Modulation of CYP1A expression in rainbow trout by a technical grade formulation of propiconazole
Abstract
In a recent survey of pesticide concentrations in aquatic ecosystems of Central America, the antifungal triazole compound propiconazole was found to be the most widely distributed pesticide. Previously, technical grade propiconazole (TGP) has been shown to modulate cytochrome P450 activity in mammals and birds. The present study investigated the concentration- and timedependent effects of TGP on hepatic cytochrome CYP1A gene expression and catalytic activity in rainbow trout (Oncorhyncus mykiss). The TGP produced both a mixed-pattern response and a biphasic response for CYP1A expression following waterborne exposure. Evidence for inhibitory complex formation with cytochrome P450 may explain the occurrence of the mixed-pattern response. To further characterize the influence of TGP on CYP1A expression, a comparison was made between TGP and analytical grade propiconazole (AGP) with in vivo and in vitro assays. This comparison demonstrated that induction of the CYP1A gene resulted from unidentified compounds in TGP. Since TGP can be coapplied with other agricultural pesticides, either inhibition or induction of CYP1A protein activity by TGP and potentially other cytochrome P450 isoenzymes may lead to unexpected toxicological interactions.
INTRODUCTION
Over the past two decades, a number of antifungal imidazole and triazole derivatives have been approved for use in agricultural and clinical settings. These fungicides were designed with the intent of inhibiting cytochrome P450-mediated ergosterol biosynthesis [1]. Inhibition of cytochrome P450- catalyzed biotransformations by these compounds is caused by interaction with the heme atom of cytochrome P450 [2]. As a result of interaction with the site of oxygen binding, the ability of cytochromes P450 to catalyze oxidative metabolism is inhibited. Propiconazole (Fig. 1a) is a triazole compound that is used as a systemic foliar fungicide. A recent survey of pesticide concentrations in Costa Rican banana plantations found that propiconazole concentrations in nearby streams were as high as 24 μg/L during the dry season [3]. In a separate study that surveyed pesticide concentrations in aquatic ecosystems of Central America, propiconazole was found to be the most widely distributed pesticide [4]. Propiconazole was present in 60% of the samples collected in effluents, 56% of the samples collected in creeks, 43% of the samples collected from main rivers, and some of the riverine sediment samples reached 130 μg/kg [4].
Presently, little is known about the effects of propiconazole and related compounds on hepatic CYP1A1 expression in fish. In an earlier study, a 14-d exposure to 20 mg of technical grade propiconazole (TGP)/L had no effect on rainbow trout (Oncorhyncus mykiss) hepatic CYP1A1 catalytic activity [5]. Technical grade propiconazole has been shown to modulate CYP1A1 expression in rat [6-8] and in bobwhite quail [7, 8]. Technical grade propiconazole, acting as either an inhibitor or an inducer of cytochrome of CYP1A1 expression or other cytochrome P450 isozymes could potentially influence the disposition, detoxification, or activation of a second compound that is a substrate for a cytochrome P450 isozyme.
The molecular mechanisms involved in CYP1A1 induction have been extensively studied in mammalian species. Induction of CYP1A1 in mammals and in fish is initiated with the binding of the aryl hydrocarbon receptor (AhR) by a ligand. Well-characterized AhR ligands include aromatic hydrocarbons, polychlorinated biphenyls, dibenzodioxins, and dibenzofuran congeners [9]. In mammalian models, the ligandbound complex translocates to the nucleus [10], binds with the AhR nuclear translocator protein, and then binds to specific DNA sequences termed dioxin responsive elements (DREs) in the regulatory region of the gene [11]. A very similar induction process of CYP1A1 is believed to occur in fish [12]. Currently, there is little information on the functionality of the teleost AhR in terms of binding affinities for nontraditional agonists, particularly at environmentally realistic concentrations.
Recently, Gooneratne et al. [13] measured the activity and specificity of two CYP1A isozymes in rainbow trout CYP1A1 and CYP1A3. Both CYP1A proteins exhibited high O-dealkylase activities for the substrates ethoxyresorufin, methoxyresorufin, and phenacetin, and both enzymes were good catalysts for the oxidation of 7,12-dimethylbenz[a]anthracene (DMBA) [13]. However, no qualitative differences could be detected between the two proteins in their ability to metabolize DMBA into individual metabolites. Since there is a 96% sequence similarity between the CYP1A1 and CYP1A3 transcripts [14], we were precluded from distinguishing between these forms with the pfP1450-3′ cDNA probe cloned from rainbow trout [15]. Therefore, we will refer to general expression of the CYP1A subfamily rather than to specific transcripts and isozymes.
