Bioaccumulation kinetics of brominated flame retardants (polybrominated diphenyl ethers) in blue mussels (Mytilus edulis)
Abstract
Baltic Sea blue mussels, Mytilus edulis, were exposed to polybrominated diphenyl ethers (PBDEs, IUPAC congeners 47, 99, and 153) and polychlorinated biphenyls (PCBs, congeners 31, 52, 77, 118, and 153) in a flow-through experimental setup for 44 d. After the exposure phase, the mussels were allowed to depurate in natural brackish water for 26 d. After analyses, uptake clearance rate coefficients (ku), depuration rate coefficients (kd), and bioaccumulation factors (BAF) were calculated. A rapid uptake of all PBDEs and PCBs was observed, especially for PBDE congeners 47 and 99 (ku 120 and 170 L/day/g dry weight, respectively). The depuration rate decreased with increasing hydrophobicity as expected for the PCBs, but for the PBDEs, depuration rate coefficients appeared to be of the same magnitude for all three congeners independently of log Kow. The BAFs obtained for PBDE 47 and PBDE 99 (1.3 × 106 and 1.4 × 106 ml/g dry weight, respectively) were higher than for all other substances in the study, severalfold higher than for PCBs of similar hydrophobicity. The presented data indicate that the bioaccumulation potential of PBDEs, extensively used as flame retardants, is similar or higher than that of PCBs for filter feeding organisms such as blue mussels.
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are widely used as additive flame retardants for plastics and textiles. As an example, the global consumption of so-called penta-BDE-mixtures, such as Bromkal 70-5DE, was 4,000 tons/year in 1992 [1]. Additive flame retardants can rather easily leak out of treated materials during the entire life cycle of the product, causing a diffuse contamination of the environment [2]. The first report of PBDEs in samples from the Swedish environment was published by Andersson and Blomkvist [3], who found high concentrations of primarily tetra-BDE in fish, especially pike (Esox lucius), from River Häggån (southern Sweden). In subsequent studies, brominated flame retardants have been found in many terrestrial and aquatic organism from America, Europe, and Asia [1]. In the Baltic Sea, PBDE has been reported in laminated sediment cores, where Nylund and coworkers [4] found that the concentrations of PBDEs increased rapidly between the years 1980 and 1987 and individual PBDEs were of the same magnitude as individual PCBs in the uppermost sediment layers. In an extensive survey, Sellström and coworkers [5] analyzed different animal samples collected from terrestrial, aquatic, and marine systems in Sweden during the 1970s and 1980s. The PBDEs were found in almost all samples but in lower concentrations in terrestrial species compared with aquatic and marine species. Further, much higher concentrations were found in some fish consumers (for example, gray seal) than in their food, indicating biomagnification of the PBDEs. Increasing concentrations of PBDEs in the environment may therefore pose a hazard to seafood consumers in the near future, and a better knowledge of the bioavailability and bioaccumulation potential of PBDEs is of interest. Two of the PBDEs (47 and 99) included in the present study are the major constituents of the commercial product Bromkal 70-5DE [6] and are found in many environmental samples [7]. It has been shown that tetra-, penta-, and hexa-BDEs are readily absorbed from the diet by pike (Esox lucius) [8] and are accumulated in organisms of different trophic levels [9]. The reported high uptake of PBDEs 99 and 153 is seemingly in conflict with an earlier proposed molecule size limit for uptake of hydrophobic organic compounds (HOCs) in organisms [10-12]. According to this, molecules with effective cross sections (ECS) larger than 9.5 Å cannot pass biological membranes. The PBDEs 99 and 153 both have ECS at 9.6 Å [8]. In contrast to the PBDEs, the bioaccumulation kinetics of PCBs have been rather extensively studied in blue mussel [13, 14]. The main objective of the present study was to determine and compare the bioaccumulation kinetics of some selected PBDEs and PCBs in blue mussels, Mytilus edulis, from the Baltic Sea.
