Implications of gut purging for tissue residues determined in bioaccumulation testing of sediment with Lumbriculus variegatus
Abstract
Bioaccumulation test procedures using the oligochaete Lumbriculus variegatus have been developed as a means of evaluating the accumulation of chemicals from freshwater sediments. To avoid including chemicals associated with gut contents as part of the measured tissue residue, a 24-h period of purging in clean water after the uptake phase of the test has been recommended. While purging acts to reduce bias from gut contents, it also has the potential to introduce bias caused by depuration of chemicals from tissues. In this paper, a series of model calculations are used to assess the expected sensitivity of measured residues of nonionic organic chemicals to the presence of sediment in the gut and to varying lengths of purging. If organisms are not purged, the predicted influence of gut contents on measured residue is not large (generally <20%) when a biota-sediment accumulation factor (BSAF) of one is assumed. However, if BSAFs substantially less than one apply, projected errors increase to 30-fold or more. To derive a better estimate of the time required for L. variegatus to clear the gut of sediment, a sediment purging experiment was conducted; results indicate that >98% of sediment had cleared the gut in 6 h (half-life = 0.98 h). Based on these results and model analyses, a much shorter purging period of 6 h, rather than 24 h, is suggested as a reasonable guideline for many test applications.
INTRODUCTION
Many environmental contaminants commonly found in sediments have the potential to accumulate through the aquatic food chain, thus providing a pathway for exposure of higher trophic levels, including humans. Bioaccumulation tests have been developed as one mechanism for assessing the extent of bioaccumulation that may occur from contaminated sediments [1-3], and these procedures have been incorporated into certain regulatory programs [4].
For freshwater sediments, the oligochaete Lumbriculus variegatus is an organism commonly used for bioaccumulation testing of sediments. Conceptually, this is a simple procedure in which oligochaetes are exposed to test sediments for 28 d, then sieved from the sediments and analyzed for contaminants of interest. The U.S. EPA and ASTM [2, 3] protocols recommend a 24-h holding period in clean water at the end of the sediment exposure to allow organisms to purge their gut of sediment prior to analysis; this purging was included to prevent sediment-bound chemicals in the gut from being measured as a part of the tissue burden.
Inclusion of a 24-h purging period in the test protocol has been questioned by some because of concerns that tissuebound chemicals will depurate from the organisms during holding in clean water, thereby underrepresenting the steadystate concentration in the organism residing in the sediment [3, 5]. Some research has suggested that shorter purging periods, such as 10 to 12 h [6, 7], might be sufficient to clear sediment from the gut of oligochaetes. Although this would reduce depuration, it also presents logistical problems to conducting the test (e.g., test manipulations required late at night), which is a serious practical consideration for a procedure to be conducted, in large part, by contract laboratories.
The purpose of this paper is to provide a quantitative examination of several alternatives for ending bioaccumulation tests with L. variegatus and to discuss their consequences for interpretation of sediment bioaccumulation data. Literature data were used to estimate depuration of nonionic organic chemicals from tissues as a function of time and Kow. A purging experiment was conducted to quantify rates of sediment elimination from the gut of L. variegatus. The goal in this analysis was not to develop a rigorous kinetic model for L. variegatus but rather to use available data and reasonable assumptions to develop practical guidance for the implementation of bioaccumulation testing.
METHODS
Sediment purging experiment
A sediment purging experiment was conducted to determine the rate at which sediment is eliminated from the gut of L. variegatus when removed from sediment and placed in clean water. Approximately 600 adult oligochaetes (>3-cm length) were added to a 19-cm-diameter crystallizing dish containing 750 ml of sediment from West Bearskin Lake (Cook County, MN, USA) with an overlying layer of 1.5 L Lake Superior water (hardness = 48 mg/L as CaCO3; alkalinity = 45 mg/L as CaCO3). Sediment from West Bearskin Lake has been used for many years in our laboratory as a control sediment and has been found to be free of toxicologically significant contamination [8]. The sediment used in this experiment had a moisture content of 79%, 7.4% TOC, and particle size dominated by silt/sand (50.5% sand, 47.2% silt, 2.3% clay). About 0.25 g of trout chow was added to the sediment as a supplemental food source. Temperature during this sediment exposure, as well as during the subsequent purging period, was 23 ± 1°C.
