Comparative studies of the reduction behavior of chromium(VI) by humic substances and their precursors
Abstract
Hexavalent chromium (Cr[VI]) is reduced by dissolved organic carbons (DOCs) such as humic substances, tannic acid (TA), and gallic acid (GA). The kinetic constants and the resulting chemical species after the reduction were compared with each other. The kinetic constants for GA and TA, which are model precursors of humic substances, were two to three orders of magnitude larger than those for the humic substances when these kinetic constants were expressed as a function of the molar concentration of the reductive functional group (Fred) in various DOCs. After the reduction of Cr(VI), the percentages of the species complexed with GA and TA were higher than those with the humic substances. This appears to be due to the formation of high molecular weight compounds by polymerization during the reduction of Cr(VI) and complexation of Cr(III) with the polymerized compounds. The UV-vis spectrophotometric data and gel permeation chromatography support this view.
INTRODUCTION
In the environment, chromium mainly exists in hexavalent (Cr[VI]) and trivalent (Cr[III]) states [1]. Cr(VI) is a strong oxidizing agent, which is also highly toxic and carcinogenic, but Cr(III) exhibits less toxicity [1]. Cr(VI), which is used extensively in the leather tanning process as well as in the electroplating industries, often flows into natural waters, such as rivers and lakes, thereby severely and negatively affecting aquatic ecosystems. To estimate the toxicity of Cr(VI) in an aquatic ecosystem, it is important to determine the chemical species of the chromium as well as the total concentration of all species. Nakayama et al. [2] reported that the distribution of chromium species in natural waters was 25 to 40% for inorganic Cr(VI), 10 to 20% for inorganic Cr(III), and 45 to 60% for Cr(III) complexed with dissolved organic carbon (DOC) such as humic substances. Moreover, the finding repeated by Nakayama et al. as well as by this laboratory found that, although inorganic Cr(III) was easily reoxidized to Cr(VI) in the presence of manganese dioxide, the rate of this oxidation was suppressed by the presence of DOCs such as humic substances. This is due to the complexation of Cr(III) with DOCs [3, 4].
In addition, Cr(VI) is reduced to Cr(III) in the presence of DOCs such as humic substances [5-10]. The source of reducing power for this resides in the hydroquinone groups in humic substances [8-10]. Several hypotheses have been proposed for the formation of humic substances. One of these is that humic substances are produced from the chemical polymerization of low molecular weight decomposition products, such as polyphenols and amino acids, which arise via the decay of dead animals, plankton, and plant material [11]. Tannic acid (TA) and gallic acid (GA), which are polyphenols that originate from dead leaves, are thought to be one of the precursors of humic substances [12]. These are also widely distributing in natural water and constitute a portion of lower molecular weight DOC, while humic substances make up the higher molecular weight fraction. Arakawa et al. [13] reported that Cr(VI) was reduced efficiently by alkaline extracts of dead leaves. This suggests that low molecular weight compounds such as TA and GA are important in the reduction of Cr(VI). Recently, we studied the influence of humic substances on the distribution of chemical species after the reduction of Cr(VI) and found that most of the Cr(VI) that had been reduced by humic substances existed as complex with humic substances [14]. Since TA and GA have functional groups (carboxyl and phenolic OH groups) that are similar to humic substances, these may have the ability to reduce and complex with Cr(VI). Therefore, it is thought that the presence of TA or GA in aquatic ecosystems influences the level of Cr(VI) present. However, the behavior of low molecular weight DOC, such as TA or GA, toward the reduction of Cr(VI) is not well understood. In the present work, we compared the rate of reduction of Cr(VI) by high molecular weight DOC (humic substances) with that of low molecular weight DOC, TA, and GA. Moreover, we evaluated the chemical species of chromium after the reduction of Cr(VI) by humic substances, TA, and GA. The polymerization of GA accompanied by the reduction of Cr(VI) was confirmed from UV-vis spectroscopy and gel chromatography.
| DOCs | % C | % H | % N | % S | % O | Reducing capacity (mmol of Cr/g of C) | Eoxp (mV vs Ag, AgCl) |
|---|---|---|---|---|---|---|---|
| SHA | 52.15 | 5.44 | 2.11 | 0.36 | 39.94 | 95 | — |
| SFA | 41.26 | 5.27 | 0.95 | 0.36 | 52.16 | 110 | +760 |
| TA | 48.72 | 3.77 | NDa | ND | 47.51 | 92 | +430 |
| GAb | 49.42 | 3.56 | 0 | 0 | 47.03 | 89 | +190 |
- aNot detected.