MATERIAL AND METHODS
Experimental organisms
Baltic Sea blue mussels, Mytilus edulis, were collected in November 1996 from a depth of approximately 5 m in the vicinity of Askö Laboratory (Trosa archipelago, northern Baltic Sea proper, Sweden). Mussels used in the experiment had an initial shell length of 20 ± 2 mm and an initial tissue dry weight of 27 ± 1.1 mg (mean ± SE, n = 30). This length interval corresponds to an age of between 5 and 7 years in the Baltic proper [15]. The mussels were held in a storage tank with a flow of natural brackish water (sand filtered, 6.8‰ S, 9.6°C) for 17 d prior to the start of the experiment. During this period, they were fed with cultured green algae, Scenedesmus obtusiusculus. The algae were cultured in a medium consisting of sterile filtered brackish water (0.22-μm pore size, Millipore, Bedford, MA, USA) and 1 ml/L of algae culture medium B-1 (BioProcess ApS, Hirtshals, Denmark) under continuous fluorescent light at 20°C. Algal cells used in the experiments were harvested during the logarithmic phase of growth.
| Systematic name | Systematic numbering | log Kow | Effective cross section (Å) |
|---|---|---|---|
| PCBs | |||
| 2,4′,5-tri-CB | PCB 31a | 5.67b | 8.7c |
| 2,2′,5,5′-tetra-CB | PCB 52a | 5.84b | 8.7d |
| 3,3′,4,4′-tetra-CB | PCB 77a | 6.36b | 8.7c |
| 2,3′,4,4′,5-penta-CB | PCB 118a | 6.74b | 8.7c |
| 2,2′,4,4′,5,5′-hexa-CB | PCB 153a | 6.92b | 8.7d |
| PBDEs | |||
| 2,2′,4,4′-tetra-BDE | PBDE 47e | 6.02f | 8.1c |
| (5.87-6.16) | |||
| 2,2′,4,4′,5-penta-BDE | PBDE 99e | 6.81f | 9.6c |
| (6.64-6.97) | |||
| 2,2′,4,4′,5,5′-hexa-BDE | PBDE 153e | 7.39f | 9.6c |
| (6.86-7.92) |
Test substances
A mixture of HOCs (five PCBs and three PBDEs, Table 1) was obtained from the Department of Environmental Chemistry, Stockholm University. The PCBs were synthesized according to the methods described by Sundström [16] and Bergman et al. [17] and the PBDEs according to Örn et al. [18]. A generator column [19] was used to prepare a water solution of the HOCs (Table 2).
Bioaccumulation
The HOC uptake study was performed from November 25, 1996, to January 8, 1997, in a flow-through aquaria system (Fig. 1) described previously [20]. In brief, the system consisted of a feed tank (30 L) that received sand-filtered brackish water pumped from an inlet at 16 m depth near the Askö Laboratory. The water was transferred from the feed tank by hydrostatic pressure to five mixing chambers (3 L) with magnetic stirrers connected to 20 exposure aquaria (2 L) (four aquaria to each mixing chamber). Peristaltic pumps (Alitea, Stockholm, Sweden) were used to pump deionized water onto the generator column, to pump the eluate with test substances into four of the mixing chambers, and to pump algal solution from the cultures into all five mixing chambers. Deionized water was pumped onto the generator column with an average speed of approximately 40 ml/h, matching the volume of eluate being pumped to the mixing chambers. The five mixing chambers received green algae S. obtusiusculus, corresponding to a nominal organic carbon concentration of 0.1 mg/L.
