Elevated trace element concentrations and standard metabolic rate in banded water snakes (Nerodia fasciata) exposed to coal combustion wastes
Abstract
Trace element concentrations in banded water snakes, Nerodia fasciata, and representative prey items from a site polluted by coal combustion wastes were compared with concentrations in conspecifics from a nearby reference site. Water snakes accumulated high concentrations of trace elements, especially arsenic (As) and selenium (Se), in the polluted habitat. In addition to being exposed to contaminants in water and sediments, snakes in the polluted site are exposed to contaminants by ingesting prey items that have elevated whole-body concentrations of trace elements, including As, cadmium (Cd), and Se. Snakes from the polluted site exhibited mean standard metabolic rates (SMR) 32% higher than snakes from the reference site. As a result, snakes from the polluted site appear to have elevated allocation of energy to maintenance and theoretically should have less energy available for growth, reproduction, and storage. Our findings are consistent with physiological responses recently documented in other organisms from the polluted site. We hypothesize that long-term exposure to coal ash–derived trace elements and the resultant accumulation of some elements are responsible for observed increases in SMR.
INTRODUCTION
Because of increasing reliance on coal for the generation of electricity, coal combustion wastes have become a ubiquitous form of global pollution. By the turn of the century, it is estimated that 120 million tons of coal fly ash will be produced annually in the United States alone [1]. The waste products, which contain high concentrations of numerous potentially toxic trace elements, including arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), and selenium (Se), typically are disposed of in surface impoundments and landfills [1-3]. Coal-fired power plants and their associated waste-disposal facilities are often associated with aquatic habitats and thus pose a significant threat to the health of aquatic wildlife. Numerous recent studies have linked coal combustion wastes to various anomalies in vertebrates [2, 4-6].
Chronic exposure to environmental pollutants, such as coal combustion wastes, is often not directly lethal but can adversely affect the morphology, physiology, genetics, and/or behavior of organisms. Such sublethal effects can directly or indirectly influence an individual's ability to survive, grow, and reproduce, thereby potentially having effects at the population and community levels. Although many effects of pollutants can be pollutant, dose, or species specific, it has generally been suggested that exposure to xenobiotics results in the activation of energetically demanding physiological responses [7]. As a result, exposure to xenobiotics can be expressed in organisms as increased oxygen consumption (standard metabolic rates, hereafter SMR) [5, 7, 8]. Indeed, recent studies on coal combustion wastes have reported that exposure to this complex mixture of pollutants results in increased SMR in an amphibian and an invertebrate [5, 9].
In the current study, we sought to determine whether the banded water snake (Nerodia fasciata) exhibits a similar increase in SMR when exposed to coal combustion wastes. Overall, reptiles have been an understudied group with regard to environmental pollutants [10]. Most studies on exposure of reptiles to trace elements report tissue concentrations but provide little insight as to how these levels might sublethally affect the biology of organisms (turtles [11-13], alligators [14, 15], and snakes [16-20]). The goals of the current study were therefore to determine the following: (1) whether water snakes inhabiting a coal ash disposal site accumulated potentially harmful trace elements, (2) whether trophic transfer of pollutants was a possible route of exposure, and (3) whether exposure to the pollutants had physiological ramifications by affecting energy allocation patterns.
MATERIALS AND METHODS
Site description
A coal-burning electric power plant with its associated coal ash settling basins is located on the U.S. Department of Energy Savannah River Site, a National Environmental Research Park near Aiken, South Carolina. Sluiced coal ash is pumped from the plant into a primary settling basin (15 ha). Surface water flows from the primary basin into a smaller, secondary basin (6 ha) and then into a 2-ha swamp before reaching Beaver Dam Creek, a tributary of the Savannah River. Sediment in the ash basin habitat has elevated levels of a number of trace elements, including Cd, As, Se, Cr, and Cu [2, 3].
The reference site is approx. 7 km south of the polluted site and consists of a series of six wetlands, none more than 150 m from another of the five adjacent wetlands. Because no barrier to snake movement exists among wetlands, the entire area was treated as a single reference site. The reference site is historically unpolluted by coal combustion wastes. Sediments and organisms sampled from this site have low concentrations of trace elements [3].