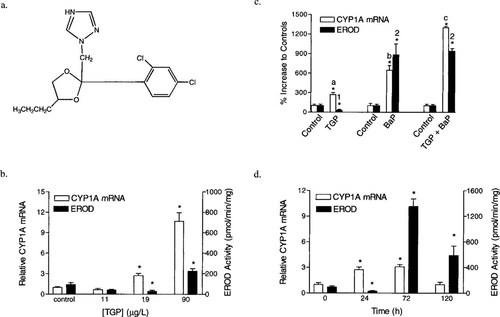
(a) Structure of the antifungal triazole compound propiconazole. (b) Concentration-response for rainbow trout hepatic CYP1A mRNA and EROD activity following 24 h of continuous waterborne technical grade propiconazole (TGP) exposure. Columns and error bars represent the mean + standard error of the mean. * represents values that are significantly different compared to their respective control values (p < 0.05). (c) Comparison of hepatic CYP1A mRNA levels and EROD activity from fish exposed to TGP, to benzo[a]pyrene (BaP), or to TGP and BaP. The CYP1A mRNA values with different letters are significantly different and EROD values with different numerals are significantly different (p ≤ 0.05). (d) Temporal response of hepatic CYP1A mRNA and EROD activity in rainbow trout following continuous waterborne TGP exposure.
The focus of the present study was to examine the concentration- and time-dependent effects of TGP on hepatic CYP1A mRNA levels and CYP1A catalytic activity in rainbow trout. Rainbow trout were chosen as the model species because the majority of research examining the effects of antifungal imidazole compounds on cytochromes P450 of fish has been done with this species [16, 17]. A comparison was made between the ability of TGP and analytical grade propiconazole (AGP) to influence AhR-activated CYP1A mRNA levels and catalytic activity in rainbow trout, to influence AhR-activated luciferase activity in recombinant mammalian cell line, and to influence transformation of a mammalian AhR to its DNA binding form with gel retardation analysis. Interaction between TGP and the CYP1A protein was investigated by conducting kinetic and spectral assays. Further, a comparison was made between the CYP1A-inducing potential of benzo[a]pyrene (BaP) and TGP.
MATERIALS AND METHODS
Chemicals
The TGP (formula weight = 342.2) for waterborne exposures was provided by Ciba-Geigy (90.2% purity; Greensboro, NC, USA). The AGP for spectral experiments and analytical standards was obtained from Research Diagnostics (99.9% purity; Tittisville, NJ, USA). C18 sep-pak columns were purchased from Phenomenex (Torrance, CA, USA). A random priming oligo-kit, restriction endonucleases, and probe-quant G-50 columns were purchased from Pharmacia (Uppsala, Sweden). Nitrocellulose BA 85 (0.2 μm) and a Turboblotter kit were purchased from Schleicher and Schuell (Keene, NH, USA). Luciferase activity was determined using the Promega luciferase assay system (Madison, WI, USA). Phenol, chloroform, acetonitrile, methanol, formamide, ficoll, guanidinium thiocyanate, sodium dodecyl sulfate, sarkosyl, glycerol, intensifying screens, and RX Fuji film were purchased from Fisher Scientific (Pittsburgh, PA, USA). The 32P-labeled nucleotide [α-32P]dCTP (3,000 Ci/nmol) was purchased from DuPont (NEN) (Wilmington, DE, USA). All other materials were purchased from Sigma Chemical (St. Louis, MO, USA).
Care and handling of fish
For waterborne exposures, rainbow trout (weight range, 50–75 g) were provided by the London Fish Hatchery (Ohio Division of Wildlife, London, OH, USA) and were held in dechlorinated water in 500-L, flow-through tanks on a 12 light: 12 dark photoperiod. For the intraperitoneal injection studies, rainbow trout (250–300 g) were purchased from Jones Fish Hatchery (Newtown, OH, USA). Fish were maintained at 12 ± 1°C in a flow-through system with carbon filtered tap water. All experiments were conducted at 12 ± 1°C.
Waterborne exposures
Waterborne exposures were performed to characterize the concentration- and time-dependent effects of TGP on CYP1A expression. Four fish were used for each exposure group and for controls. Livers from individual fish were subdivided to obtain parallel measurements for CYP1A mRNA levels and for CYP1A catalytic activity. Exposure concentrations were maintained with a dilutor system [18] at 12°C under low-level, cool, white fluorescent lighting. For quantification, TGP was extracted from the water by passing 200 ml of exposure water through a C18 sep-pak column and eluted with 1.0 ml of acetonitrile. Extracts were measured with a high pressure liquid chromatography (HPLC) system equipped with a spectrophotometric detector at 268 nm. A 100-μl sample was injected onto a pre-equilibrated C18 reverse-phase column (4.6 × 150 mm, 5 micron) and eluted isocratically over 6 min at 30°C at a flow rate of 1.0 ml/min with a mobile phase containing 80% acetonitrile:20% water. The mean retention time under these conditions for AGP and TGP was 3.6 min. The TGP was identified, and water concentrations were calculated by comparison to retention times and responses of AGP standards. The limit of detection with this method was 2 μg/L in the exposure tanks.
The BaP exposure and BaP-TGP coexposure concentrations were also maintained with a proportional dilutor system. The BaP water concentrations were measured using reversephase HPLC with a fluorescence detector at an excitation wavelength of 285 nm and an emission wavelength of 405 nm. A 100-μl sample was injected onto a pre-equilibrated C18 column (4.6 × 150 mm, = micron) and eluted isocratically with a mobile phase containing 80% acetonitrile:20% water at a flow rate of 1.0 ml/min. The mean retention time for BaP was 10.3 min with a limit of detection of 0.10 μg BaP/L.