| Substance | Column loading (mg) | Water concn. (ng/L) |
|---|---|---|
| PCB 31 | 0.85 | 5.22 ± 2.2 |
| PCB 52 | 0.94 | 3.69 ± 1.6 |
| PCB 77 | 0.87 | 0.44 ± 0.2 |
| PCB 118 | 0.96 | 0.38 ± 0.2 |
| PCB 153 | 1.09 | 0.49 ± 0.1 |
| PBDE 47 | 1.58 | 0.31 ± 0.2 |
| PBDE 99 | 1.72 | 0.070 ± 0.05 |
| PBDE 153 | 1.33 | 0.086 ± 0.11 |
The system was stabilized for 3 d before the mussels were added. At the start of the experiment, 40 mussels were placed in each of the 20 aquaria. Four aquaria connected to the mixing chamber did not receive any test substance and were used as control for measurements of mussel condition and HOC background concentration in mussel tissue. When the experiment started, the water had a temperature of 9.6°C and a salinity of 6.8‰. At the termination of the experiment, the temperature had decreased to 7.6°C, whereas the salinity remained constant. The average brackish water flow rate to each mixing chamber was approximately 5.3 L/h and to each aquarium about 1.3 L/h. The study was performed in a constant temperature room at 7°C with dimmed lighting.
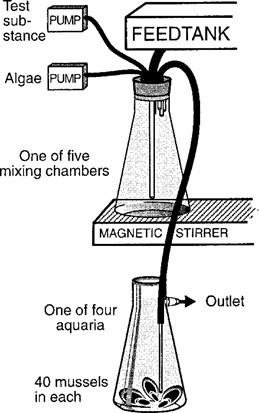
The design of the flow-through bioaccumulation system.
Depuration
The HOC depuration study was performed between January 8 and February 3, 1997 (day 44 to day 70 of the whole study). Remaining mussels from the uptake study were removed from the aquaria, placed on petri dishes (one petri dish for each aquarium), and transferred to two 50-L tanks. These tanks received a constant flow of uncontaminated natural sand-filtered brackish water at a rate of 16 L/h. During the depuration period, the mussels were fed S. obtusiusculus at approximately the same rate as during the exposure period.
Sampling
Mussel samples for the HOC analyses were taken at days 0, 3, 7, 15, and 44 during the bioaccumulation phase and at days 49, 56, and 70 during the depuration phase. Initial samples (t = 0) were taken from the storage tank before the experiment started and consisted of 150 mussels divided into three samples of 50 mussels each. At each sampling occasion, samples of 24 to 40 mussels were taken from each of two exposure aquaria (connected with different mixing chambers) or, during depuration, from each of two petri dishes. In addition, a pooled sample of 21 mussels from the two remaining petri dishes with control mussels was taken at the termination of the experiment (day 70). The soft tissues of the mussels were removed and placed in glass scintillation vials (one for each sample) and stored at −20°C. Any dead mussels were excluded from the samples.
The condition of the mussels was determined at days 0, 15, 32, 44, and 70 for the nonexposed control mussels and at days 44 and 70 for exposed mussels. Each sample consisted of 30 to 40 individuals, giving a total number of 243 mussels used for condition measurements. Shell length and soft tissue dry weight (constant weight at 60°C) was determined, and a body condition index (BCI) was calculated as BCI = 100 × tissue dry weight (mg)/shell length (mm).
Water samples, 800 ml for total HOC (i.e., particle associated plus apparently dissolved) and 10 ml for organic carbon analysis, were taken from the outlets of the mixing chambers at the same occasions as the mussel sampling for HOC analysis. In addition, samples for HOC analysis were also taken from the algal culture (two samples) and from the brackish water (one sample). All water samples for HOC analysis were stored in 1-L glass bottles at −20°C until extraction. Additional samples of effluent water from the depuration tanks were also taken for organic carbon analysis.
Carbon analysis
Organic carbon content was determined as total (TOC) and dissolved (DOC) organic carbon on unfiltered and filtered (GF/F, 0.7-μm cutoff, Whatman, Maidstone, UK) samples, respectively, with a total carbon analyzer (TOC 5000, Shimadzu, Japan). Particulate organic carbon (POC) was calculated by subtracting DOC from TOC. The TOC concentration in the water was 5.1 ± 0.09 mg/L, DOC was 5.0 ± 0.07 mg/L, and POC was 0.08 ± 0.07 mg/L (mean ± SE; n = 26). There was no significant difference between the mixing chambers, including the tanks in the depuration study, for any of the variables (p > 0.05; ANOVA). The carbon concentration in the experimental water was similar to the normal situation in the Baltic Sea, where DOC usually varies between 4 and 7 mg/L and POC seldom exceeds 1 mg/L [21].