| Prey type | Site | Arsenic | Cadmium | Chromium | Copper | Selenium |
|---|---|---|---|---|---|---|
| Bullfrog tadpoles | AB | 31.87 ± 0.15 | 1.28 ± 0.03 | 9.55 ± 1.53 | 26.18 ± 8.54 | 26.56 ± 0.59 |
| 2 per site | REF | 3.84 ± 0.36 | 0.38 ± 0.00 | 12.80 ± 4.59 | 26.98 ± 3.53 | 3.43 ± 0.21 |
| Bullfrog metamorphs | AB | 15.55 ± 4.35 | 0.80 ± 0.04 | 1.58 ± 0.22 | 13.79 ± 0.35 | 26.85 ± 0.25 |
| 2 per site | REF | 0.27 ± 0.06 | 0.11 ± 0.00 | 1.06 ± 0.03 | 6.33 ± 0.63 | 1.55 ± 0.08 |
| Southern toad adults | AB | 2.87 ± 1.03 | 0.15 ± 0.04 | 3.27 ± 1.31 | 15.52 ± 4.02 | 16.84 ± 4.71 |
| 3 per site | REF | 0.16 ± 0.03 | 0.13 ± 0.03 | 1.66 ± 0.35 | 19.18 ± 1.02 | 1.99 ± 0.13 |
| Green treefrog adults | AB | 1.01 ± 0.16 | 0.28 ± 0.05 | 7.86 ± 1.65 | 19.82 ± 1.20 | 9.82 ± 0.61 |
| 3 per site | REF | 0.29 ± 0.05 | 0.13 ± 0.03 | 5.79 ± 0.79 | 29.10 ± 3.30 | 1.12 ± 0.04 |
| Bluegill sunfish juveniles | AB | 2.61 ± 0.39 | 0.75 ± 0.05 | 2.38 ± 0.12 | 1.02 ± 0.28 | 19.52 ± 0.90 |
| 3 per site | REF | 0.43 ± 0.04 | 0.01 ± 0.01 | 2.25 ± 0.05 | 0.21 ± 0.01 | 2.02 ± 0.05 |
| Mosquitofish adults | AB | 2.89 ± 0.91 | 0.32 ± 0.19 | 1.56 ± 0.23 | 4.97 ± 0.36 | 14.28 ± 2.68 |
| 3 per site | REF | 0.40 ± 0.01 | 0.12 ± 0.05 | 1.65 ± 0.23 | 6.73 ± 1.42 | 1.82 ± 0.21 |
| Red-fin pickerel juveniles | AB | NA | NA | NA | NA | NA |
| 3 (Ref only) | REF | 0.33 ± 0.16 | 0.06 ± 0.02 | 1.11 ± 0.32 | 2.62 ± 0.36 | 1.32 ± 0.33 |
| Largemouth bass juveniles | AB | 1.92 ± 0.61 | 0.31 ± 0.24 | 1.27 ± 0.45 | 4.20 ± 1.59 | 18.32 ± 1.49 |
| 3 (AB only) | REF | NA | NA | NA | NA | NA |
Animal collection
Banded water snakes were collected by hand and in minnow traps between April 12 and June 21, 1997, at the reference site as well as in the coal ash settling basins and swamp at the polluted site. All snakes that had visible physical contusions (presumably from traps) were excluded from the study to prevent possible alterations in metabolic rates due to physical trauma. We used 8 and 17 snakes from the polluted site and reference site, respectively, to determine SMR. A subset of snakes from the SMR study and a subset of animals not used for analysis of SMR (total N = 5 per site) were fasted for 7 d to void gut contents and then sacrificed for trace element analysis. Common prey items for N. fasciata from each site were determined by palpating snakes after capture. Prey items included bullfrogs (Rana catesbeiana), green treefrogs (Hylacinerea), southern toads (Bufo terrestris), bluegill sunfish (Lepomis macrochirus), mosquitofish (Gambusia affinis), red-fin pickerel (Esox americanus), and largemouth bass (Micropterus salmoides). Although pickerel and bass inhabit only the reference site and polluted site, respectively, they were included in the analysis because they occupy a similar trophic level and represent an important portion of the water snake diet. Prey items from each site were collected by hand and in minnow traps and were immediately sacrificed to prevent them from voiding gut contents. Thus, whole-body trace element concentrations of prey items accurately reflect the contaminant dose that a snake would ingest in the field.
Trace element analysis
Snake livers and whole prey items were lyopholized, homogenized, and sent to the University of Georgia Crop and Soil Science Center for analysis. Samples were digested using HNO3/H2O2 and then analyzed for As, Cd, Cr, Cu, and Se using inductively coupled plasma mass spectroscopy. Elements analyzed were those known to be commonly associated with coal combustion wastes [2, 3]. Some prey items, such as tadpoles and mosquitofish, were small; therefore, groups of three to five individuals were pooled for trace element analysis.