Quantification of CYP1A mRNA levels
Total RNA was isolated by acid guanidinium thiocyanatephenol- chloroform extraction [19] and quantified spectrophotometrically at 260 nm. For slot blots, 5 mg of formaldehydedenatured RNA in a final volume of 39 μl was mixed with 2 volumes of 203 standard saline citrate (SSC), and samples were then applied to the slot blot [20]. Each well was washed twice with 500 μl 10 × SSC, and the filter was air dried and baked at 80°C for 2 h. Membranes were prehybridized at 42°C in 5 × SSC, 0.1% sodium dodecyl sulfate, 5 × Dendardt's solution [20], 50% formamide, and 100 μg/ml yeast tRNA for 24 h. The CYP1A rainbow trout pfP1450-3′ cDNA [10] (ATCC 37652) was excised from the plasmid pUC18 with EcoR1 and PstII. The 1.5-kb insert was labeled with [α-32P]dCTP by the random priming method [21], unincorporated nucleotides were removed with a probe-quant G-50 column, and 1 × 106 cpm/ ml was used for hybridization. Hybridizations were conducted at 42°C for 24 h, and the final wash was in 0.2 × SSC, 0.1% sodium dodecyl sulfate at 65°C over 30 min. Hybridization to a cDNA specific for r18 S RNAs from Xenopus laevis was used to confirm that equal amounts of RNA were applied to each lane and each slot. Membranes were stripped by washing in stripping buffer (0.1 × SSC, 0.1% sodium dodecyl sulfate) at 100°C for 30 min. Hybridized filters were exposed to preflashed Fuji RX film at 280°C with intensifying screens. Optical densities of CYP1A mRNA and rRNA bands were compared with the National Institute of Health Image gel analysis software package (version 1.60) obtained from the National Institute of Health Image homepage at http://rsb.info.nih.gov/nih-image.
Ethoxyresorufin-O-deethylase and NADPH cytochrome c reductase activity
Microsomes were isolated from livers as previously described [22], resuspended in 0.1 M Tris, 20% v/v glycerol (pH 7.8), and stored at 280°C for a period of 4 to 6 weeks. A modification of the catalytic method described by Burke and Mayer [23] was performed to measure ethoxyresorufin-O-deethylase (EROD) activity. Incubations were performed in a volume of 500 μl containing 0.1 M Tris (pH 7.8), 0.25 mg/ml microsomal protein, and 2.5 μM ethoxyresorufin. The assays were initiated after a 1-min preincubation with 2.5 μl of 50 mM NADPH at 25°C and ended after 0.5 min with the addition of 1 volume of ice-cold acetonitrile. Incubation vials were centrifuged to remove precipitated microsomal protein and measurement of resorufin concentrations were made as described by Levine et al. [22]. Formation of resorufin was linear over the incubation period. Incubations without NADPH and incubations without protein served as negative controls.
The NADPH cytochrome c reductase activity was measured with fish that were not previously exposed to propiconazole. The NADPH cytochrome c reductase activity was measured with a reaction mixture containing 970 μl of horse heart cytochrome c (1.1 mg/ml in 0.1 M Tris buffer, pH 7.8, 1 mM KCN) and 5 μl of microsomal suspension (14 μg protein). The TGP was added in 5 μl of methanol to a final concentration of 6 μM, and samples were incubated at 25°C for 2 min. The reaction was started with 20 μl of NADPH (3.6 mg/ml) and the reduction of cytochrome c was measured continuously at 550 nm for 1 min (e = 21.1/mM/cm). Control assays were incubated with 5 μl of methanol alone before the reaction was started. Measurements were conducted with a dual-beam spectrophotometer.
Intraperitoneal injections
To compare the CYP1A-inducing potential between TGP and AGP, fish were injected with dimethyl sulfoxide (DMSO) (1 μl/g), 100 mg AGP/kg (1 μl/g) in DMSO, or 100 mg TGP/ kg (1 ml/g) in DMSO. Livers from individual fish were subdivided 24-h postinjection to obtain parallel measurements for CYP1A mRNA levels and for EROD activity.
Measurement of AhR-mediated luciferase activity in the recombinant mouse H1L.1c2 cell clone
Cell line. Mouse hepatoma (hepa1c1c7) cells, stably transfected with the halogenated aromatic hydrocarbon-inducible luciferase expression vector pGudLuc1.1, referred to as H1L.1c2 cells, were prepared as previously described [24]. This vector contains the firefly luciferase gene under halogenated aromatic hydrocarbon-inducible control of four DREs such that, when exposed to AhR ligands, induction of luciferase occurs in a time-, dose-, and AhR-dependent manner [24]. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-inducible luciferase activity is detectable at 2 h and peaks at 4 h [24].
Induction protocol. Twenty-four-well plates of stable cell clones were incubated with DMSO alone, with AGP in DMSO, with TGP in DMSO, or with TCDD, which served as a positive control (at the indicated concentration) for 4 h at 37°C. After the induction incubation period, cells were washed with phosphate-buffered saline and then lysed using Promega lysis reagent as described by the manufacturer. The lysates were centrifuged and 35 μl of the cleared lysate was added to a 96- well plate. Luciferase activity was determined using the Promega luciferase assay system in an automated microplate luminometer, in which 100 μl of luciferase substrate was injected into each well. Light output was integrated over 20 sec after a delay of 10 sec to allow the light output to reach maximal intensity.
