Field transplantation of the freshwater bivalve Corbicula fluminea along a polymetallic contamination gradient (river Lot, France): II. Metallothionein response to metal exposure
Abstract
Specimens of the Asiatic clam Corbicula fluminea were transplanted from a clean lacustrine site to four stations along a polymetallic pollution gradient in the river Lot, France, downstream from an old Zn ore treatment facility (see Part I). From April to September 1996, we studied Cd and Zn bioaccumulation and the metallothionein-like metal-binding protein (MT) concentrations by subsampling the cages at t = 0, 21, 49, 85, 120, and 150 d. Marked differences were observed among the four stations. At the most polluted station Riou-Mort, MT concentrations did not increase despite very rapid metal accumulation; all mollusks died between days 49 and 85, suggesting that the metal detoxification mechanisms were overwhelmed at this station. At the next station downstream, the final levels of bioaccumulated metal after 150 d were as high as those at the Riou-Mort station (t = 21 d), but in this case the MT concentrations also increased progressively with positive correlations between MT and metal concentrations; no mortality was observed, but a significant growth inhibition was revealed in comparison to the reference site, with a lack of correlation between MT (as a stress response) and reduced growth. Subcellular metal partitioning, as determined by size-exclusion chromatography, revealed that most of the Cd was sequestered by MT (70% of cytosolic Cd). In contrast, most of the Zn was bound to low molecular weight proteins (70%, ≤ 6.5 kD), the MT fraction representing only 12% of cytosolic Zn. These data show the marked role of MT toward Cd bioaccumulation and toxic effects on this freshwater bivalve species.
INTRODUCTION
Results obtained during the 5-month transplantation of Corbicula fluminea along a pollution gradient on the river Lot [1] confirm the high bioaccumulation capacities of metals in benthic bivalves, especially for Cd. Bioaccumulation results from the availability of metals binding and crossing biological barriers at the interface between the soft body and the surrounding medium within the palleal cavity: gills, mantle, gut wall, and elsewhere. After their transport across the cellular membrane, intracellular storage and sequestration of metals are based on a sequence of events more or less specific for each element (essential or toxic metals). Among the best-studied intracellular structures involved in metal storage in aquatic invertebrates are vesicle-bound granules and metal-binding proteins [2, 3]. Among the cytosolic ligands, metallothioneins (MTs) are low molecular weight proteins (6–7 kD) first discovered by Margoshes and Vallee in 1957 [4] in horse kidney cortex. They were then described in many animal species (mammals, reptiles, amphibians, invertebrates, and so on) as well as in plants [5-8]. They possess a high proportion of cysteine residues (30% of total amino acids) placed in specific sequences that allow them to be classified into three classes according to their homology with mammalian MTs [9, 10]; MTs in bivalves generally belong to class I, with a high level of similarity with mammalian MTs [11, 12]. The primary role of MTs in cellular metabolism is believed to be their participation in the homeostasis of essential metals such as Cu and Zn [11]. They are also responsible for protective action against toxic metals, such as Cd, Ag, or Hg, because of their capacity to sequester these metals and decrease their bioavailability to other cellular ligands. The metals present different affinity constants for the thiol sites located in the two clusters of these globular proteins, following the sequence: Hg(II) ≫ Cu(I), Ag(I), Bi(III) ≫ Cd(II) > Pb(II) > Zn(II) > Co(II) > Fe(II) [13]. Many of these metals are able to induce the synthesis of MTs by a transcriptional activation system using regulatory elements and factors activated by the concentration of free Zn in the cytosol [3, 11, 14].
Numerous research studies have been carried out on the use of MT as biomarkers in relation to the contamination of aquatic species by heavy metals [11, 12, 15, 16]. However, MTs have complex biosynthetic regulation mechanisms. Indeed, they are inducible by a wide range of other factors, such as hormones, second messengers, cytotoxic agents, physical stress, and so on [17, 18].