HOC extraction and cleanup
Samples of wet mussel tissue were extracted with some minor modifications to the method described by Jensen et al. [22]. Prior to the extractions, PCB 189 was added to the solvent (hexane/ether) as an internal standard. Extractions were carried out with 25 ml of a hexane/ether (9:1) mixture, added twice. The aqueous phase consisted of 50 ml of a 0.9% NaCl/0.1 M H3PO4 solution. For evaporation of solvents, a rotary evaporator was used instead of a water bath. The extractable organic content (EOC) of the sample was gravimetrically determined. In order to check the efficiency of the extractions for the different substances, three samples consisting of nonexposed mussels taken from the storage tank were analyzed as extraction controls. A mixture of HOCs in known amounts was added to each of these samples prior to extraction, and the samples were then treated in the same way as all other mussel samples. The results from extraction controls showed a rather large range of recovery for the different congeners. Recovery for PCBs 31, 52, 77, 118, and 153 was 114, 123, 114, 129, and 140%, respectively, and for PBDEs 47, 99, and 153, the recovery was 102, 73, and 46%, respectively. In an earlier study [8], where the same extraction and cleanup methods were used on samples of pike, the interval was much smaller, varying between 87 and 103% (Burreau, personal communication). One possible explanation for this discrepancy is that there are differences in the composition of pike and mussel tissues (e.g., lipids), which result in different efficiencies of the cleanup procedure. However, the recovery was similar in all three mussel extraction control samples and all HOC concentrations presented are corrected for recovery.
The water samples were extracted with n-hexane in flasks on a shaker apparatus. The water was extracted twice with 200 ml hexane for approximately 3 h, and PCB 189 was added to the solvent in the first extraction as an internal standard. After the extraction, the combined hexane extracts were reduced to a smaller volume (<1 ml) using a rotary evaporator. In order to evaluate whether the extraction time (23 × h) was sufficient, four of the samples were extracted for 3 × 3 hours, and each fraction was analyzed separately. For all congeners, more than 90% of the total was found in the first two fractions.
The cleanup procedure applied was modified from Smith et al. [23], applied by Zebühr et al. [24] for PCBs and by Burreau et al. [8] for PBDEs. The extract residues were cleaned up on an open column (10-mm inner diameter) containing, from bottom to top, 10 mm neutral silica gel (10% deactivated with water [w/w]), 30 mm KOH-impregnated silica, 30 mm H2SO4-impregnated silica, and 30 mm neutral silica gel. At the top, 5 to 10 mm of anhydrous Na2SO4 was added to reduce the water content of the samples. The HOCs were eluted with 50 ml n-hexane. The hexane was then evaporated to a smaller volume (<0.5 ml) and the samples were transferred to glass vials and stored at −20°C prior to analysis.
HOC analysis
The samples were analyzed on a gas chromatograph (GC 8060, Fisons Instruments, Altrincham, UK) coupled to a mass spectrometer (MD 800, Fisons). The samples were injected on the column at a GC oven temperature of 65°C that was held for 1 min. The temperature was then raised to 210°C at 20°C/ min, followed by a raise of 10°C/min up to 300°C, a temperature that was held for 10 min. The instrument was tuned and calibrated by using a reference gas including mass units ranging from 69 to 502. In addition, an external standard mixture including all studied analytes was run each day on the GC-MS before sample injection in order to establish the response factors used in the concentration calculations. Procedural blanks for the mussel extractions were analyzed and the levels were found insignificant. The limits of detection for the various analytes were 0.3 pg for PCBs 31, 77, and 118; 0.4 pg for PCB 52; and 0.5 pg for PCB 153. For PBDEs 47, 99, and 153, limits of detection were 0.6, 1.4, and 2.7 pg, respectively.