Oligochaetes remained in the West Bearskin sediment for 48 h, after which they were sieved from the sediment with a stainless steel sieve and placed in a shallow tray containing Lake Superior water. Four groups of 10 organisms each were removed immediately for weight determination; from the remaining organisms, 28 additional groups of 10 organisms were isolated and placed in separate, 300-ml-high form beakers, which were subsequently placed in an intermittent water renewal system [9] receiving Lake Superior water. Groups of four beakers were removed for weight analysis at 1, 2, 4, 6, 8, 12, and 24 h after the initial sieving. Automatic water renewal occurred during hours 2 and 14 of this purging period.
Weight determinations were made in aluminum weighing pans that were ashed for 2 h at 550°C prior to use. Organisms were transferred from the beakers to the weighing pans with a pipette; after transfer, the pipette was used to remove excess water from the pans. Pans were placed in a drying oven at 60°C for 96 to 120 h, weighed (±0.01 mg), then ashed for 2 h at 550°C and weighed again. Finally, residual ash was removed from the pans by brushing with a small brush, then carefully wiping the pan with a moistened tissue and allowing the pan to dry. Weight of the pan after removal of the ash was used to determine total dry weight and ash weight by comparison with weights at previous steps.
Predicting depuration of nonionic organic chemicals
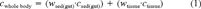 (1)
(1) (2)
(2) (3)
(3) (4)
(4) (5)
(5) (6)
(6) (7)
(7)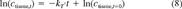 (8)
(8)RESULTS AND DISCUSSION
Literature estimates of the mass contribution of sediment to overall body mass of oligochaetes are typically in the range of 10 to 20% [7, 10, 13]; Norberg-King and Dawson [14] compared dry weight and ash-free dry weight in oligochaetes reared in four sediments and estimated that sediment contributed from 7 to 37% of total mass, with an average of 20%. Errors in dry weight-based chemical concentrations were estimated for gut sediment weights of 5 to 25% of total dry weight. When test animals are not purged, the predicted errors in chemical concentrations are independent of Kow but are dependent on both the proportion of organic carbon in the sediment and the proportion of organism mass comprised of sediment (Fig. 1a). Because of the assumed condition that BSAF = 1, there is no error when fOC = 0.05, which is the same as the assumed lipid content of the oligochaetes. Interestingly, errors were not particularly large over this range of conditions, with most values falling between 80 and 120% of the true tissue concentration. When lipid-normalized chemical concentrations are calculated for oligochaetes that have not been purged, errors tend uniformly toward overestimation and are greater in magnitude than for mass-based concentrations (Fig. 1b).
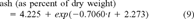 (9)
(9)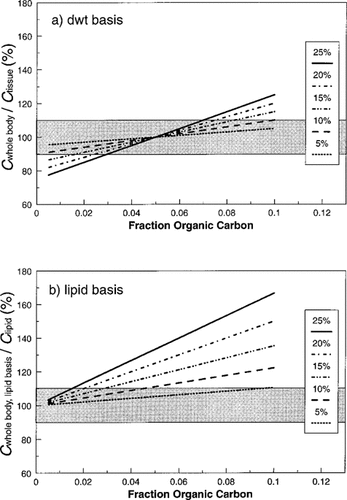
Projected whole-body concentrations of nonionic organic chemicals in oligochaetes as a function of sediment content of the gut (5–25% of total dry weight) and sediment organic carbon, assuming BSAF = 1. Results are expressed on a dry weight (a) or lipidnormalized (b) basis relative to the true concentration in tissue or lipid. Shaded areas represent ± 10% of the initial tissue concentration (ctissue,t=0).