Protein correction. Protein concentrations were determined in each well using a modification of the fluorescamine bioassay [25]. Briefly, fluorescamine in acetone was added to all wells, including a standard curve of 0 to 50 μg bovine serum albumin assayed in triplicate. Subsequent fluorescence was quantified using a microplate fluorometer at 400 nm excitation and 460 nm emission wavelengths. Protein concentrations were estimated from the standard protein curve. All samples were expressed as relative light units per milligram of protein.
Gel retardation analysis
Gel retardation assays were performed as described by Washburn et al. [26]. A complementary pair of synthetic oligonucleotides containing the sequence 5′-GATCTGGCTCTTCTCACGCAACTCCG-3′ and 5′-GATCCGGAGTTGCGTGAGAAGAGCCA-3′ (corresponding to the AhR binding site on the DRE3 and designated as the DRE oligonucleotide) was synthesized, purified, annealed, and radiolabeled with [γ32P]ATP as described by Helferich and Denison [27]. Guinea pig hepatic cytosol (16 mg protein/ml) was incubated with DMSO alone, 100 μM AGP in DMSO, 100 μM TGP in DMSO, or 1 nM TCDD in DMSO for 2 h at 25°C. The 100 μM concentrations of propiconazole were calculated based on purity. After incubation, the samples were mixed with ficoll sample buffer (to 10% [v/v]) and loaded onto a 4% nondenaturing polyacrylamide gel that had been pre-electrophoresed for 1 h. The samples were subjected to electrophoresis with continual recycling of a Tris-acetate-EDTA buffer. The gels were then dried and protein-DNA complexes visualized with autoradiography.
Optical difference spectroscopy and EROD kinetic assays
Hepatic microsomes isolated from rainbow trout that were given a 1-d exposure to 1.18 μg BaP/L were diluted to a concentration of 1 mg protein/ml in 50 mM Tris (pH 7.8) and divided equally between the sample and the reference cuvettes. Either TGP or AGP dissolved in methanol was added to a final concentration of 10 μM in 2-ml aliquots to the sample cuvette, and an equal amount of methanol was added to the reference cuvette. Difference spectra were recorded following a 10-min equilibration period. To determine if cytochrome P450-bound propiconazole could be displaced by CO, the sample and reference cuvettes were bubbled with CO for 30 s, the sample cuvette was reduced with 1 mg of sodium dithionite, and the difference spectra were recorded following a 10-min equilibration period. Spectral assays were conducted with a dualbeam spectrophotometer.
The EROD kinetic assays with hepatic microsomes were performed with two TGP concentrations (10−2 and 10−1 μM) and seven ethoxyresorufin substrate concentrations (range = 31–2,500 nM). Microsomes were isolated from rainbow trout that received 24 h of waterborne exposure to 1.18 μg BaP/L. The CYP1A-induced fish were used in this portion of the study because the intrinsically low activity of uninduced microsomes precluded detection of decreases of EROD activity over a large range of inhibitor concentrations. Control incubation mixtures contained the same volume of vehicle (5 μl of methanol) that was used for additions of TGP. The EROD assays for inhibition and kinetic assays were performed as described within, but TGP was added to the incubation mixture prior to the addition of protein, ethoxyresorufin, and NADPH. Formation of resorufin in the kinetic assays was linear for each substrate concentration over the incubation period.
Chromatographic analysis of TGP
The TGP and AGP standards (100 μM) were prepared in DMSO. A 40 μl aliquot was injected onto a C18 reverse-phase column (4.6 × 150 mm, 5 micron) and eluted with a linear gradient from 30% acetonitrile:70% water to 70% acetonitrile: 30% water over 45 min at 30°C with a flow rate of 1.0 ml/min. The eluent was monitored continuously with a spectrophotometric detector at 268 nm.
Statistics
Exposure concentrations, EROD activity, relative mRNA levels, and NADPH cytochrome P450 reductase activity are reported as means ± standard error of the mean (SEM). Oneway ANOVA with Dunnett's t test was used to detect differences between control and TGP treatment groups for CYP1A mRNA levels, EROD activity, NADPH cytochrome c reductase activity, or luciferase activity (α = 0.05) [28]. One-way analysis of variance with Duncan's multiple range test was used to identify significant differences between EROD activity and CYP1A mRNA levels for fish exposed to TGP alone, exposed to BaP alone, or coexposed to TGP and BaP (α = 0.05) [28]. The Student's t test was used to determine if fish coexposed to TGP and BaP had an additive increase in CYP1A mRNA and EROD activity relative to fish exposed to TGP alone and to BaP alone. For this analysis, the mean level of CYP1A mRNA and EROD activity for fish exposed to TGP alone and to BaP alone were added together and a pooled standard deviation was used to construct a 95% confidence interval that was then used to compare with the 95% confidence interval from fish coexposed to BaP and TGP. Apparent Vmax and apparent Km values ± asymptotic standard error were calculated using a nonlinear regression model that fit the Michaelis-Menten equation using the SAS NLIN procedure [28]. Statistical differences between apparent Vmax and apparent Km values were determined by comparing asymptotic 95% confidence intervals. For secondary plots, the log of (1 + [TGP nM]) transform was used to linearize the data so that linear regression could be applied to determine the x-intercept ± SEM [29]. The Vmax and Km values derived by nonlinear regression were used to construct the secondary plots.