Calculations and statistical analyses
The BCI-data was analyzed by linear regression to test if mussel condition changed with time and by analysis of covariance (ANCOVA) to test for differences between treatments. A first-order growth coefficient (g) was calculated using linear regression on natural log-transformed BCI data. Linear regression was also used to determine the change in mussel dry weight with time. This regression was then used to calculate the dry weight of mussels used in the HOC analyses. The HOC concentration in mussel tissue (Cm) was based on dry weight (ng/g dry weight) since extractable organic content normalization (ng/g EOC) introduced higher variability in the data.
Depuration rate coefficients (kd) were calculated from the decline in HOC concentration in mussels during the depuration phase by linear regression of natural log-concentration versus time. The kd values were adjusted for mussel growth during the experiment by adding the calculated growth coefficient (kd + g).
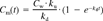
Bioaccumulation factors (BAFs) were calculated as the ratio of the uptake clearance rate coefficient to the depuration rate coefficient (BAF = ku/kd). In order to estimate the uncertainty in calculated ku and BAF values, extreme limits, based on the distribution of values (95% CI) for water concentrations and depuration rates, were simulated in KaleidaGraph using the formulas for calculation of ku and BAF. Depuration half-lives (t1/2 = −ln 0.5/kd) and theoretical time to reach 90% of steady-state tissue concentrations (t90 = −ln 0.1/kd) were also calculated.
RESULTS AND DISCUSSION
Mussel growth and mortality
A slight decrease in body condition index of the mussels was observed during the 70 d of the study, resulting in a negative growth coefficient, g = -0.003 ± 0.001 (g ± SE; linear regression, p < 0.0006; df = 1: 242). This indicates a moderate starvation of all the experimental mussels, and no influence of HOC exposure on the growth of the mussels was observed (no difference in BCI between exposed and non-exposed mussels, ANCOVA; p > 0.05; df = 1: 140). The overall mortality was 1.5% (0–3 individuals per aquarium, 12 individuals all together), and no difference between treatments was observed.
HOC background
Small amounts of some of the studied PCB congeners were detected in the algal culture and the brackish water. In the algal culture (n = 2), the concentration of PCB 52 was 1.5 ± 0.2 ng/L and PCB 153 was 0.44 ± 0.03 ng/L (mean ± SE). With a dilution factor of 103, as used in this experiment, this cannot be considered an important source of PCBs to the mussels. In the natural brackish water sample (n = 1), the concentrations of PCBs 31, 52, and 153 were 0.08, 0.08, and 0.13 ng/L, respectively. No PBDEs could be detected in the algal or brackish water samples.
In the control mussels, four of the five analyzed PCB congeners were detected, indicating the presence of these substances in the Baltic Sea. However, measured concentrations of PCBs were lower than previously reported in mussels from the same area (e.g., [25]). No PBDEs could be detected, that is, the levels were less than 0.004, 0.008, and 0.015 ng/g dry wt for PBDEs 47, 99, and 153, respectively. This might indicate that this area is rather unaffected by PBDE pollution. The background levels of PCBs were less than 10% of the accumulated residues and cannot be suspected to have interfered with the determination of PCBs in the depuration study.
HOC accumulation and depuration
The concentration of PCBs and PBDEs in the experimental water samples (Table 2) varied to some extent over the exposure time. A problem with the generator column between days 41 and 44 lead to drastically lowered HOC concentrations in the exposure water. All water samples taken at day 44 were therefore excluded from the calculation of mean water concentrations. However, all exposure aquaria were similarly affected by this malfunction and HOC concentrations found in the replicate mussel samples at day 44 were generally in good agreement with each other.
All PCBs and PBDEs used in the study were taken up by the mussels and accumulated in the organisms. The observed uptake of PBDEs 99 and 153 indicates that the earlier suggested ECS limit for HOC uptake in organisms (9.5 Å) does not block the uptake of these substances in mussels. Calculated uptake clearance rate coefficients (ku) were markedly higher for PBDEs 47 and 99 than for all the other substances in the study (Table 3 and Figure 2). Comparison of uptake clearance rates between PCBs and PBDEs of similar hydrophobicity shows a significantly higher ku value for the PBDEs. For example, PBDE 47 has a six to seven times higher ku value than PCBs 52 and 77, and PBDE 99 has a 12 to 30 times higher ku value than PCBs 118 and 153. It can be pointed out that the log Kow values used for the PBDEs are mean values from rather broad intervals (Table 1), but this does not affect the interpretation of the results.
