ||
| 3.92 × 1011 | 16.3 × 1013 | 4.5 × 1014 | 0.0024 |
| 5.93 × 1011 | 12.8 × 1013 | 4.5 × 1014 | 0.0046 |
Experiments were performed for all reactant and reference compounds to determine if wall loss to the reactor surface (or dark reactions) was significant over the course of the experiment. After filling the reactor with the reactant and reference compound for each experiment, the reactor was allowed to sit for several hours, and this process was repeated several times to pretreat the wall surface. The rate of wall loss, kw, was then determined by filling the reactor with reactant, reference, and ethyl nitrite and sampling the reactor in the dark for several hours to determine an initial wall loss. The reactor was irradiated for 30 s and the wall loss measured again over several hours. A first-order wall loss rate could then be determined.
For kinetic studies, increasing irradiation times of 15 s to 3 min were used for testing naphthalene and biphenyl. Irradiation times were 30 s for phenanthrene and 15 to 30 s for linalool.
Analytical methods
An initial concentration measurement of the SOC in the bench-top reactor was made prior to irradiation by adsorbing the analytes onto a pre-trap packed with Tenax-TA (Alltech, Deerfield, IL, USA) and then thermally desorbing the analytes (200°C) onto a cryotrap (−65°C) [19]. Ten- to 100-ml gas samples were withdrawn from the reactor through a septa into the pretrap by using a syringe. A calibration curve for each reactant was generated by spiking liquid standard solutions onto the glass wool portion of the Tenax pretrap, removing the solvent with a helium flush, and then desorbing analyte onto the cryotrap. All samples were analyzed by gas chromatography using a mass selective detector (Hewlett Packard, Palo Alto, CA, USA; 5890/5971A MSD) and a J&W Scientific (Folsom, CA, USA) DB-5ms column. Gas chromatography temperature programs varied depending on the analyte. Products were identified by GC retention time and mass spectra.
The relative disappearance of reactant and reference compounds was measured using various SPME fibers. Using SPME, analytes are adsorbed onto a fused silica fiber coated with different phases of varying thicknesses. The fiber is housed in a needle and is exposed to the gas mixture in the reactor when expelled. The needle is then inserted into the GC injection port, the fiber expelled, and the analytes thermally desorbed. A lengthy desorption and cryotrapping period is not needed (as with Tenax methods), and the GC program does not need to be modified, with the exception of operating in the splitless mode during the desorption of the fiber.
Experiments were done to determine which fiber coatings and exposure times were appropriate for the reactant and reference compounds. A 100-m polydimethylsiloxane (Supelco, Bellefonte, PA, USA) fiber was used for sampling all SOCs and reference compounds, excluding propene. A 75-m Carboxen polydimethylsiloxane (Supelco) fiber was used for sampling propene, as it had a higher partition coefficient for propene.
The SPME fibers were exposed for 30 s to 1 min (depending on the analyte) to the gas mixture inside the reactor, through a septa, and were immediately inserted into the GC injection port for desorption and analysis. The same SPME fiber was used throughout an experiment in order to eliminate variation due to differences in fiber coating. The variation between injections from the same fiber was found to be approximately 10% relative standard deviation. Fibers were changed after approximately 50 injections. Reactant concentrations of 30 pg/cm3 were easily detected using SPME.
Relative rate determination
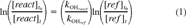 (1)
(1)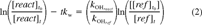 (2)
(2)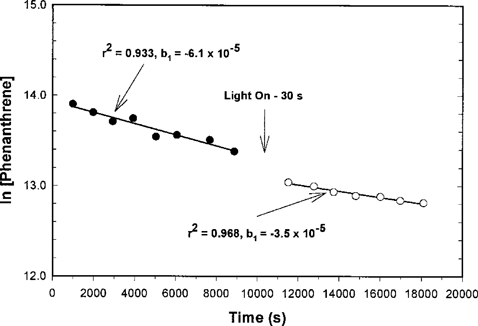
Phenanthrene wall loss before (6.1 × 10−5 L/s) and after (3.5 × 10−5 L/s) 30-s irradiation.
Chemicals
The following chemicals were obtained from Aldrich Chemical Company (Milwaukee, WI, USA) (purity is noted in parentheses): naphthalene (99+%), biphenyl (99%), phenanthrene (98%), nonane (anhydrous, 99+%), styrene (99+%), propene (99+%), and ethyl nitrite in ethanol (10–20% wt solution). Diluent gases were zero grade air and nitrogen (Praxair, Cincinnati, OH, USA).
Kinetic modeling
The Syracuse Research Corporation (SRC, Syracuse, NY, USA) structure-activity relationship program, AOPWIN, was used to predict reactant-OH rate constants [24, 25]. This program is based on structure-activity relationships originally developed by Atkinson [26].