RESULTS
Concentration response
Rainbow trout were given waterborne exposures to environmentally realistic waterborne concentrations of TGP to characterize concentration- and time-dependent effects on CYP1A expression. The CYP1A expression demonstrated a concentration-dependent response following 24 h of exposure to TGP. The median exposure concentration (18.9 ± 1.6 μg/L) increased relative CYP1A mRNA levels 300%, but EROD activity was inhibited by greater than 50% relative to controls (Fig. 1b). The highest exposure concentration (90.4 ± 6.1 μg/L) increased relative CYP1A mRNA levels 1,100%, but EROD activity was increased by only 200% relative to controls. To test for inhibition of NADPH reductase activity, a concentration of 6 μM TGP was used. This concentration is slightly above the IC50 for hepatic microsomal EROD activity but does not cause inhibition of NADPH cytochrome c reductase. Levels of NADPH cytochrome c reductase activity were not significantly different. Values were 1.34 ± 0.05 nanomoles/ min/mg protein and 1.35 ± 0.07 nanomoles/min/mg protein for microsomes preincubated with carrier solvent alone and for microsomes preincubated with TGP, respectively.
Coexposures to TGP and BaP
Rainbow trout were exposed to TGP, BaP, or TGP and BaP for 24 h in order to compare the abilities of these two compounds to modulate CYP1A expression. Exposure to 21 μg TGP/L caused a 300% increase in CYP1A mRNA levels but significant inhibition of EROD activity (Fig. 1c). In contrast to the results with TGP, exposure to BaP increased both CYP1A mRNA levels and EROD activity (Fig. 1c). Exposure to 1.23 μg BaP/L, which is a concentration near the water solubility for BaP, produced an 800% increase in CYP1A mRNA levels and a 1,000% increase in EROD activity. The combined CYP1A mRNA level for fish exposed to TGP alone and for fish exposed to BaP alone added up to an 1,100% increase over control levels. Correspondingly, coexposure to 21 μg TGP/L and 1.42 μg BaP/L produced an additive increase (1,300%) in CYP1A mRNA levels (Fig. 1c). However, EROD activity for fish coexposed to TGP and BaP did not exhibit an additive increase (Fig. 1c). In other words, there was not a significant difference in EROD activity between fish exposed to BaP alone and fish coexposed to TGP and BaP. This nonadditive increase in EROD activity for fish coexposed to TGP and BaP appears to be the result of partial inhibition of EROD activity by TGP.
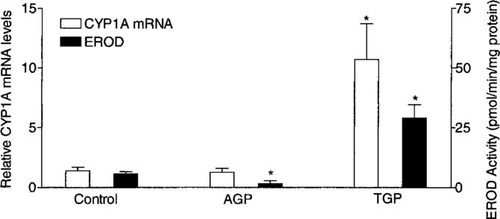
Modulation of hepatic CYP1A mRNA levels and EROD activity for rainbow trout 24-h postinjection with either 100 mg analytical grade propiconazole (AGP)/kg or 100 mg technical grade propiconazole (TGP)/kg in dimethyl sulfoxide (DMSO). The CYP1A mRNA values and EROD activity values (for exposed groups) with an asterisk are significantly different from control values (p < 0.05).
Temporal response
Rainbow trout CYP1A mRNA levels and EROD activity demonstrated a time-dependent response over 120 h of continuous exposure to a mean concentration of 18.8 ± 1.1 μg TGP/L (Fig. 1d). Relative CYP1A mRNA levels were induced 300% after 24 and 72 h of exposure but decreased to basal levels after 120 h of exposure. In comparison, EROD activity was initially inhibited after 24 h of exposure, became highly induced after 72 h of exposure, but then decreased by 50% after 120 h of exposure. Both the concentration- and timedependent exposures demonstrated that TGP contained an AhR agonist.
Comparison between the CYP1A-inducing potential of TGP and AGP
To determine if induction of CYP1A mRNA levels resulted from the presence of an AhR agonist in TGP, rainbow trout received intraperitoneal injections with either TGP (90.2% purity) or intraperitoneal injections with AGP (99.9% purity). Injections of 100 mg AGP/kg did not affect CYP1A mRNA levels but caused a >80% inhibition of EROD activity relative to controls (Fig. 2). In contrast, fish that received injections of 100 mg TGP/kg had a 1,100% increase in CYP1A mRNA levels and a lower but significant (500%) increase in EROD activity. This comparison demonstrated that the increase in CYP1A mRNA levels caused by TGP resulted from an AhR agonist in TGP.