Given that our sediment purging study involved a single sediment, it is unknown whether sediments with differing physical characteristics or chemical contamination would show markedly different clearance rates. However, Kukkonen and Landrum [6] and Brooke et al. [7] evaluated several sediments with varying composition and contamination and showed that, despite the varying characteristics, gut purging in all sediments was complete after 10 to 12 h. While there were no published observations for purging times as short as 6 h, these studies provide evidence that gut purging is not delayed to a large extent by these factors.
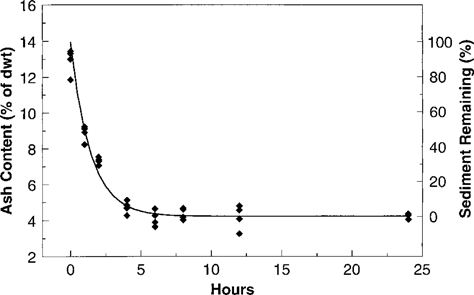
Results of the L. variegatus gut purging experiment. Regression equation is ash (as percent of dry weight) = 4.225 + exp(–0.7060.t + 2.273) (r2 = 0.977). Scale for sediment remaining in the gut was derived assuming a starting ash content of 13.93% (y intercept of regression) and an ash content of 4.225% when purging is complete.
In addition to providing time to clear sediment from the gut, holding organisms in clean water also allows depuration of tissue-bound chemicals. For nonionic organic chemicals, the depuration rate is related to Kow. As comprehensive data were not available specifically for L. variegatus or oligochaetes in general, the relationship developed by Gobas et al. [12] was used to estimate depuration constants as a function of Kow. Depuration rates predicted from this relationship were evaluated using clean water depuration data for L. variegatus exposed to fluoranthene [15], pyrene [6, 16], anthracene [16], fluorene [5; personal communication, B.R. Sheedy, U.S. EPA, Duluth, MN, USA], and benzo(a)pyrene [10] (Fig. 3). In addition, data for depuration of a variety of chlorinated organic compounds by other oligochaetes (Limnodrilus hoffmeisteri and Tubifex tubifex) were included [13, 17; as analyzed in 18] (these latter experiments involved purging in clean sediment rather than water only, which is thought to increase elimination rate [6]). Overall, these data suggest that the depuration constants derived by Gobas et al. [12] provide a reasonable representation of depuration from L. variegatus. It is worth noting, however, that the analysis would be strengthened by additional data specifically for L. variegatus and chemicals between log Kow three and five, for which depuration is greatest in the first 24 h.
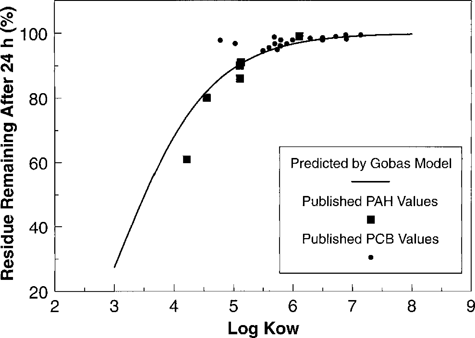
Predicted depuration of nonionic organic chemicals from tissue as predicted from Kow based on a model by Gobas et al. [12] for guppies. Data points represent L. variegatus depuration data for wateronly depuration of PAHs [5, 6, 10, 15, 16] and clean sediment depuration of organochlorines by Limnodrilus hoffmeisteri and Tubifex tubifex [13, 17, 18].
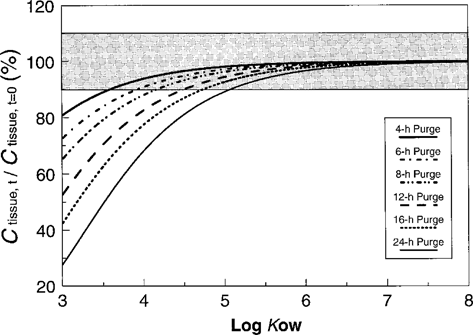
Predicted depuration of nonionic organic chemicals from tissue as a function of Kow and duration of purging, assuming no contribution from sediment in the gut. Shaded area represents ± 10% of ctissue,t=0.