The aim of this study was to analyze the variations in MT concentrations in the whole soft body of C. fluminea during transplantation phases along four stations located along a polymetallic pollution gradient of the river Lot and their role in Cd and Zn bioaccumulation. Two complementary approaches were adopted: quantification of MTs using a mercury saturation assay, and purification and identification using low-pressure liquid chromatography and metal determinations in the different cytosolic protein fractions to estimate Cd and Zn burdens sequestered by MTs.
MATERIALS AND METHODS
Sampling procedure
Four adult C. fluminea specimens (1.5–2-cm shell length) were collected from the cage at each station, from April to September 1996, after 21, 49, 85, 120, and 150 d. The clams were rinsed in river water, dried on absorbent paper sheets, and immediately sealed in a polyethylene bag filled with nitrogen. They were then stored on dry ice until their transfer to the laboratory and subsequent freezing at −60°C. Freezing under anoxic conditions is necessary to avoid MT oxidation, which could result in polymerization of the molecules and interfere with the isolation and quantification procedures [19]. Metallothionein analyses were conducted at the whole organism level; after thawing, the soft bodies were dried on absorbent paper and weighed (fresh weight [fresh wt]). Four organisms (two pooled groups of two organisms each) were analyzed for each sampling time.
Metallothionein determination by Hg saturation assay
Metallothionein concentrations were determined using the mercury saturation assay adapted from Dutton et al. [20] and Couillard et al. [21]. Several important changes were made to the procedure, notably the replacement of 203Hg with cold inorganic Hg [22].
The mollusk tissues were homogenized in 20-ml polypropylene tubes with a tissue grinder (Ultra-Turrax T-25, IKA-labor-technick, Staufen, Germany) in an ice-cold Tris-HCl 25 mM buffer (Sigma, St. Quentin Fallavier, France; pH 7.2 at 20°C), with a dilution of 4 with buffer, on a w/w basis. This step was performed in a glove bag filled with nitrogen (Atmosbag, Aldrich Chemical, Milwaukee, WI, USA). The homogenized samples were kept on ice to inhibit protease activity. Aliquots of 1.5 ml of the homogenates were placed in microtubes (Eppendorf, Hamburg, Germany) and centrifuged at 20,000 g for 60 min at 4°C (Sigma 3K12, rotor 12154). To 200 μl of supernatant was added a 200-μl HgCl2 (Merck, Darmstadt, Germany) solution containing 50 mg of Hg per liter in 10% trichloroacetic acid (Sigma). This step ensures that the high molecular weight proteins were precipitated and that the MTs were saturated by Hg(II). After incubating for 10 min, a 400 μl solution of pig-blood hemolysate was added to scavenge excess Hg not bound to the MTs and the resulting suspension rapidly centrifuged at 20,000 g for 20 min to minimize transfer of the Hg(II) from the MTs to the hemoglobin. The final supernatant was quantitatively recovered and digested in 3 ml of pure HNO3 for 15 min before Hg determination.
At the same time, three reference samples or blanks—200 μl Tris-HCl buffer + 200 μl HgCl2 solution + 400 μl pigblood hemolysate—were prepared to monitor the Hg complexation efficiency of the hemoglobin. Under our experimental conditions, an average burden of only 12.2 ± 6.4 ng of Hg (n = 20) was measured in these reference samples, compared to the 10,000 ng initially added (0.12%). The mean of the three blank values measured for each analytical run was deducted from the Hg burdens measured in each sample.
A recovery percentage from purified rabbit liver MT (Sigma M-7641) was systematically determined. The standard MT solution was prepared in the homogenization buffer at a concentration of 10 μg of MT per milliliter and stored in 1.5-ml polypropylene tubes under nitrogen at −60°C. This internal standard enabled us to determine the ratio between the binding sites measured after Hg saturation and the potential binding sites indicated by the supplier and previously verified by Cd and Zn determinations on MT solution samples. Under our experimental conditions, the average value of the recovery percentage was 99.4 ± 8.7% (n = 20).