A possible explanation for the observed higher bioavailability (i.e., higher ku values) of PBDEs compared to PCBs can be different partitioning behavior of these contaminant groups since the bioavailability of HOCs in consumed food may differ from the bioavailability of dissolved contaminants [26]. Very rapid sorption of HOCs including PCBs has been well demonstrated in aquatic systems [e.g., 27] and can be assumed to have occurred also in our flow-through experiment. Algal sorption of HOCs was not studied specifically in the present experiment. However, in previous studies, we have studied the sorption of 14C-labeled PCBs [28] and 14C-labeled PBDE 47 (Gilek and Björk, unpublished data) to the green algae Scenedesmus sp. The carbon-normalized partitioning coefficients (log KPOC) of the three studied PCB congeners were found to be in the range of 5.3 to 5.6, 5.4 to 5.6, and 6.1 to 7.0 ml/g for PCBs 31, 49 (with a similar log Kow value as PCB 52), and 153, respectively. Assuming a similar partitioning behavior of PCB 52 to that of PCB 49, the fraction of PCB 52 associated to algae in the present experiment can be estimated to be in the range of 2.0 to 3.2%. This can be compared with the measured log KPOC of 5.3 to 5.4 for PBDE 47, which indicates an algal partitioning of 1.6 to 2.0%. To summarize, there is an indication that algal sorption is lower for PBDE 47 than for PCB 52. Given a higher bioavailability of dissolved HOCs [26], this could result in different uptake rates of these two compounds. However, it is still unclear if this is the sole cause for the observed generally faster uptake of PBDEs than of PCBs.
| Substance | ku (L/d/g dry wt.) | kd (d−1) | t1/2 (d) | t90 (d) | BAF × 105 (ml/g dry wt.) |
|---|---|---|---|---|---|
| PCB 31 | 14 {27; 8.7} | 0.129 ± (0.028) | 5.4 | 17.8 | 1.1 {2.1; 0.7} |
| PCB 52 | 20 {38; 12} | 0.118 ± (0.021) | 5.9 | 19.5 | 1.7 {3.2; 1.0} |
| PCB 77 | 17 {41; 9.2} | 0.073 ± (0.019) | 9.5 | 31.5 | 2.3 {5.6; 1.3} |
| PCB 118 | 14 {43; 7.2} | 0.052 ± (0.018) | 13.3 | 44.3 | 2.7 {8.2; 1.4} |
| PCB 153 | 5.7 {9.8; 3.5} | 0.027 ± (0.014) | 25.7 | 85.3 | 2.1 {3.6; 1.3} |
| PBDE 47 | 120 {289; 67} | 0.090 ± (0.017) | 7.7 | 25.6 | 13 {3.2; 0.7} |
| PBDE 99 | 170 {678; 75} | 0.123 ± (0.045) | 5.6 | 18.7 | 14 {5.5; 0.6} |
| PBDE 153 | 19 {; 7.5} | 0.086 ± (0.008) | 8.1 | 26.8 | 2.2 {; 0.9} |
For the least hydrophobic PCB congener, PCB 31, the highest concentrations in the mussels were observed after 15 d of exposure, which is in agreement with the expected time to reach 90% of steady-state tissue concentration for this congener. However, substantially lower concentrations of PCB 31 in the mussels on day 44 indicate a decline in exposure. One possible explanation for this is that PCB 31, with a relatively high water solubility, might have been depleted in the generator column, thus leading to low concentration in the experimental water in the late part of the exposure phase.