RESULTS AND DISCUSSION
Wall loss experiments
Figure 1 shows the first order wall loss rate (kw) for phenanthrene. When slight differences in the wall loss rates before and after the 30-s irradiation were measured, we chose to use the wall loss rate measured after the 30-s irradiation because we felt that this was more representative for our photolysis studies (repeated irradiation). Because the rate of wall loss was found to be dependent on the history of the use of the reactor, we measured the wall loss rate and reaction rate with OH radicals in consecutive experiments for reactant and reference pairs.
Wall loss in the reactor was significant for biphenyl and phenanthrene, with rates of 4.9 × 10−5 L/s and 3.5 × 10−5 L/s, respectively. We did not observe wall loss for naphthalene, linalool, or the reference compounds (nonane, propene, and styrene). Our measured wall loss rates are similar to those previously measured for other reactants in other systems [16, 17]. Based on the vapor pressure of our reactants (Table 2), it appears that wall loss is significant in our reactor for semivolatile compounds with solid vapor pressures less than ˜7 Pa. However, chemical structure and functional groups may also affect wall loss.
Relative rate experiments
Based on the results of our wall loss studies, the measured biphenyl- and phenanthrene-OH rate constants (kOH) were determined by correcting for wall loss using Equation 2, and the naphthalene- and linalool-OH rate constants were determined using Equation 1 (no wall loss correction). These data are shown in Figures Fig. 2., Fig. 3.. In general, the linear regressions indicate good correlation and y-intercepts (b0) close to zero. Figures Fig. 2., Fig. 3. also give the rate constant ratios for the reactant and reference compounds (b1) and the 95% confidence interval of this ratio.
| Reactant | Vapor pressure (Pa) | kOH this work [cm3/molecule/s] (95% confidence limits) | kOH literature [cm3/molecule/s] (95% confidence limits) | kOH estimated using structured-reactivity relationshipa [cm3/molecule/s] |
|---|---|---|---|---|
| Naphthalene | 10.4b | 2.73 (2.36–3.10) × 10−11c | 2.42 (2.23–2.61) × 10−11c | 2.2 × 10−11 |
| 2.59 (2.35–2.83) × 10−11d | ||||
| Linalool | 7.7e | 2.17 (1.89–2.45) × 10−10f | 1.59 (1.19–1.99) × 10−10d | 1.2 × 10−10 |
| 1.93 (1.69–2.17) × 10−10g | ||||
| Biphenyl | 1.3b | 7.44 (5.54–9.34) × 10−12c | 6.7 (5.9–7.7) × 10−12h | 6.8 × 10−12 |
| 8.5 (7.7–9.3) × 10−12i | ||||
| Phenanthrene | 0.02b | 1.33 (0.97–1.69) × 10−11c | 1.27 (1.04–1.5) × 10−11j | 1.3 × 10−11 |
| 1.73 (1.52–1.94) × 10−11j | 3.4 (2.2–4.6) × 10−11j | |||
| 1.56 (1.36–1.76) × 10−11k |
- akOH estimated using the SRC AOPWIN software based on [23-27].
- b Vapor pressures from [30].
- c Reactant kOH using nonane as the reference.
- d Reactant kOH using isoprene as the reference.
- e Linalool solid vapor pressure estimated using SRC software.
- f Linalool kOH rate calculated using a styrene kOH rate of 5.87 × 10−11 cm3/molecule/s [22].
- g Linalool kOH rate calculated using a styrene kOH rate of 5.2 × 10−11 cm3/molecule/s [23].
- h Reactant kOH using isobutane as the reference.
- i Reactant kOH using cyclohexane as the reference.
- j Reactant kOH using propene as the reference.
- k Absolute measurement of reactant kOH rate.
The measured rate constants are summarized in Table 2 along with kOH values from the literature and kOH values predicted by the SRC AOPWIN software program. The reference compounds used for the various studies are also indicated in Table 2.
There is excellent agreement between our values and those in the literature, indicating that bench-top reactors, SPME, and ethyl nitrite as the source of OH radicals can be used to determine SOC-OH rate constants as long as any wall loss is corrected for using Equation 2. The percent difference between our kOH values and the literature values range from 4% for phenanthrene to 29% for linalool (Table 2). The discrepancy between our kOH values and those in the literature can, in some cases, be attributed to the use of different reference compounds and the assumed kOH reference values. This can be seen in our phenanthrene kOH data relative to nonane and propene in Table 2. The SRC AOPWIN program predicts kOH values close to experimental kOH values for these reactants. However, the SRC program tends to predict kOH values in the lower end of the range of data listed in Table 2.