Metallothionein concentrations in C. fluminea samples were expressed as nanomoles of Hg-binding sites per gram (fw): [(nanograms of Hg in sample)/(milliliters of supernatant)] × [(tissue dilution)/(Hg molar mass)]. Because of the fact that the exact quantity of Hg binding sites per MT molecule is unknown for this species, MT concentrations cannot be expressed directly in moles of MT per gram (fresh wt).
Purification and identification of MTs
The cytosolic fractions were obtained by homogenization at 4°C in a Tris-HCl 25 mM buffer (pH 7.2 at 20°C), with 2 mM dithiothreitol (DTT, antioxidant, Sigma), 0.2‰ sodium azide (NaN3, antimicrobial agent, Sigma), and 1 mMPefabloc-Sc (protease inhibitor, Interchim, Montlueon, France); followed by centrifugation at 20,000 g for 30 min (Sigma 3K12, rotor 12111) and ultracentrifugation of the supernatant at 100,000 g for 90 min (Beckman L5-50B, Palo Alto, CA, USA, rotor 55-2Ti), both conducted at 4°C. The upper lipid layer was gently separated from the aqueous phase by aspiration and discarded.
A Sephacryl S-100 HR column (Pharmacia, Orsay, France, 1.6 × 100 cm) was equilibrated with a degassed 10 mM Tris-HCl buffer, with 150 mM NaCl, 1 mM DTT, and 0.2‰ NaN3 (pH 7.5 at 25°C). Calibration for protein-molecular weight estimation was based on blue dextran (2,000 kD), which corresponds to the void volume, bovine albumin (66 kD), carbonic anhydrase (29 kD), cytochrome c (12.4 kD), and aprotinin (6.5 kD) (Sigma). Aliquots of cytosol (2 ml) were eluted with the equilibration buffer at 4°C at a constant flow (40 ml/h) controlled by a peristaltic pump (Gilson Minipuls 3 Villiers-Le-Bel, France). Fractions of 7 ml were collected and metals were measured by atomic absorption spectrophotometry. No acidic digestion was carried out before analysis; a specific matrix modifier was used for Cd determination ((NH4)2HPO4, 0.4%) to separate the nonspecific absorption resulting from salt concentration in the samples. Fractions containing MTs were identified by the Cd peak corresponding to the elution fraction of the purified rabbit liver MT.
From metal determinations on samples collected at the different stages of the MT purification (initial homogenate, pellets, 100,000 g supernatant, and eluate fractions), an estimate of metal recovery could be made at the end of the separation procedure. The recovery obtained after chromatography was 93 ± 5% for Cd and 101 ± 6% for Zn (n = 5).
Metal determination
After digestion of biological samples, the total Cd and Zn concentrations were measured by atomic absorption spectrophotometry as described previously in the companion paper (Part I).
Total Hg determination at the end of the mercury saturation assay was carried out by flameless atomic absorption spectrometry (CETAC M-6000 Mercury Analyzer, Varian, Walnut Creek, CA, USA) after dilution of the digestates up to 20 ml with ultrapure water. A bromine salt treatment was applied before the addition of stannous chloride [23]. The detection limit was 0.01 μg of Hg per liter.
The validity of the analytical method was checked periodically by means of a standard biological reference material from lobster hepatopancreas (Tort-2, CNRC, Ottawa, ON, Canada). Values were consistently within the certified ranges for each element (data not shown).

Metallothionein concentrations and burdens in the whole soft bodies of C. fluminea during the 5 months of transplantation at the four stations. BP = Boisse-Penchot, RM = Riou-Mort, B = Bouillac, C = Cajarc. The curves correspond to the regression models and the symbols represent measured values (two replicate samples of two pooled animals each).
Data treatment
Results of MT concentrations were treated by linear regression for each station as a function of the transplantation durations, with STAT-ITCF (Paris, France). Comparison among MT data from the four stations was based on the nonparametric Kruskal-Wallis one-way analysis of variance by ranks and on the Wilcoxon-Mann-Whitney test. An alpha risk equal to 0.05 was adopted for the statistical significance of the effects observed. Simple linear correlations among fresh weight of the bivalves, Cd or Zn bioaccumulation, and MT concentrations were determined with Excel 5.0.