The aryl hydrocarbon receptor (AhR)-mediated induction of luciferase activity in a recombinant cell line following a 4-h incubation period with analytical grade propiconazole (AGP) (100 μM), technical grade propiconazole (TGP) (100 μM), or tetrachlorodibenzo-p-dioxin (TCDD) (1 nM). Luciferase activity values with an asterisk are significantly different from incubations that received dimethyl sulfoxide (DMSO) alone (p < 0.05).
An additional comparison between AGP and TGP was conducted with a recombinant mammalian cell line. This cell line contains the firefly luciferase gene under the control of several DREs that respond to AhR agonists with a concentration-, time-, and AhR-specific induction of luciferase activity [24]. Cells were incubated with carrier alone, 100 μM AGP, 100 mM TGP, or 1 nM TCDD. Incubations with AGP and TGP resulted in a 3- and a 33-fold increase in luciferase activity, respectively, relative to controls (Fig. 3), which was approximately 1 and 44% of the relative increase in luciferase activity, respectively, resulting from exposure to 1 nM TCDD.
The induction of luciferase activity by TGP suggests involvement of the AhR but does not directly confirm it. In order to assess the ability of these formulations to transform the AhR into its DNA-binding form, we carried out gel retardation analysis. Although this assay does not directly confirm the ability of an AhR agonist to induce CYP1A expression, the positive correlation between the relative induction of luciferase activity in the recombinant cell line and the relative ability of AGP and TGP to stimulate AhR transformation and DNA binding (Fig. 4) suggest that TGP contains an AhR agonist. The inability of concentrations of 200 μM AGP and TGP to increase AhR:DRE complex formation over the 100-μM concentration appears to be due to solubility limitations of propiconazole at concentrations >100 μM.
Optical difference spectra and inhibition of in vitro EROD activity
Difference spectroscopy and kinetic assays were used to determine the mechanism of EROD inhibition by propiconazole. Addition of either AGP or TGP to hepatic microsomes produced a type II difference spectrum characterized by an absorbance maxima at 425–427 nm and an absorbance minima at 407–411 nm. The TGP and AGP were completely displaced from cytochromes P450 after bubbling for 30 s with CO. Displacement of the type II difference spectra after bubbling with CO demonstrated that TGP and AGP directly and reversibly bound to the heme moiety of cytochromes P450.
The EROD kinetic assays were performed to characterize the mechanism of inhibition by TGP on CYP1A catalytic activity. The EROD activity in the presence and absence of TGP exhibited Michaelis-Menten-type kinetics (Fig. 5a). Increases in TGP concentration caused apparent Vmax values to decrease but did not cause a significant effect on apparent Km values (Fig. 5b). The lack of significant differences in apparent Km values along with a concentration-dependent decrease in apparent Vmax values, resulting from increasing TGP concentrations, were indicative of purely noncompetitive inhibition of EROD activity [30]. In noncompetitive inhibition, the inhibitor binds to the free enzyme, characterized by the inhibition constant KI, and the enzyme-substrate complex, characterized by the inhibition constant KIS, with equal affinity [29]. Secondary plots were constructed to further illustrate that TGP had an equal affinity for the free enzyme and the enzyme-substrate complex (KI = KIS) [29]. The value for KIS = 4.4 ± 0.2 μM (Fig. 5c) and the value for KI = 4.7 ± 0.1 mM (Fig. 5d), which were not significantly different values.
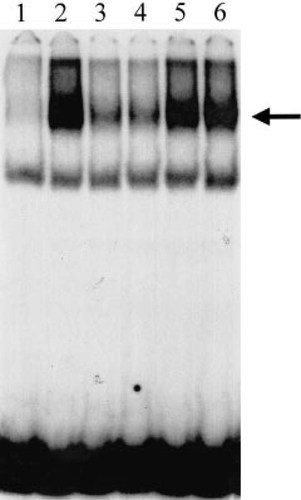
Gel mobility shift induced by tetrachlorodibenzo-p-dioxin (TCDD), analytical grade propiconazole (AGP), and technical grade propiconazole (TGP). The arrow indicates the retarded band induced by ligand-aryl hydrocarbon receptor (AhR)-dioxin responsive elements (DRE) complex formation. Lane 1 represents dimethyl sulfoxide (DMSO) alone, lane 2 represents 1 nM TCDD in DMSO, lanes 3 and 4 represent 100 μM and 200 μM AGP in DMSO, respectively, and lanes 5 and 6 represent 100 μM and 200 μM TGP in DMSO, respectively.
Chromatographic analysis of TGP
Reverse-phase HPLC and spectrophotometric analysis of AGP resolved two primary peaks that appear at 23.9 and 24.1 min and may represent the two diastereomers of propiconazole [31, 32] (Fig. 6). However, since analytical grade standards for the isomers of propiconazole were not available, we were precluded from determining if these two peaks represented the diastereomers. Analysis of TGP resolved several small peaks with shorter retention times (14.2, 18.6, 19.0, and 22.1 min) than what are believed to be the two isomers of propiconazole (Fig. 6). The predominant peak in TGP had a retention time of 24.1 min, which is the same retention time as the predominant peak in the AGP formulation.