- b% C, % H, and % O of GA are calculated values.
EXPERIMENTAL
Material
Humic and fulvic acid (SHA, SFA) were extracted from peat soil (Shin-Shinotsu, Hokkaido, Japan) and purified according to the procedure recommended by the International Humic Substances Society (IHSS) [15]. An alkaline extract of peat was acidified with HCl up to pH <1. The precipitate and supernatant were separated by centrifugation (3,500 rpm, 15 min), and the precipitate was transferred into a dialysis tube (MW < 1,000 cut-off; Spectra/Pore, Houston, TX, USA). Dialysis was carried out against distilled water for 2 to 3 weeks with stirring. Finally, powdered humic acid (HA) was obtained by lyophilization. The supernatant was passed through an Amberite XAD-2 column (Organo, Tokyo, Japan; 50 mm ϕ × 320 mm). The fulvic acid (FA) was eluted by an aqueous solution of 0.1 mol/L NaOH. After neutralization of the fraction, the aqueous solution of FA was passed through a Sephadex G-10 column (50 mm ϕ × 330 mm; Pharmacia Biotech AB, Uppsala, Sweden), in which water was used as an eluent. The FA fraction was shaken with an H+-type cation exchanger (Amberite IR-500), and the aqueous phase was then evaporated. Finally, powdered FA was obtained by lyophilization.
Tannic acid and gallic acid were obtained from Wako Pure Chemical Industries (Osaka, Japan). These humic and fulvic acids were stored as a basic stock solution (0.01 mol/L NaOH) in a refrigerator.
Table 1 shows the results of elemental analysis and the values of reducing capacities of the DOCs. The reducing capacities were determined by a modified Walkley–Black method [16]. This method measures the amount of Cr(VI) reduced by DOCs under extremely acidic conditions. A mixture of 0.4 ml of 1.00 g/L DOCs and 0.2 ml of 0.10 mol/L Cr(VI) solution was added to 2.0 ml of concentrated H2SO4 in a glass vessel. The reaction vessel was kept at 90°C in a water bath, and the concentration of remaining Cr(VI) was monitored.
METHODS
Determination of oxidation potential by cyclic voltammetry
The oxidation potential (Eoxp) of each DOC was determined by cyclic voltammetry (CV) according to the method of Helburn and MacCarthy [17]. The pH of 0.1 mol/L KCl aqueous solution containing 1 g/L DOC was adjusted to 9 by 0.05 mol/L HEPES buffer. Five-milliliter aliquots of the solution were pipetted into an electrolysis glass cell and then deaerated by argon for 15 min. Subsequently, the CV voltammograms were recorded by scanning the potential from −1.0 to +0.9 V (vs Ag/AgCl) at 50 mV/s. A gold disk electrode for a working electrode, a platinum wire for a counter electrode, and an Ag/AgCl electrode for a reference electrode were used. The measurements were performed at room temperature (22°C).
Kinetic measurement
Since the reduction rate of Cr(VI) by humic substances is very slow [10], the temperature for the reaction was set at 50°C in this study in order to accelerate the reaction. The pH of the sample solutions, including 40 mg/L of each DOC, was adjusted by using sulfuric acid and sodium hydroxide. The pH of the sample was also checked at the end of the reaction in order to confirm a constant pH. The deviation of pH was about ±0.2 to 0.3. Fifty-milliliter aliquots of the sample solutions in the Erlenmeyer flask were stirred in a water bath. After reaching constant temperature, 5 mmol/L Cr(VI) was added by a micropipette to give 50 to 100 μmol/L Cr(VI) in the solution. Two-milliliter aliquots of the solution were pipetted into a 10-ml measuring flask to which 0.1 ml of concentrated sulfuric acid and 0.1 ml of 0.25% diphenylcarbazide (DPC) in acetone was added, and this solution was then diluted with water. After 20 to 30 minutes, the concentrations of Cr(VI) in the sample solutions were determined by measuring the absorbance at 542 nm. The reproducibilities of these experiments for GA and TA were about 10%. The statistical tool used in this work for curve fitting is ORIGIN version 4.1J (Microcal Software, Northampton, MA, USA).