Using the depuration constants from Equation 7, depuration as a function of Kow was plotted for a series of purging periods (Fig. 4) assuming no contribution from sediment in the gut. For compounds with a log Kow greater than five, all values are projected to be within 10% of the initial concentration for depuration times up to 24 h. For compounds with a log Kow less than five, however, substantial differences exist. To retain 90% of the initial concentration of compounds with log Kow > 4, depuration for 8 h or less is required. Even after just 4 h of depuration, projected concentration of chemicals with log Kow of three are only 80% of the initial concentration.
Given the opposing influences of gut purging and chemical depuration, selecting the appropriate procedure for bioaccumulation testing involves balancing the diminishing bias from gut contents against the growing bias from depuration. This issue does not have a single solution because it depends on the chemicals of interest, the required accuracy, and other variables that may vary from study to study. Many chemicals typically of interest in bioaccumulation studies (e.g., DDT, endrin, dieldrin, many PCBs, higher molecular weight PAHs) have log Kow values greater than five, in which case 90% accuracy with little or no bias from gut contents can be achieved with longer purging periods such as 24 h. Nonetheless, results of the gut purging experiment suggest that elimination of sediment is essentially complete within 6 h, which may make this shorter depuration preferable in many instances.
A critical factor in this analysis is the sensitivity of modeled concentrations to model assumptions. The most critical of these appears to be the assumption that the BSAF = 1. Chemicals with very high Kow may not reach equilibrium between sediment and tissue within the 28-d duration of the L. variegatus bioaccumulation test and hence would show BSAF values of less than one [3]. Growth dilution could also lower BSAF values. Perhaps more significantly, studies by Mc-Groddy and Farrington [19] and others have indicated that, in certain sediments, the apparent partitioning of PAHs between sediment organic carbon and pore water leads to much higher KOC values than would be calculated from literature relationships [20], with resulting “apparent” KOC values as much as three orders of magnitude higher. On the presumption that the interstitial water is indicative of the true chemical activity of the PAH in sediment [20], this suggests that BSAF values could be as much as three orders of magnitude below the value of one assumed in the analyses presented above.
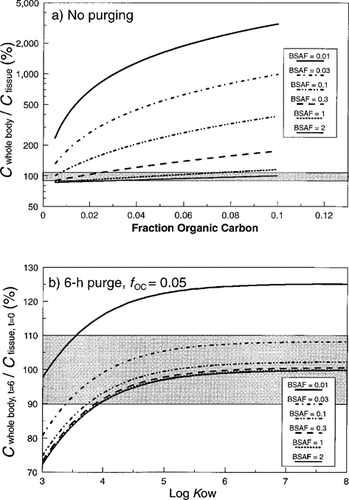
Effect of varying BSAF on predicted whole-body residues of nonionic organic chemicals in L. variegatus, assuming a gut sediment content of 15% dry weight. Panel (a) presents expected concentrations at time = 0 (no purging) as a function of sediment organic carbon. Panel (b) shows expected cwhole body after a 6-h purging period as a function of Kow, assuming fOC = 0.05. Shaded areas represent ± 10% of the initial concentration in tissue (ctissue,t=0); note differences in scale between panels.