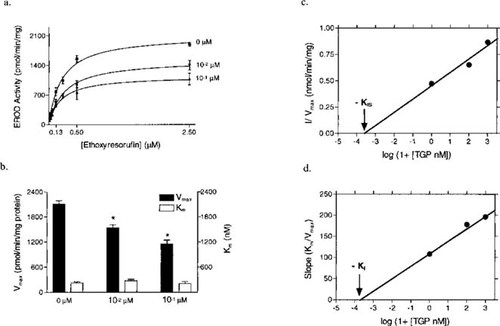
(a) Michaelis-Menten plot of in vitro inhibition of rainbow trout hepatic EROD activity by technical grade propiconazole (TGP). Each point represents the mean of three separate determinations ± the standard error of the mean (SEM). (b) Graph of kinetic values for TGP inhibition of in vitro rainbow trout hepatic EROD activity + SEM. (c and d) Secondary plots for determination of the inhibitor constants for inhibition of rainbow trout EROD activity.
DISCUSSION
In vivo, TGP produced concentration- and time-dependent modulation of CYP1A activity. A mixed-pattern response for CYP1A expression was observed after 24 h of exposure to concentrations of 19 μg/L or 90 μg/L (Fig. 1b). The mixedpattern response for fish exposed to 19 μg/L was characterized by a 300% increase in CYP1A mRNA levels but a 70% inhibition of EROD activity. In a similar manner, fish exposed to 90 μg/L had a greater than 1,000% increase in CYP1A mRNA levels but only a modest 200% increase in EROD activity. In vitro, TGP formed a type II binding spectra and acted as a purely noncompetitive reversible inhibitor of CYP1A catalytic activity. Noncompetitive in vitro inhibition of EROD activity may explain the lack of correlation between CYP1A mRNA levels and EROD activity in vivo. In addition, coexposure to BaP and TGP created an additive increase in CYP1A mRNA levels but a nonadditive increase in EROD activity. This again may indicate partial inhibition of CYP1A catalytic activity by an environmentally realistic concentration of TGP. A biphasic pattern has also been reported for the relationship between CYP1A protein levels and CYP1A catalytic activity of rat and bobwhite quail that were treated with TGP [7].
In addition to showing a mixed-pattern response, EROD activity for fish exposed to 19 μg TGP/L demonstrated a biphasic pattern with respect to time (Fig. 1d). The biphasic pattern was characterized by initial inhibition of EROD activity followed by induction of EROD activity. Correspondingly, Niemeegers et al. [33] demonstrated that administration of the clinically used antifungal imidazole compound clotrimazole resulted in a mixed-pattern response with rats. In that study, clotrimazole caused initial inhibition of cytochrome P450 activity, but induction was evident 120 h after administration of the dose. In another study, it was shown that induction of cytochrome P450 in rats by the agriculturally used antifungal imidazole compound prochloraz can only be fully recognized 48 h after administration of the dose [34]. This lag period coincided with the time when the prochloraz elimination rate was highest.
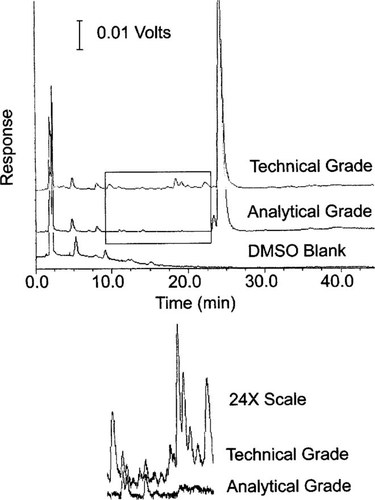
Reverse-phase high-pressure liquid chromatographic analysis of dimethyl sulfoxide (DMSO), analytical grade propiconazole (AGP) in dimethyl DMSO, and technical grade propiconazole (TGP) in DMSO. The two formulations were eluted with a linear gradient from 30% acetonitrile:70% water to 70% acetonitrile:30% water over 45 min with a flow rate of 1.0 ml/min. The eluent was monitored continuously with a spectrophotometric detector at 268 nm.
The CYP1A mRNA levels also demonstrated a biphasic pattern with respect to time, which was characterized by initial induction of CYP1A mRNA levels followed by a return of CYP1A mRNA to basal levels after 120 h of exposure. The return of CYP1A mRNA to basal levels after 120 h of exposure may have resulted from lower hepatic concentrations of TGP at this time relative to hepatic concentrations between 24 and 72 h of exposure when CYP1A mRNA levels were induced (Fig. 1d). Frequently, compounds that are P450 substrates can induce their own cytochrome P450-mediated oxidative metabolism. Perhaps TGP is capable of inducing its own metabolism and overcoming initial inhibition of EROD activity during the first 24 h of exposure [35]. The agriculturally used antifungal imidazole compound prochloraz was shown to be metabolized to glucuro-conjugated metabolites and to a lesser extent hydroxy metabolites [36]. Since hepatic propiconazole concentrations and metabolic products of propiconazole were not measured, we were unable to determine the cause of the biphasic pattern for CYP1A mRNA levels.