Speciation analysis for chromium after reduction by cation exchange/DPC colorimetric method
The chemical species of Cr(VI) after reduction by DOCs were determined using a cation exchange/DPC colorimetric method reported previously [14]. The sample solution at pH 4.9, containing 40 mg/L DOC and 100 μmol/L Cr(VI), was shaken for 1 week in a water bath at 50°C. A 5-ml aliquot of the solution was passed through the column packed with 0.4 ml of the cation exchanger, Sulphopropyl Sephadex C-25 (C-25), and the effluent was then collected. In this procedure, the cationic species, inorganic Cr(III), were retained on the C-25, but the anionic species, inorganic Cr(VI) and Cr(III) complex with DOCs, passed though the column. The concentrations of Cr(VI) in the effluent were determined by the DPC colorimetric method, and the total concentration of Cr in the effluent was determined by the ICP-AES method. The concentration of Cr(III) complex species can be calculated by subtracting the concentration of inorganic Cr(VI) from total concentration of Cr in the effluent. The ability of each DOC to complex with Cr(III) was also evaluated by the above method using the C-25 resin [18].
UV-vis spectrometry. A UV-2000 type spectrophotometer (Shimadzu, Kyoto, Japan) was used to measure the UV-vis absorption spectra of the solutions. The concentrations of HA, FA, and TA were 20 mg/L and that of GA was 4 mg/L. The time dependency in the spectrum of GA from 400 to 200 nm was observed by reacting 25 μmol/L Cr(VI) and 4 mg/L GA (pH 4.9, 50°C).
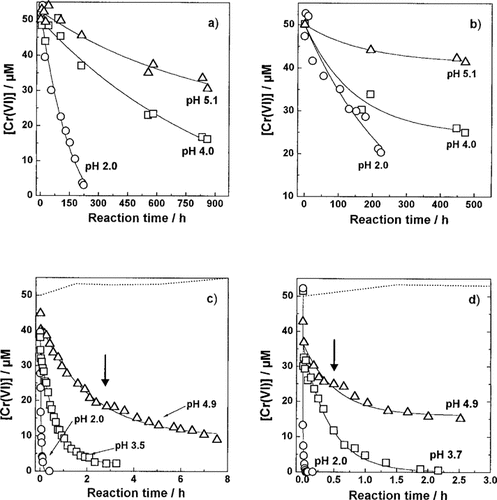
Reduction of Cr(VI) by various DOCs. DOC, 20 mg/L; Cr(VI)add, 50 μM; temperature, 50°C. SHA (a), SFA (b), TA (c), GA (d). Dashed line shows the control (without DOC).
Gel chromatography. Gel chromatography was carried out to confirm the polymerization of GA that accompanied the reduction of Cr(VI). The glass column (25 mm ϕ × 500 mm length) filled with Sephadex G-10 (exclusion limit 700, Pharmacia) was used for this. Blue dextran (MW > 2 × 106) was used to determine the void volume of this column. Pure water was used as the mobile phase, the flow rate of which was 1 ml/min. The solution (pH 4.9) containing 200 mg/L GA and 1 mmol/L Cr(VI) was shaken about 6 d at 50°C, and then 2-ml aliquots of the solution were injected on the column and fractions of the effluent were collected at 5 min intervals. The absorbance of each fraction was measured at 260 nm, and the concentration of Cr in each fraction was determined by flame atomic absorption spectrometry.