RESULTS AND DISCUSSION
Metallothionien concentrations in the bivalves
Figure 1 represents MT concentrations measured at the whole soft body level of C. fluminea from the four stations during the 150-d caging phase. Note that a very low dispersion is observed between the two samples analyzed for each sampling time. Data treatment based on the two nonparametric tests shows that the MT concentrations from bivalves collected at the Bouillac station are significantly different from the three other stations.
At the reference site Boisse-Penchot and at the Cajarc station, a 2.5-fold increase in MT concentrations was observed between 0 and 21 d, followed by a slight increase after 150 d. It is worth mentioning that the values of time 0 were obtained directly from the bivalves collected in the Cazaux-Sanguinet freshwater lake (Aquitaine, France) before their transplantation to the river Lot. Thus, the increase in MT concentrations during the first phase may be explained at least in part by the effects of the transplantation itself (stress effects, modification of abiotic conditions, change in nutritional supplies, and so on). According to this hypothesis, the MT concentrations in the organisms at the time of their transplantation to the different stations (real time 0 of caging) would be greater than those measured when the bivalves were first collected, thus reducing or eliminating the increase observed between 0 and 21 d. The reproductive cycle of C. fluminea may also have played a role during this period. Indeed, field studies on the Garonne river showed two spawning periods for C. fluminea, in May and September [24], and seasonal variations in MT concentrations conducted at the Cazaux-Sanguinet site revealed maximum values just before the spawning period and corresponding to maximum gonadal development [25]. Hormonal secretions, generally described in the literature as agents of induction of MT biosynthesis, could explain these results [18]. Samples taken after 21 d (May 1996) should correspond to the first phase of high MT concentrations. Embryos incubated in the gills of the individuals collected after 150 d at the two stations (September) support the hypothesis of an increase in MT concentrations in September, in direct relation to the reproductive cycle of the mollusks.
In the Riou-Mort, MT concentrations were close to the values measured at Boisse-Penchot, in marked contrast to the very high Cd and Zn bioaccumulations found in the bivalves (a 30-fold increase for Cd and a sixfold increase for Zn after 21 d compared with initial concentrations). As previously mentioned [1], the mollusks were under acute contamination conditions, leading to the death of all organisms 49 to 85 d after transplantation. These conditions suggest a spillover situation: the defense capacities of the bivalves, when confronted with the massive uptake of metals in cells and tissues, are overwhelmed, resulting in rapid and severe structural and functional damage. This theory was first described by Winge et al. [25], who stipulated that once the MTs in the cytosol are saturated by metals and the intracellular fluxes of toxic elements are beyond their biosynthesis capacities, sequestration by MTs and thus protection are overwhelmed and metals exert toxicity [2, 12, 26]. However, factors other than Cd and Zn may contribute to the toxic effects observed at this station. Other elements associated with industrial discharges or organic contaminants could have generated complementary toxic effects, able to inhibit MT production directly or indirectly or to induce structural or functional disturbances in their own right [1].
At the Bouillac station, MT concentrations increased progressively as a function of time, as the kinetics were close to those of Cd and Zn accumulations previously observed [1]: maximum values were obtained after 150 d, reaching 110 nmol sites per gram (fresh wt) (a ninefold increase compared with time 0). No mortality was observed during the 150 d of caging at this station, although metal concentrations at the end of the transplantation phase were as high as those measured in the bivalves collected at Riou-Mort after 21 and 49 d, where complete mortality occurred. However, mollusk growth at the Bouillac station was severely suppressed (−40% relative to Boisse-Penchot, after 150 d), suggesting that protection by these cytosolic proteins through metal sequestration mechanisms was not efficient enough to avoid all metabolic disturbances. We should mention that embryos incubated in the gills were not observed after 150 d, unlike the situation at the reference site Boisse-Penchot.