To determine if induction of CYP1A mRNA by TGP resulted from unidentified compounds in TGP, the TGP and the AGP formulations were compared with in vivo and in vitro analyses. For in vivo tests, a concentration of 100 mg/kg was chosen because concentrations ≤50 mg/kg of clotrimazole (99.9% purity) were shown to inhibit gizzard shad and rainbow trout EROD activity [17, 37]. Fish given intraperitoneal injections of 100 μg AGP/kg showed significant inhibition of hepatic EROD activity, but CYP1A mRNA levels were not affected (Fig. 2). In comparison, intraperitoneal injections with 100 mg TGP/kg caused high induction of CYP1A mRNA levels but only modest induction of EROD activity; this pattern was almost identical to the pattern for the highest tested waterborne TGP concentration and may again illustrate partial inhibition of EROD activity by TGP (Fig. 1b). Rainbow trout given intraperitoneal injections with 100 mg clotrimazole/kg had a nearly duplicate CYP1A expression pattern to rainbow trout that received injections with 100 mg AGP/kg [17]. The comparison between clotrimazole and AGP suggests that highly pure formulations of antifungal imidazole and triazole compounds are not inducers of constitutive CYP1A gene expression in fish but rather that they only influence CYP1A catalytic activity in fish.
In contrast to the results from intraperitoneal injections, AGP caused a small increase in AhR activated luciferase reporter activity (Fig. 3) and to a small degree activated the AhR to its active DNA-binding form (Fig. 4). The AhR was activated in the recombinant cell line and the gel retardation analysis but not in the whole fish, resulting in a higher affinity of the mammalian AhR for propiconazole relative to the AhR from rainbow trout. Previously, it was shown that the AhR from scup has different ligand-binding characteristics compared to the mammalian AhR [38]. This study determined that several polychlorinated biphenyl congeners were mixed-type inducers of CYP1A and CYP2B in mammals but were relatively ineffective inducers of CYP1A expression in scup. It was proposed that CYP1A induction in fish requires compounds with a greater degree of planarity compared with mammals. This in turn suggests that the AhR in scup and perhaps other species of fish may have different ligand-binding properties than in mammalian species. Collectively, the results from in vivo and in vitro analyses provide evidence that activation of CYP1A expression in rainbow trout by TGP resulted from unidentified compounds that have an affinity for AhR. Two by-products, a symmetrical 1,3,4-triazole racemic-constitutional isomer and a propiconazole ditriazole analogue, were isolated in the synthesis of propiconazole [31]. Whether these by-products or other constituents were responsible for AhR dependent activation of CYP1A gene expression requires further investigation.
Antifungal imidazole compounds have been shown to interfere with a number of cytochrome P450 reactions, and propiconazole appears to interact with cytochromes P450 in a similar way as imidazole compounds [17, 37]. A number of antifungal imidazole derivatives inhibit cytochrome P450-mediated steroid metabolism [39, 40] as well as xenobiotic metabolism in rats [41-43]. Prochloraz caused a concentrationdependent inhibition of ovarian microsomal aromatase activity and follicular oestradiol secretion in rainbow trout [44]. Prochloraz and clotrimazole have also been shown to inhibit cytochrome P450-mediated xenobiotic metabolism in rainbow trout [16] and gizzard shad [37], respectively. Because propiconazole interacts with cytochrome P450 in a similar way as other antifungal imidazole compounds, propiconazole and/ or unidentified compounds within the TGP have the potential to modulate a wide range of cytochrome P450-dependent processes in wildlife.
Environmental consequences
Since antifungal imidazole compounds are widely used in agriculture, the mixed pattern of inhibition and induction of CYP1A catalytic activity, as well as potentially other cytochrome P450 isoenzymes produced by this class of compounds in mammals [7], birds [7], and fish, may lead to unexpected toxicological responses when coapplied with other chemicals. With the red-legged partridge, either penconazole or prochloraz in combination with the organohosphate insecticide malathion enhanced malathion toxicity by increasing oxon formation [45, 46]. A similar experiment done with fathead minnows showed that pretreatment with TGP can enhance in vitro hepatic parathion activation rates as well as enhance the acute toxicity of parathion [47]. With the honeybee, the presence of either TGP or prochloraz increased the toxicity of the pyrethroid insecticide λ-cyhalothrin 9- and 17-fold, respectively, by delaying the metabolism, detoxification, and elimination of the insecticide [48]. In contrast to the work done with redlegged partridge [45, 46], Ronis and Badger reported that TGP did not effect parathion-induced inhibition of serum cholinesterase activity and that TGP lowered the activation:deactivation ratio for parathion in rat [49].
Presently, there is the potential for coapplication of TGP with a number of different organophosphate insecticides on banana plantations [50]. The complex pattern of inhibition or induction of CYP1A protein activity and/or potentially other cytochrome P450 isoenzymes by TGP may lead to unexpected toxicological interactions with other pesticides that were not predicted from single-compound-only hazard assessments. Further studies will be conducted to characterize the unidentified compounds in TGP that were responsible for CYP1A induction.
Acknowledgements
Portions of this research were supported through a U.S. Environmental Protection Agency Fellowship awarded to S. Levine, by National Institutes of Environmental Health Sciences grant ES07685, and by Superfund research support grant ES04699. The authors would like to acknowledge Dr. Jay Gooch for his valuable comments on this manuscript.