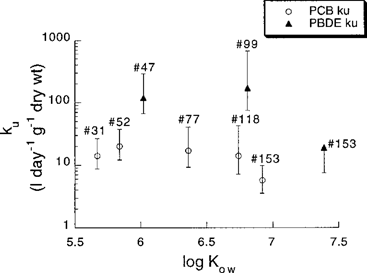
The relationship between uptake clearance rates (ku) (L/d/g dry wt.) and log Kow for the PCBs and PBDEs used in the study. Error bars for ku represent simulated extreme limits based on the distribution of values (95% CI) for water concentrations (Table 2) and depuration rate coefficients (Table 3 and Fig. 3) used in the calculations of ku. Upper limit for ku for the most hydrophobic PBDE congener is missing. Log Kow values for PCBs are taken from Hawker and Connell [35] and for PBDEs are mean values of the interval given by Watanabe and Tatsukawa [36].
In general, depuration data showed good fit to the used first-order model (0.79 ≤ r2 ≤ 0.98, p < 0.05). Calculated depuration rate coefficients (kd) decreased with increasing hydrophobicity (Kow) for the PCBs (Fig. 3), and the lowest depuration rate of all was observed for PCB 153. The depuration rates obtained for the PCBs in the present study are in agreement with depuration data for mussels reported by others (e.g., [14, 29, 30]) and is consistent with the general assumption that depuration rates of hydrophobic substances are inversely related to log Kow (e.g., [31]). However, the PBDEs deviate from this expected pattern and, instead of decreasing with increased Kow, the depuration rates for PBDEs appear to be independent of Kow. Hence, PBDE 47 has a depuration rate comparable with those of PCBs 52 and 77, whereas the more hydrophobic PBDEs (99 and 153) have higher kd values compared with corresponding PCBs. Recent studies indicate that higher brominated PBDE congeners under certain conditions may undergo debromination, thus forming smaller, less brominated substances [32]. If the penta- and hexa-BDEs in the present study have, to a certain extent, undergone debromination in the depuration phase, this would result in higher kd values than expected compared with PCBs of similar hydrophobicity. A similar result would also appear if the substances were actively degraded by metabolic processes in the organisms. Very little is known about active metabolism of PBDEs in bivalves, but generally, metabolism in mussels is not considered important for aromatic substances [33].
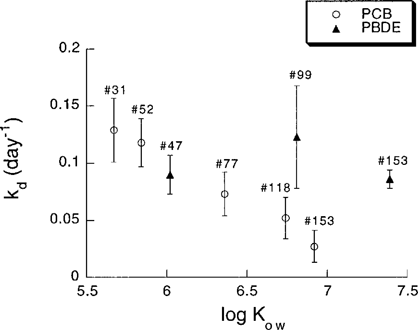
The relationship between depuration rate coefficients (kd) (d−1) and log Kow for the PCBs and PBDEs used in the study. Error bars for kd represent 95% confidence intervals. Log Kow values for PCBs are taken from Hawker and Connell [35] and for PBDEs are mean values of the interval given by Watanabe and Tatsukawa [36].
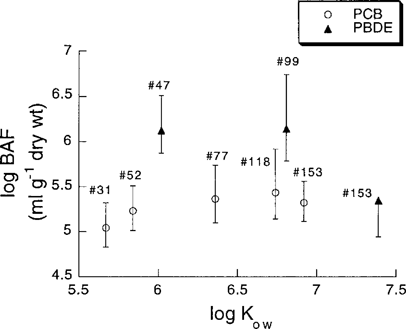
The relationship between bioaccumulation factor (BAF) (ml/g dry wt.) and log Kow for the PCBs and the PBDEs in Baltic Sea blue mussels (M. edulis). Error bars represent simulated extreme limits based on the distribution of values (95% CI) for water concentrations (Table 2) and depuration rate coefficients (Table 3 and Fig. 3) used in the calculations of ku. Log Kow values for PCBs are taken from Hawker and Connell [35] and for PBDEs are mean values of the interval given by Watanabe and Tatsukawa [36].
The bioaccumulation potential is highest for two of the PBDEs, 47 and 99. Calculated BAFs (Table 3 and Fig. 4) were six to eight times higher for PBDE 47 than for PCBs 52 and 77, and BAF for PBDE 99 was five to seven times higher than for PCBs 118 and 153. Calculated BAFs increased slightly with increasing log Kow for the lower chlorinated PCBs, whereas no clear trend was observed for the PBDEs. However, the most hydrophobic congener, PBDE 153, showed a lower BAF than the other PBDEs but comparable to that of all PCBs.