Measurement of SMR
After capture, all snakes were returned to the laboratory, where they were acclimated in individual containers at approx. 25°C for 5 d. Snakes were given access to water, but food was not provided to allow snakes to void gut contents. Individual snakes were placed in 1- to 3-L flasks (depending on the size of the snake), and walls of flasks were covered to prevent visual disturbances. Flasks were placed in an environmental chamber (25°C) and connected to individual channels on a computer-controlled, indirect, closed-circuit respirometer (Micro-Oxymax, Columbus Instruments, Columbus, OH, USA). The first channel for each trial was connected to a flask containing an 8.4-V battery (Procell Zinc Air Medical Battery, DA146, Duracell, Bethel, CT, USA) that consumed a known amount of oxygen per minute. Oxygen concentration in each flask was determined at 0.5–1.0-h intervals for at least 24 h. Sex, snout-vent length, total length, and mass were determined for each snake subsequent to sacrifice for trace element analysis or release at the site of capture.
Data analyses
Trace element concentrations of selected prey items from polluted and reference sites were not statistically compared because of small sample sizes (Table 1). Trace element concentrations in livers of snakes were log transformed prior to comparison using one-way analysis of variance (ANOVA). Because multiple element concentrations were obtained from the same liver samples, critical values were adjusted downward using a sequential Bonferroni adjustment. Minimum critical value as determined by Bonferroni adjustment is p < 0.01. To determine relationships with snake body size, we also compared snake body mass with hepatic trace element concentrations (As, Cd, and Se) using linear regression.
Because SMR is a measure of metabolic rate at rest, the highest 50% of oxygen consumption values for each individual were deleted prior to analysis to ensure that unobserved periods of activity were excluded from estimates of SMR [5]. Comparisons were then made on the basis of the mean O2 consumption rate from the lower 50% of measurements for each individual. Data were tested for normality and homoscedasticity and transformed by square roots prior to analysis. Rather than using mass-divided oxygen consumption values, data were analyzed using analysis of covariance (ANCOVA) with snake body mass as the covariate because scaling relationships between mass and oxygen consumption are typically described by a scaling exponent less than 1.0 [21, 22]. Additionally, we calculated 95% confidence intervals about the slope of the regression line comparing body mass and O2 consumption for snakes from each site [23]. Nonoverlap of confidence intervals was used to judge significant differences between slopes.
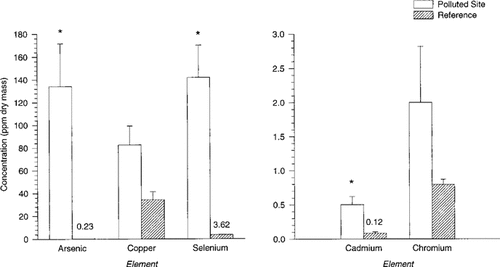
Trace element concentrations of livers from water snakes (Nerodia fasciata) inhabiting a coal ash–polluted site and a reference site (N = 5 per site). Data are means (ppm dry mass) ± 1 SE. Arsenic, selenium, and cadmium concentrations in snake livers from the reference site are low; therefore, concentrations are included as superscripts above the corresponding bar. * identifies significant differences between sites (p < 0.001).
RESULTS
Trace element concentrations
Arsenic, cadmium, and selenium concentrations were higher in all prey items sampled from the polluted site compared to prey items from the reference site (Table 1). Highest concentrations of As, Cd, and Se were found in bullfrog tadpoles and metamorphs from the polluted site. Correspondingly, As, Cd, and Se were significantly higher in livers of snakes from the polluted site compared to snakes from the reference site (As: F1,9 = 399.85, p < 0.001; Cd: F1,9 = 31.95, p < 0.001; Se: F1,9 = 286.98, p < 0.001; Fig. 1). Only Se concentrations showed a significant linear relationship with snake body mass (mass = −104 + 1.81[Se]; r2 = 92.2%, p = 0.009). Neither Cd nor As was significantly linearly related to snake body mass (p = 0.061 and p = 0.413, respectively).
Standard metabolic rates
Mean SMR of snakes from the polluted site was significantly higher than mean SMR of snakes from the reference site (mean oxygen consumption = 5.79 ± 1.17 and 3.69 ± 0.63 ml/h, respectively; F1,24 = 116.70, p < 0.001). Average mass-divided values for oxygen consumption were 32% higher in snakes from the polluted habitat than in snakes from the reference site (0.0959 ± 0.0066 and 0.0726 ± 0.0080 ml/g/h, respectively). Although the slope of the relationship between body mass and oxygen consumption was greater for snakes from the polluted site than for reference snakes, the difference between slopes was not statistically significant (p > 0.05; Fig. 2).