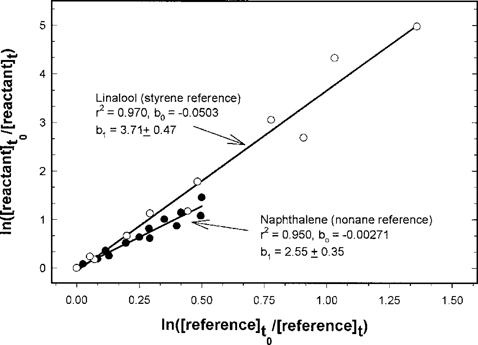
Plot of Equation 1 for linalool relative to styrene and naphthalene relative to nonane, where r2 is the correlation coefficient, b0 is the y-intercept, and b1 is the slope (rate constant ratio) ± the 95% confidence interval of the linear regression.
Identification of products
Although our experiments were not optimized for SOC-OH reaction product identification, we were able to use SPME for sampling reaction products amenable to GC analysis. Previous methods required the collection of relatively large-volume samples from the reactor in order to identify products [27]. Because of the selectivity and sensitivity of SPME, we believe it can be a useful and simple tool for collecting and identifying reaction products.
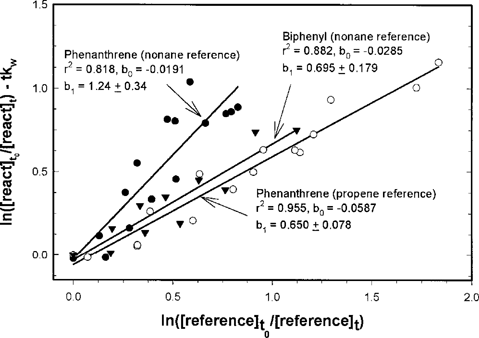
Plot of Equation 2 for biphenyl relative to nonane and phenanthrene relative to nonane and propene, where r2 is the correlation coefficient, b0 is the y-intercept, and b1 is the slope (rate constant ratio) ± the 95% confidence interval of the linear regression.
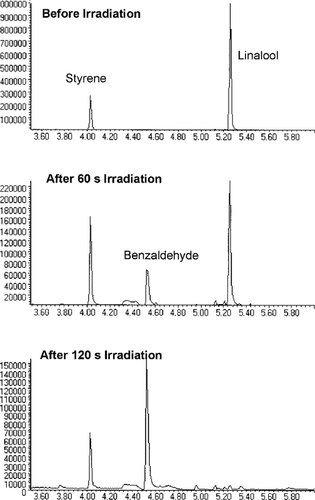
Chromatograms generated by solid-phase microextraction/gas chromatography/mass spectrometry showing the disappearance of the reference (styrene) and reactant (linalool) and the formation of a product (benzaldehyde) with repeated irradiations. Benzaldehyde is a product of the OH radical addition to the ethylene group of styrene [23].
Of the experiments we conducted, the most evident product was the formation of benzaldehyde from the OH radical addition to the vinyl group of styrene. This is shown in Figure 4 and has been previously measured [23]. The 2-hydroxy-1-phenyl-ethanone was also measured as a reaction product of styrene with OH radical in our experiments. Acetone is expected to be a reaction product of linalool with OH radical [27] but was not measured because of our GC temperature program. However, we did measure 6-methyl-5-hepten-2-one as a product of this reaction [27]. Finally nonanone was identified as a possible product of the reaction of nonane with OH radical.
This work shows the feasibility of using relatively nonpolar SPME fibers to collect and identify volatile reaction products. It is possible that more polar fibers, combined with reversephase liquid chromatographic mass spectrometry, could be used to identify more polar, less volatile products without analyte derivitization [28].
Atmospheric half-lives
To put all of the kOH values in Table 2 in perspective, we used the values to calculate the range of atmospheric half-lives for the reactants. We assumed the 24-h average OH radical concentration was 1 × 106 molecules/cm3 [29] and that gas-phase direct photolysis and reaction with O3 and NO3 radicals were of minor importance. This results in an atmospheric half-life of 0.26 to 0.36 d for naphthalene, 0.033 to 0.067 d for linalool, 0.86 to 1.5 d for biphenyl, and 0.17 to 0.83 d for phenanthrene.
The experimental and predicted kOH values for these reactants show these compounds do not meet the atmospheric half-life cutoff (greater than 2 d) for toxic substances. As such, the global presence of these compounds in the atmosphere is primarily due to regional combustion sources (naphthalene, biphenyl, and phenanthrene) and regional biogenic emissions (linalool).
This study shows that bench-top reactors, combined with corrections for reactant wall loss and simplified analytical tools (such as SPME), can be used to measure SOC-OH rate constants. Further studies are needed to determine if the reactant wall loss correction used here is applicable for compounds with vapor pressures less than 1 × 10−2 Pa.
Acknowledgements
The authors thank Jay Shi and William Begley.