In most cases (both for PCBs and PBDEs), accumulation data (see two examples in Fig. 5) were better described by a linear uptake model (higher r2 values) than by the first-order bioaccumulation model. Apparently, steady-state tissue concentrations were not reached during the bioaccumulation experiment; the uptake was still in its linear phase at day 44. In contrast to this, calculations of the time to reach 90% of steady state (t90), based on depuration data, indicate that the exposure phase was sufficiently long to attain concentrations close to steady state, except for the most hydrophobic congeners (Table 3). It is unclear why tissue concentrations did not reach steady state in the present experiment. One possible explanation to the apparently linear shape of the accumulation curves is that exposure to the test substances increased over time. There were no indications of significantly increased (or decreased) HOC water concentrations over time, and water concentrations could be described as average values (Table 2). Alternatively, mussels may have changed their feeding physiology (e.g., ventilation rate, consumption rate, and gut passage time) during the experiment as an adaptation to the low food availability in the experimental water [34]. Low food availability may, for example, lead to longer gut passage times of ingested food [34] and thereby higher assimilation efficiencies of carbon and HOCs.
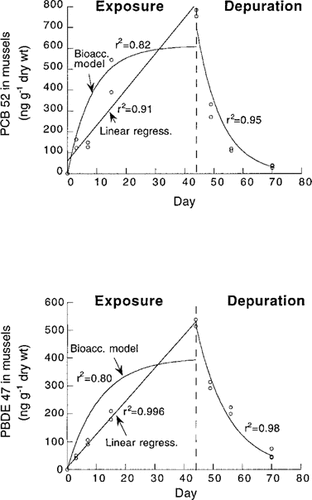
Tissue concentration (ng/g dry wt.) of PCB 52 and PBDE 47 in Baltic Sea blue mussels (M. edulis) in the 70 d flow-through exposure and depuration study. Note the differences in scales on the y-axis for the two congeners.
Uptake rate coefficients were also calculated based on an assumption of initial linear uptake rates during the first 3 d of exposure, that is, assuming very low initial elimination that can be neglected. A comparison of these ku values with those calculated according to the bioaccumulation model reveals that there is no significant difference between the data from the two models when looking at the PCBs. However, for the PBDEs, the initial linear uptake rate assumption resulted in ku values, and hence BAFs, that were a factor of two to four lower compared with the bioaccumulation model. These BAFs are, however, still notably higher for the tetra- and penta-BDE congeners than for their corresponding PCBs.
CONCLUSIONS
The studied PCBs and PBDEs are bioavailable substances that are rapidly taken up and bioaccumulated by the blue mussels. Uptake is efficient also for those of the substances that have ECS values >9.5 Å. Uptake clearance rate coefficients and bioaccumulation factors were especially high for PBDEs 47 and 99 and were much higher for these substances than for the PCBs included in the study. After uptake, the elimination of PBDEs 99 and 153 was faster than expected based on their hydrophobicity and compared with the PCBs. This suggests that the amounts of PBDEs 99 and 153 in the tissues rather rapidly reach steady state or decline if exposure decreases. The high bioaccumulation potential of the PBDE congeners in this study indicates that these substances may reach high concentrations in aquatic organisms. High concentrations of mainly PBDE 47 have indeed been detected in several environmental samples and, since the concentration of PBDEs in the environment seems to be increasing, these substances deserve more attention. In particular, more information is needed on the partitioning behavior, bioavailability, metabolism, and, most importantly, the ecological effects of PBDEs.
Acknowledgements
The study received financial support from the Swedish Environmental Protection Agency. Åke Bergman and coworkers at the Department of Environmental Chemistry, Stockholm University, are thanked for providing us with PBDEs and PCBs. We also wish to thank the Stockholm Center for Marine Research for providing facilities and technical support at the Askö Laboratory and for funding travelling expenses.