DISCUSSION
Water snakes inhabiting the coal ash–polluted habitat are potentially exposed to trace elements suspended or dissolved in water, bound in the sediments, and through ingestion of contaminated prey items. Studies on As and Se indicate that organisms in polluted aquatic habitats often bioaccumulate high levels of certain trace elements via trophic transfer [24-28]. On the basis of the substantially elevated levels of As, Cd, and Se found in prey items at the polluted site, it is possible that much of the elements concentrated in the hepatic tissue of water snakes was accumulated from their diet. It is apparent that water snakes, like other secondary consumers, can accumulate high levels of As and Se [24, 27, 28]. To our knowledge, the levels of As and Se we report in water snakes from the coal ash–polluted site are the highest yet documented in a reptile or amphibian.
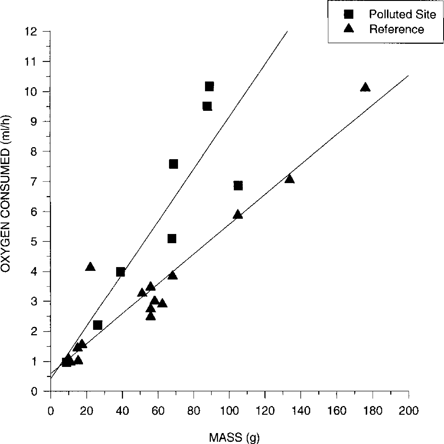
Oxygen consumption (ml O2/h) of water snakes (Nerodia fasciata) inhabiting a coal ash–polluted site and a reference site plotted against body mass (g) (N = 8 and 17, respectively). Polluted site: ml O2/h = 1.00 + 0.021 (mass); reference site: ml O2/h = 1.06 + 0.012 (mass). All measurements were taken of postabsorptive snakes at 25°C.
Arsenic, selenium, and cadmium have been shown to adversely affect many organisms at concentrations much lower than those found in coal ash–exposed water snakes and their prey items. For example, dietary As levels of 30 ppm, approximately that of bullfrog tadpoles consumed by snakes in our study, were enough to decrease growth in mallard ducklings [25]. Decreased growth is only one of many known responses of vertebrates to As exposure; others include liver damage, weakness, paralysis, carcinogenesis, mutagenesis, and teratogenesis [27, 29]. Only one previous study has documented As levels in a snake species. Winger et al. [18] found low As levels (0.06 ppm wet weight) in water snakes inhabiting a relatively uncontaminated ecosystem.
Cadmium levels previously reported for two snake species, Pituophis melanoleucus (hatchlings) and Nerodia sp., were only a fraction of the levels we found in N. fasciata in the polluted site and were even lower than the levels we documented in snakes from the reference site [18, 20]. However, caution must be exercised in making these comparisons because different tissues were used in each study. Nevertheless, cadmium levels in snakes from the coal ash–polluted habitat are more than four times the hepatic concentrations found in conspecifics from the reference site (Fig. 1). Our findings corroborate other studies that indicate that although cadmium can accumulate in vertebrate tissue, it is not thought to biomagnify [30]. However, in relatively small quantities, Cd is considered to be among the most toxic heavy metals [31], and is often associated with renal failure, respiratory disorders, and aberrant reproductive function [32, 33].
Selenium levels in water snakes from the coal ash–polluted site are more than 10 times the levels that induce severe reproductive problems in other vertebrate species [34]. We also found that hepatic Se levels increase with snake body size. It is possible that larger snakes have higher Se loads because of prolonged residence in the polluted habitat and/or ingestion of larger quantities of contaminated prey. Of the few other studies that have examined Se levels in snakes, only one study, by Ohlendorf et al. [19], clearly indicated that snakes in Se-contaminated habitats incorporate high levels of selenium in their tissues. Terrestrial snakes (P. melanoleucus) captured near Kesterson Reservoir, an area heavily contaminated with Se from agricultural drainwater, had liver Se concentrations of 10.9 ppm [19]. Interestingly, the site described by Ohlendorf et al. [19] had sediment Se concentrations 20 times higher than sediment levels in the coal ash–polluted habitat utilized in our study [3, 35]. It might be that Se is more bioavailable to water snakes than P. melanoleucus because water snakes feed on aquatic organisms, such as fish and amphibians, which are relatively spatially confined and readily accumulate selenium [2, 3, 19, 24]. On the other hand, P. melanoleucus from Kesterson feed mainly on terrestrial rodents, birds, and bird eggs. Although rodents in Kesterson can have elevated tissue concentrations of Se [36], the combined vagility of P. melanoleucus and their prey might prevent the snakes from being consistently exposed to high dietary concentrations of Se [19].