RESULTS AND DISCUSSION
Reduction rate of chromium(VI) by humic substances and their precursors
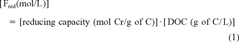 (1)
(1)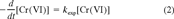 (2)
(2)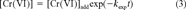 (3)
(3) (4)
(4) (5)
(5)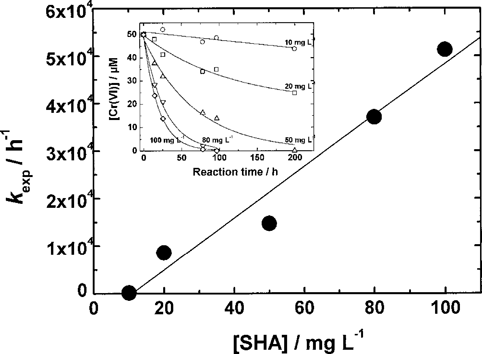
Relationships between kexp and concentration of SHA. Insertion figure: Kinetic curves of Cr(VI) reduction by SHA. Cr(VI)add, 50 μM; temperature, 50°C; pH, 2.0.
Equation 5 shows a linear relationship between the kexp values and the concentrations of the DOCs. Such a linear relationship was reported in the case of the reduction of Cr(VI) by various phenolic compounds [8]. As shown in Figure 2, we also confirmed this linearity for the case of SHA. The kexp and kFred values, which were calculated by Equation 5, are summarized in Table 2. The kFred values increased with decreasing pH, which can be explained by the decrease in the redox potential of Cr(VI) [10]. The order of kFred at pH 5, which is near the pH of natural water, was GA > TA > SFA > SHA. The values for TA and GA were larger by two to three orders of magnitude than those for the humic substances. As shown in Figure 1, the reduction of Cr(VI) by the humic substances was not complete even after several hundred hours. However, the reduction of Cr(VI) by TA and GA was complete in several to 10 h. The Cr(VI) reduction rate for the low molecular weight DOC such as TA and GA was much faster than that for the humic substances.
| DOCs | pH | [Fred]/mmol/L | kexp/h−1 | log kArOH/M−1h−1 |
|---|---|---|---|---|
| SHA | 2.0 | 2.0 | 6.0 × 103 | 6.5 |
| 4.0 | 2.0 | 1.2 × 103 | 5.8 | |
| 5.1 | 2.0 | 1.0 × 103 | 5.7 | |
| SFA | 2.0 | 1.8 | 6.8 × 103 | 6.6 |
| 3.9 | 1.8 | 5.6 × 103 | 6.5 | |
| 5.0 | 1.8 | 3.5 × 103 | 6.3 | |
| TA | 2.0 | 1.8 | 2.3 × 107 | 10 |
| 3.5 | 1.8 | 1.6 × 106 | 8.9 | |
| 4.9 | 1.8 | 4.7 × 105 | 8.4 | |
| GA | 2.1 | 1.8 | 1.4 × 108 | 11 |
| 3.7 | 1.8 | 2.3 × 106 | 9.1 | |
| 4.9 | 1.8 | 1.9 × 106 | 9.0 |
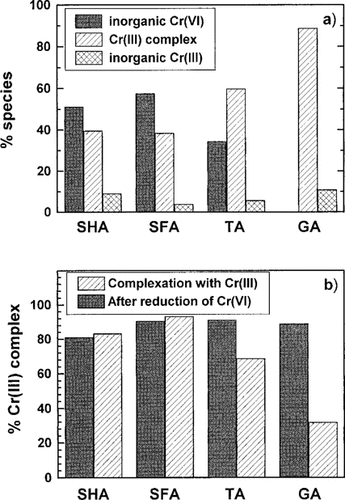
Results of speciation analyses of Cr. DOC, 20 mg/L; Cr(VI)add or Cr(III)add, 100 μM; pH, 4.9; reaction time, 170 h; temperature, 50°C. Cr species after the reduction (a), comparison of amounts of complex species produced by reduction of Cr(VI) and complexation with Cr(III) (b).