These deviations have profound influences on the concentrations predicted to occur in sediment bioaccumulation tests. Because a BSAF substantially less than one means that the concentration of chemical will be much higher in the organic carbon of the sediment than in tissue lipid, the influence of gut-borne sediment on whole-body concentrations increases dramatically (Fig. 5a). Whereas whole-body concentrations of chemicals deviated from the true value by only about 15% for a gut sediment content of 15% and a BSAF of one (Fig. 1a), changing the BSAF to 0.01 increases the projected error to a more than 30-fold error in high TOC sediments (Fig. 5a). Purging for as little as 6 h eliminates the majority of the bias from low BSAFs (Fig. 5b). Assuming a gut sediment weight of 15% and an fOC of 0.05, errors in whole-body residue after 6 h of purging would be expected to exceed 10% only for BSAFs less than 0.02.
Biota-sediment accumulation factors slightly higher than one (e.g., 1.7) have been reported and are thought to represent an upper bound for BSAF values for nonionic organic chemicals accumulated directly by benthic invertebrates, at least where active uptake is not involved [3]. As indicated by Figure 5, increasing the assumed BSAF from one to two has relatively little effect on predicted responses.
Metabolism was not explicitly considered in this analysis, although oligochaetes are not thought to possess a large capacity for metabolism of most xenobiotic chemicals [21, 22]. Metabolism of chemicals in the tissue would act to decrease the BSAF, thereby increasing the importance of purging, although it would simultaneously speed elimination of chemical from the tissue during the purging period. For chemicals/sediments showing small BSAF values, measurement of chemical concentration in sediment pore water would likely provide insights as to whether low accumulation rates were caused by unusual partitioning behavior in the sediment or by metabolism.
Bioaccumulation tests can also be used to assess the accumulation of metals from sediment. Several authors have reported errors in analyses of metal bioaccumulation by invertebrates stemming from gut contents [23-26]. In addition, there is evidence that bulk sediment concentrations of metals do not correlate directly with the bioaccumulative fraction of metals in sediment [27], which would further confound the interpretation of tissue analyses that included sediment from the gut. Although the evaluation we have presented is oriented toward nonionic organic chemicals, the resultant purging recommendations should be applicable to studies focusing on accumulation of sediment-associated metals as well.
The impetus for this analysis was the uncertainty surrounding the consequences of alternative methods for terminating bioaccumulation tests with L. variegatus. The original guidance published by the U.S. EPA and ASTM [2, 3] prescribed a 24-h period for elimination of gut contents. Studies by Kukkonen and Landrum [10] and Brooke et al. [7] measured elimination of sediment after 10 to 12 h and found that purging was essentially completed, indicating that this shorter period of gut purging would be sufficient and perhaps preferable in order to reduce depuration of chemicals from tissue. Although technically preferable to a 24-h period, purging periods of 10 to 12 h would be logistically difficult, particularly for a test protocol that is used widely in a regulatory context. In the present study, we evaluated sediment purging more rigorously between 0 and 12 h and found that only 6 h were required for >98% purging of gut contents. Shortening the purge period to 6 h further reduces potential errors from chemical depuration, particularly for compounds with log Kow < 5.
As illustrated in our analyses, the appropriateness of any protocol for ending the L. variegatus bioaccumulation test will depend on the accuracy required, the chemicals being measured, and the characteristics of the test sediments. In general, however, we believe that the use of a 6-h purging period in clean water will provide a reasonable balance among the biases presented by chemical depuration and sediment in the gut. From a practical standpoint, a 6-h purging period has the added advantage of allowing bioaccumulation tests to be ended within the confines of a normal workday. Nonetheless, where chemicals of interest have log Kow > 5, longer purging periods, up to 24 h, can probably be used with only minimal errors. For chemicals with log Kow substantially less than four or which may be rapidly metabolized, kinetic studies may be required to derive accurate estimates of tissue burdens.
Acknowledgements
We thank J.W. Nichols for help in evaluating bioaccumulation models and for review of the manuscript. G.T. Ankley, C.G. Ingersoll, and S.A. Diamond also provided helpful comments on earlier versions of this manuscript. This manuscript was reviewed in accordance with U.S. EPA policy. Mention of trade names does not imply endorsement by the U.S. EPA or the federal government.