Identification of accumulated elements in animal tissues provides a measure of the extent of pollutant exposure experienced by organisms but fails to provide insight into the biological significance of exposure to pollutants. It is important that investigators examine physiological and/or ecological correlates in conjunction with tissue concentrations of contaminants. For example, we found significant physiological ramifications of exposure to coal ash–derived pollutants, as illustrated by significantly higher mean SMR (a measure of maintenance expenditures) in water snakes from the polluted site. Water snakes from the polluted site had mean (mass-divided) SMR 32% higher than conspecifics from the reference site. In reptiles, the portion of the energy budget allocated to maintenance requires nearly 85% of the total energy assimilated [37]. The remaining portion of the energy budget is allocated to production (growth, reproduction, and storage). Because maintenance costs represent such a large proportion of the overall energy budget, even a slight increase in allocation to maintenance should substantially reduce total energy available for production. Because processes related to a depleted production budget are those that directly or indirectly affect population dynamics (i.e., reproduction, growth, storage, and dispersal), the elevation in maintenance expenditures reported here could have important ramifications at higher levels of organization.
To maintain energy balance in the coal ash–polluted habitat, snakes must overcome elevated maintenance demands either by altering energy allocation patterns or by increasing total energy assimilation. However, both of these modifications would likely be detrimental to the affected organisms. For example, if total energy assimilated does not change, increasing the amount of energy allocated to maintenance results in a disproportional decrease in allocation of energy to growth, reproduction, and storage [5]. On the other hand, affected individuals could theoretically overcome the higher-than-normal expenditures on maintenance by increasing the amount of total energy assimilated. However, increasing energy assimilation would require increased foraging time and related energy expenditures (activity costs) as well as increased susceptibility to predation. Furthermore, in the ash-polluted habitat in which prey items are heavily contaminated (Table 1), increased assimilation of energy could be deleterious because it would likely result in increased trophic uptake of pollutants.
Different pollutants can trigger different metabolic responses in various organisms. Some pollutants are thought to cause organisms to increase their oxygen consumption [5, 7, 8], whereas other pollutants are known to cause a decrease in overall oxygen consumption [8]. It appears that increased SMR associated with exposure to coal combustion wastes might be a generalized response across taxa. Water snakes, a secondary consumer, exhibit a similar metabolic response as those responses observed in various invertebrate and amphibian species. For example, freshwater shrimp (Palaemonetes paludosus) exhibited 50% increases in SMR when transplanted to the polluted habitat [9]. In addition, larval bullfrogs (Rana catesbeiana) exhibited increases in SMR ranging from 30 to 175% after field exposures to the coal ash–contaminated habitats [5]. Increased metabolic rate in such a diversity of species is probably symptomatic of systemic problems triggered by coal combustion wastes [7]. Activation of cellular repair mechanisms, increased metallothionein production, and increased transport and excretion of pollutants are only a few of the possible energetically costly processes that might be employed by coal ash–exposed organisms.
The results of our study suggest that water snakes are valuable for studying physiological responses to sublethal levels of pollutants because of their propensity to bioaccumulate contaminants from aquatic habitats. Aquatic snakes can provide vital insight as to how pollutants move through and affect communities because they are an important trophic link to terrestrial, avian, and aquatic carnivores. In comparison to some mammals and most birds, aquatic snakes have a relatively small home range; this makes comparisons of conditions in multiple habitats within a relatively small geographic area possible. Also, unlike most mammalian and avian secondary consumers, many aquatic snakes can be maintained in experimental settings with relative ease and be utilized in controlled laboratory exposures to pollutants. Unfortunately, water snakes have rarely been examined by ecotoxicologists despite the traits that make them useful for aquatic toxicological studies [16, 18, 38-40]. To understand how pollutants affect reptiles, it is critical that future integrative studies not only examine quantities of xenobiotics accumulated in reptilian tissues but also determine the biological consequences of these levels.
Acknowledgements
We thank Christopher Beck, Rebecca Yeomans, Mary Mendonça, Mike Dorcas, Lara Hopkins, and Chris Leary for their insightful comments on the manuscript. Gina Coffman, Barb Dietsch, and Christopher Beck offered assistance with other portions of the study. W. Hopkins, C. Rowe, and J. Congdon were supported during this project by U.S. Department of Energy Financial Assistance Award DE-FC09-96SR18546 to the University of Georgia Research Foundation.