Correlations among metal accumulations (Cd and Zn), MT concentrations, and weight of the soft bodies
Correlations between metal accumulations and MT concentrations at the whole soft body level were studied by simple linear regression, using the average values for each sampling point (Fig. 2). They were significant in the mollusks transplanted to the Bouillac station for Cd (r = 0.94, α = 0.02) and Zn (r = 0.93, α = 0.05), which is consistent with Cd and Zn accumulations and MT concentrations previously observed. In contrast, for all the other stations, correlations were not statistically significant.
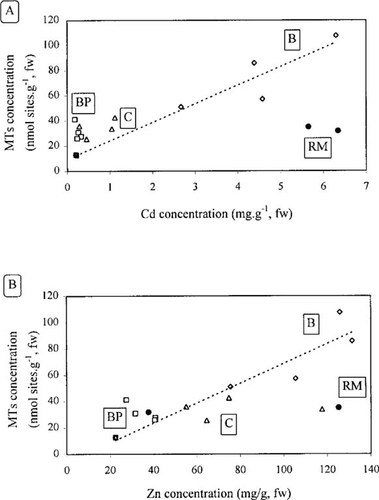
Correlations between metallothionein concentrations and Cd (A) and Zn (B) bioaccumulation, based on average values. Significant correlations are represented by linear models (α = 0.05). Correlation coefficients: (A)rB = 0.94; (B)rB = 0.93. BP = Boisse-Penchot, RM = Riou-Mort; B = Bouillac, C = Cajarc.
Linear regression analysis of MT concentrations with growth, based on the fresh weight of the soft bodies, showed significant correlations for bivalves collected at the Bouillac and Boisse-Penchot stations (Fig. 3). Nevertheless, despite a marked growth rate at the reference site (threefold during the 150-d transplantation), the MT increase was very slight compared with the Bouillac station (twofold and ninefold, respectively). At the Cajarc station, where the weight increase was the highest (fourfold), no significant correlation was observed between the two criteria. For the bivalves collected at the RM station during the two first sampling points, the absence of correlation could be directly linked to the severe exposure conditions, leading to a marked growth inhibition and jointly to biochemical perturbations, without significant differences of MT concentrations between the Riou-Mort and Boisse-Penchot stations.
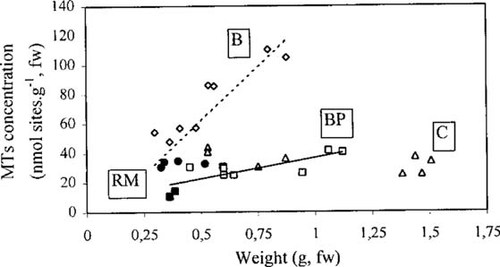
Correlations between metallothionein concentrations and soft body weight values of C. fluminea. Significant correlations are represented by linear models (α = 0.05), and the symbols correspond to measured values (two replicate samples of two pooled animals). Correlation coefficients: rB = 0.83; rBP = 0.80. BP = Boisse-Penchot; RM = Riou-Mort; B = Boillac; C = Cajarc.
Comparative analysis of Cd and Zn sequestration in the cytosolic protein fractions
Transplanted mollusks at the Bouillac station after 21 and 150 d were selected for the study of Cd and Zn distribution between pellets and cytosol fractions. Three major protein fractions containing Cd and Zn were observed in the cytosol: HMW (high apparent molecular weight proteins or void volume, ≥100 kD), fraction A (corresponding to the elution volume of the purified rabbit liver MT, apparent molecular weight around 18 kD), and fraction B (corresponding to proteins or small peptides, ≤6.5 kD) [22]. Table 1 represents Cd and Zn concentrations in the soft body homogenates, metal percentages in the cytosol phase, and relative burdens in the three protein fractions of the cytosol, after 21- and 150-d transplantation.