In Table 2, although the concentration of the reductive functional groups for each DOC, [Fred], was similar, the kFred values were quite different. It has been reported that a positive correlation exists between the oxidation rate for a phenol derivative and the oxidation potential [19]. Thus, such differences in the reaction rates may be dependent on the oxidation potential of each DOC. We then determined the oxidation peak potential (Eoxp) for these DOCs by CV (Table 2). We could not evaluate Eoxp for SHA because a clear oxidation peak was not observed. The order of Eoxp was GA < TA < SFA, and this order was consistent with that of the kFred values. This suggests that the DOC having the higher oxidation potential was hardly oxidized and, as a result, the reduction rate for Cr(VI) with the DOC becomes slower.
Speciation analysis of chromium after reduction of chromium(VI) with DOCs
Figure 3a shows the results of the analysis of Cr species after the reduction of Cr(VI) with each DOC at pH 5 for 1 week. In the case of SHA and SFA, about 50 to 60% of the Cr was found to be the unreduced Cr(VI) species and about 40% was complexed with the humic substances. In the case of TA, about 60% of the Cr was observed to be complexed with TA, and the ratio of the species complexed with TA was higher than those for the humic substances. In the case of GA, Cr(VI) was almost completely reduced and about 90% of the Cr was found to be complexed with GA. The ratio of the complexed species in the case of GA was the largest of these four DOCs.
Figure 3b shows a comparison of the percentages of the complexed Cr(III) species when Cr(VI) or Cr(III) were reacted with the DOCs. The percentages after the reduction of Cr(VI) were calculated as the percentages of the complexed species of the total Cr(III) species. In the cases of SHA and SFA, large differences were not observed between the percentages after the reduction of Cr(VI) and complexation with Cr(III). However, in GA and TA, the percentages of the complexed species after the reaction with Cr(VI) were larger than those after the complexation with Cr(III), especially in the case of GA; although the percentage of the complexed species after complexation with Cr(III) was 32%, the percentages of the complexed species after the reduction of Cr(VI) were about 90%, or 2.8-fold larger than those for the complexation with Cr(III). Therefore, TA and GA are more effective in decreasing the toxicity of Cr(VI) by the rapid reduction of Cr(VI), followed by complexation with the resulting Cr(III) produced by the reduction.
Oxidative polymerization of GA during reduction of chromium(VI)
In Figure 1c and d, the series of the kinetic data points for TA and GA deviated slightly from the calculated curve, which is indicated by arrows. These deviations can be attributed to different reducing sites for Cr(VI), which results in the reduction of Cr(VI) at a slower rate [10]. From the data of Eoxp in Table 1 and kFred in Table 2, the higher oxidative potential of these compounds showed a larger molecule size and a slower reduction rate. Therefore, the deviation in Figure 1c and d suggests the production of larger molecular weight compounds from TA or GA. Phenolic compounds such as catechol are oxidized and then polymerized easily by dissolved oxygen [20, 21]. The TA and GA would also be expected to result in higher molecular weight materials by the oxidative polymerization during the reduction of Cr(VI). We attempted to confirm this for the polymerization of GA by UV-vis spectrophotometry and gel permeation chromatography.
UV-vis spectrum. Figure 4a shows the time dependency of a UV-vis spectrum of GA for a reaction of GA and Cr(VI) at 50°C. As described in the literature [22], two peaks were observed at 260 and 210 nm on the initial spectrum of GA. However, the absorbances at 260 and 210 nm decreased and the absorbance at 320 and 230 nm increased with increasing reaction time. After 50 min, the sharp peaks in the spectrum disappeared and the absorbance increased linearly with decrease in wavelength.
Figure 4b shows UV-vis spectra of the DOCs in the absence of Cr(VI). The TA showed two shoulders in the spectrum at 360 and 270 nm. However, humic substances, such as SHA and SFA, had no peaks in their spectrum. Comparing the curves in Figure 4a and b, it was found that the spectrum of GA changed to the TA type (Fig. 4a, 5 min) and finally the humic type (Fig. 4a, 51 min). These results suggest that GA is polymerized and converted to a high molecular weight compound as humic substances during the reduction of Cr(VI).
Gel chromatography. In order to clarify the polymerization of GA that occurs during the reduction of Cr(VI), we compared the molecular weight distribution of GA before and after the reduction of Cr(VI) by using gel permeation chromatography with a Sephadex G-10 column. The void volume for the column, which was measured by blue dextran (MW > 2 × 106), was 76 ml. The elution volume (Ve) for the GA solution after the reduction of Cr(VI) was 76 ml both when the detection was carried out by measurement of the absorbance at 260 nm (Fig. 5a) and Cr by AAS (Fig. 5b). These Ve values coincided with the void volume (76 ml). On the other hand, the Ve values of GA itself (Fig. 5c) and Cr(VI) itself (Fig. 5d) were 350 ml and 145 ml, respectively. Therefore, it is thought that the molecular weight of GA after the reduction of Cr(VI) increases. These results suggest that GA was polymerized during the reduction of Cr(VI), producing high molecular weight compounds. Moreover, the comparison of the chromatograms in Figure 5b and d showed that the location of Cr in the gel chromatograph changed to that of the high molecular weight compounds after the reduction by GA. This result suggests that Cr, after the reduction, was taken up by the polymerized GA and appeared as the complexed species.
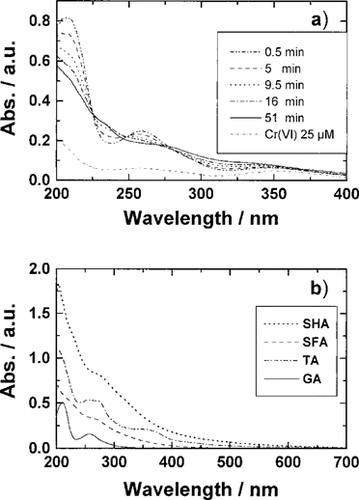
Effect of Cr(VI) on UV-vis spectra of GA (a) and UV-vis spectra of DOCs (b). (a) GA, 4 mg/L; Cr(VI)add, 25 μM; pH, 4.9; temperature, 50°C. (b) SHA, SFA, TA, 20 mg/L; GA, 4 mg/L; pH, 8.
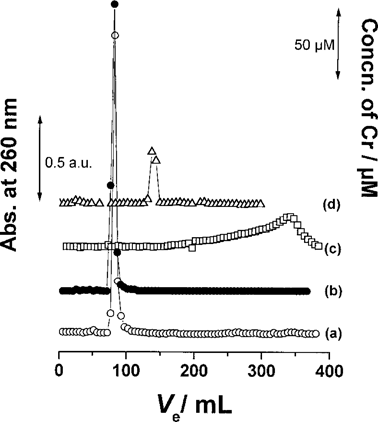
Sephadex G-10 gel chromatograms of GA, Cr(VI), and their mixture. GA, 200 mg/L; Cr(VI)add, 1 mM; pH, 4.8; reaction time of the mixture, 145 h. GA + Cr(VI); absorption at 260 nm of GA (a), GA + Cr(VI); concentration of Cr (b), GA (c), Cr(VI) (d).
CONCLUSION
The reduction kinetics of Cr(VI) by TA or GA as representative low molecular weight DOC and humic substances as the high molecular weight DOC were compared. The kinetic constants for the lower molecular weight DOC were two to three orders of magnitude larger than those for the humic substances. The kinetic constant was dependent on the oxidation potentials of the DOCs. The lower molecular weight compounds had the smaller oxidation potential and facilitated the reduction of Cr(VI). From the results of chemical speciation analysis for chromium after the reduction by each DOC, TA and GA resulted in a low toxic Cr(III) complex compared with the humic substances. Moreover, it was found by the studies of UV-vis spectrophotometry and gel chromatography that GA was oxidatively polymerized to high molecular weight compounds such as humic-like substances. During this oxidative polymerization, the Cr(III) produced by the reduction was taken up into the polymerized GA as the complexed species. Although humic substances play an important role in natural remediation of Cr(VI) in water environments, the precursors of humic substances, such as TA and GA, are also important for lowering the toxicity of Cr(VI) through reduction and the complex formation.