After 21-d transplantation, 71% of the total Cd bioaccumulated in the whole soft body was associated with the cytosol phase, which was consistent with results previously obtained after contamination of the bivalves in experimental microcosms [24]. Fraction A dominated, containing 69% of cytosolic Cd and 49% of the total Cd bioaccumulated at the whole organism level. Metallothionein fractions have been shown to sequester <10% of the total Cd bioaccumulated by aquatic mollusks [16, 27]. After an experimental contamination of C. fluminea by Cd at a concentration of 25 μg/L for 30 d, the MT fractions represented a maximum of 27% of the total Cd bioaccumulated at the whole soft body level [28]. However, in terrestrial gastropods, around 90% of the total Cd bioaccumulated in the midgut gland is associated with the cytosol phase [29]. In our study, the HMW fraction contained only 10% of cytosolic Cd, corresponding to 7.3% of the total burden in the soft body. Fraction B (≤6.5 kD) bound 17% of the cytosolic Cd or 12% of the total Cd. The presence of this fraction of low apparent molecular weight had already been observed, though not systematically, after experimental exposure of C. fluminea to Cd and is likely to be glutathione or some other peptide-metal complex [24, 28]. Cd has also been shown to bind DTT, an antioxidant of low molecular weight (150 D) containing two SH sites per molecule and thus constituting a chelator agent that is able to displace sulfur-seeking (soft) metals bound to other cellular ligands. Lobel [30] pointed out such an interaction between DTT and the subcellular distribution of zinc in the cytosol of mussel kidney (Mytilus edulis). Several tests were carried out under our experimental conditions, notably the elution of the purified rabbit liver MT (Sigma) in the presence or absence of DTT. Results showed that fraction B was absent under these two conditions, demonstrating the ineffectiveness of DTT in displacing the Cd bound to thiol sites on MT. The DTT concentration used under our conditions (2 mM) is low, given that the literature describes the appearance of this interaction at concentrations higher than 4 mM. It should also be noted that the importance of fraction B varies markedly with exposure conditions of the bivalves (see results after 150 d—Table 1). In numerous cases, this fraction cannot be observed after chromatographic separation of the cytosol, despite high levels of Cd contamination in the bivalves. These results suggest that the appearance of fraction B is not an artifact of DTT, but that it rather reflects a distinct fraction originally present in the cytosol.
| Metal | Exposure duration (d) | Tissue concentration (μg/g, fw) | Percentage in the cytosol (concentration) | HMW%/cytosol%/total | Fraction A%/cytosol%/total | Fraction B%/cytosol%/total | Recovery (%) |
|---|---|---|---|---|---|---|---|
| Cd | 21 | 2.4 | 70.7 | 10.3 | 68.7 | 17.9 | 93.6 |
| (1.7) | 7.3 | 48.6 | 12.7 | ||||
| 150 | 7.3 | 66.5 | 12.7 | 60.6 | 26.7 | 92.4 | |
| (5.2) | 8.5 | 40.3 | 17.8 | ||||
| Zn | 21 | 61.1 | 39.9 | 19.5 | 12.1 | 68.4 | 104.7 |
| (33.3) | 7.8 | 4.8 | 27.3 | ||||
| 150 | 123.5 | 13.6 | 40.7 | 37.1 | 22.2 | 97.1 | |
| (17.8) | 5.6 | 5.1 | 3 |
Zn distribution between the cytosol and pellet phases and among the different cytosolic protein fractions was markedly different from that of Cd (Table 1). Only 40% of Zn was present in the cytosol, the majority being sequestered by the pellets (membranes, nuclei, cellular organites, and so on). In the cytosol, fraction A contained only 12% of Zn that corresponded to only 5% of the total burden bioaccumulated in the soft body. The majority of cytosolic Zn was associated with fraction B, which represented approximately 70% of cytosolic Zn and 27% of total Zn. The void volume contained only 20% of cytosolic Zn and 8% of total Zn. Fraction B thus played a predominant role in the distribution of Zn among the cytosolic proteins, comparable to that of fraction A for Cd. Tests conducted with the purified rabbit liver MT in the presence and absence of DTT led to similar conclusions as for Cd: the antioxidant in our experimental conditions did not displace the Zn bound to the MT. Moreover, although this fraction is often detected during Zn determination in the samples of cytosol, its quantitative importance is highly variable, with no direct relation with metal concentration in the soft bodies of the bivalves. In terrestrial invertebrates, the presence of a low molecular weight fraction sequestering a high proportion of Zn, as opposed to Cd, has already been described, notably in the midgut gland of the snail Helix pomatia [31].
After 150-d transplantation at the Bouillac station (Table 1), Cd distribution between cytosol and pellets phases of the whole soft body of C. fluminea was comparable to that after 21 d, with 67% of Cd in the cytosol. This was the same for the different cytosolic protein fractions: HMW, fraction A, and fraction B bound 13, 61, and 27% of cytosolic Cd, respectively, with an increase in the relative importance of fraction B over fraction A. Thus, despite an accumulation of Cd in the whole soft body of the mollusks, which was three times higher after 150 d than after 21 d, Cd distribution was similar for the two exposure durations, fractions A and B representing again around 60% of the total Cd bioaccumulated in the soft body. In contrast, the Zn distribution was clearly different from that determined after the 21-d transplantation (Table 1). First, the percentage of Zn associated with the cytosol decreased markedly, from 40 to 14%, whereas accumulation at the whole soft body level had doubled. Thus, the Zn concentration in the cytosol was reduced by half (18 vs 33 μg of Zn per gram, fw). The distribution among the different cytosolic protein fractions was also strongly modified, with equivalent percentages for the HMW and A fractions (41 and 37% in the cytosol), and slightly lower for the fraction B (22%). Thus, fraction A bound the same proportion of total Zn (5%), but the Zn associated with fraction B decreased markedly, from 27 to 3% between 21 and 150 d. These results highlight variations in the quantitative role of fraction B in metal binding in the cytosol compartment of the soft body of C. fluminea. Research is currently under way to determine the nature of these low molecular weight ligands.
CONCLUSION
This field study of the links between Cd and Zn bioaccumulation and MT expression in the whole soft body of C. fluminea after transplantation along the polymetallic pollution gradient of the river Lot (France) has led to several important conclusions from an ecotoxicologic point of view. The MTs play an important role in the Cd sequestration capacities at the whole soft body level of C. fluminea. The maximum increase in MT concentrations reached a factor close to 9 after 150 d of caging at the Bouillac station, compared with the values measured at time 0, with positive correlations between MT and metal concentrations in the soft bodies. Fraction A, corresponding to the elution volume of the purified rabbit liver MT (Sigma), represented up to 50% of the total Cd bioaccumulated in the mollusks. The role of MTs is closely dependent on the exposure conditions of the bivalves. Under extreme conditions, metal bioaccumulation was presumably too rapid, leading to very high concentrations in the soft body (6.7 μg of Cd per gram, fw, and 120 μg of Zn per gram, fw) after 21 d, without any significant difference in MT concentrations in comparison with reference organisms. A spillover situation can thus be hypothesized: the defense capacities of the bivalves, when confronted with the massive influx of metals in cells and tissues, were overwhelmed, leading to rapid and severe structural and functional damage, and leading to the death of the organisms between 49- and 85-d transplantation. Results relative to Zn showed very different phenomena with respect to bioaccumulation as well as in the role of MTs in metal storage. Indeed, a significant increase in Zn concentrations was observed in the soft body of the mollusks transplanted to the three stations situated downstream from the contamination source. The proportion of the total Zn burden present in the cytosol was markedly lower than for Cd, the role of the MT fractions being less important for Zn.
Acknowledgements
This work was supported by the Ministère de l'Environnement, Agence de l'Eau Adour-Garonne, Aquitaine Region and Feder Aquitaine, within the Groupement d'Intérět Scientifique Ecologie de l'Eau du Bassin Adour Garonne. Henry Bouillard provided excellent technical assistance in the field. The authors thank Peter G.C. Campbell, INRS Eau, University of Quebec, for helpful comments on the manuscript.




