Effects of terbufos on juvenile red swamp crayfish (Procambarus clarkii): Differential routes of exposure†
Technical contribution 4262, South Carolina Agricultural Experiment Station and TIWET contribution 9615, Clemson University. Pendleton, SC, USA.
Abstract
During rain events, terbufos may be transported into aquatic ecosystems via agricultural runoff. Because crayfish are closely associated with sediment, they may be exposed to aqueous terbufos through the gills or by ingesting contaminated sediment, detritus, or plants. Bioavailability of terbufos in food and toxicity of terbufos to crayfish were examined by measuring mortality and acetylcholinesterase (AChE) inhibition and by observing behavior. A 96-h aqueous exposure and a 12-h dietary exposure of juvenile red swamp crayfish, Procambarus clarkii, to terbufos produced median lethal concentrations (LC50s) of 5.9 μg/L (95% CI: 4.4, 8.1) and 4.4 μg/g pellet (95% CI: 2.9, 6.7), respectively. Aberrant behavior, such as loss of motor control and equilibrium, was noted at concentrations almost 50% of the aqueous LC50 and 80% of the dietary LC50. As concentration increased, AChE activity decreased and mortality and aberrant behavior increased. I50s, the percentage of AChE inhibition at which 50% of the crayfish died, for aqueous and dietary exposures were 76.5% (95% CI: 67.4, 85.6) and 86.1% (95% CI: 78.1, 94.1) of control activity, respectively. Based on present study results, terbufos is available for uptake by crayfish through ingestion and causes detrimental effects at concentrations less than expected in agricultural runoff.
INTRODUCTION
During rain events, pesticides may be transported with agricultural runoff into aquatic environments, depending on soil type, environmental conditions, and chemical characteristics [1, 2]. Pesticides in runoff have been the cause of numerous fish kills [3, 4], and organophosphorus (OP) insecticides, in particular, have produced mortality and detrimental effects in aquatic plants, zooplankton, insects, crustaceans, fish, and waterfowl [5-7].
OPs inhibit acetylcholinesterase (AChE), an enzyme that hydrolyzes a neurotransmitter, acetylcholine (ACh). Many studies have correlated AChE inhibition with mortality and sublethal responses in fish and aquatic invertebrates exposed to OPs, and generally, decreasing levels of AChE activity correspond to increasing exposure concentrations of OPs [8-11].
Many benthic and epibenthic organisms, such as crayfish, utilize sediment for food, habitat, protection from cold weather and predators, and brooding eggs. Crayfish are considered critical for maintaining energy flow throughout aquatic ecosytems by ingesting dead organic material, aquatic macrophytes, and invertebrates, and transferring this energy among various trophic levels [12-15]. Crayfish are also widely distributed throughout the United States and can be found in numerous aquatic environments such as farm ponds, rivers, and lakes [16]. Because of their distribution and habitat utilization, cray-fish may be readily exposed to OPs in the water or by ingesting contaminated sediment, detritus, plants, and invertebrates.
One of the most commonly used granular OPs, terbufos (S-tert-butylthiomethyl O,O-diethyl phosphorodithioate; Counter 15G), is widely used to control corn rootworm. Terbufos is nonpolar, relatively water insoluble, and binds readily to organic carbon in soil. The log Kow and water solubility are 4.52 and 15 mg/L, respectively [17, 18]. Terbufos is applied in or above soil at the time of planting. At an application rate of 11.4 g/100 m, planted in a band 18 cm wide and 10 cm deep, the expected theoretical concentration of terbufos in soil is 6 μg/g [19]. Based on studies conducted with a rainfall simulator, terbufos runoff concentrations may range from 0.03 mg/m2 to 8.40 mg/m2, depending upon soil tillage practices [1], and may occur in concentrations up to 1.2 mg/L in runoff. Because terbufos and its metabolites may persist in soil at concentrations of 4–6 μg/g weeks after application, a rain event may produce runoff containing potentially toxic terbufos concentrations. When this runoff reaches the aquatic ecosystem, terbufos and its oxidation products may adsorb onto plants, detritus, and sediment, or may remain in the water at lethal concentrations.
Because crayfish are key aquatic organisms, assessment of ecological risk is needed for effective management and prevention of harmful population reductions caused by OP exposure. The purpose of this study was to examine the potential toxicity of agricultural runoff to juvenile crayfish. The objectives of this research were to determine the bioavailability of terbufos in crayfish food and measure acute aqueous and dietary terbufos exposures by observing mortality and aberrant behavior and by measuring AChE activity.
MATERIALS AND METHODS
Analytical methods and studies
To estimate aqueous and dietary terbufos exposures, desorption of terbufos from pellets and terbufos degradation in water were measured. Extraction efficiency of terbufos from water was measured for three replicates of 250 ml moderately hard reconstituted water (hardness ∼110 mg/L CaCO3) fortified with 80 μl of 510 mg/L terbufos in hexane (98% terbufos, American Cyanamid, Princeton, NJ, USA). To verify the spiking concentration, a hexane spike check was made by fortifying hexane with the same volume of acetone stock used to fortify the water. Terbufos was trapped on a C18 solid-phase extraction (SPE) column conditioned with hexane and removed with 3 ml 25:75 acetone: hexane. Extracts were stored at 4°C pending analysis. Samples were analyzed on a Hewlett-Packard (Avondale, PA, USA) 5890 gas chromatograph with a flame photometric detector in the phosphorus mode. Recoveries were calculated by dividing terbufos in the sample by terbufos in the spike check.
Terbufos degradation in water was measured to determine length of exposure in water. Two liters of moderately hard reconstituted water were fortified with 500 μl of 238 mg/L terbufos in hexane and mixed well. Fortified water was poured into six 1-L beakers, with 250 ml of water per beaker. All beakers were covered with aluminum foil to prevent evaporation. Four 50-ml water samples were taken at 3, 6, 24, 48, 72, and 96 h and extracted at the time of collection, as described previously. Extracts were stored at 4°C pending analysis. Terbufos half-life in water was calculated by plotting the natural log (concentration at time t [Ct] divided by the initial concentration [C0]) against time and using linear regression analysis to generate an equation.
The extraction efficiency of terbufos from pellets was measured for five Ziegler® crayfish food pellets fortified with 10 μl of 32.5 mg/L terbufos in acetone and allowed to dry approximately 15 min. One drop of water (approximately 5 μl) was added to wet each pellet. Immediately after wetting, pellets were placed in 10 ml 25:75 acetone: hexane with 1 g sodium sulfate and allowed to shake on an orbital shaker for 1 h. Extracts were filtered through sodium sulfate, rinsed with hexane, and evaporated with nitrogen to a 2-ml final volume. A spike check was made and recoveries calculated as previously described. Samples were stored at 4°C pending analysis.
Terbufos desorption from pellets was measured to estimate terbufos concentrations in food and water. These values were used to estimate exposure during the 48-h dietary toxicity test. Ten Ziegler crayfish food pellets were spiked with 10 μl each of 203-mg/L terbufos in acetone and allowed to dry for approximately 15 min. Each pellet was placed into a 1 -L beaker filled with 250 ml of moderately hard reconstituted water. Five beakers, with 10 pellets each, were covered with aluminum foil and allowed to sit for 4 h. At 4 h, water was decanted from the pellets and extracted in four 50-ml increments and one 45-ml increment. The extract was brought to a 2-ml final volume with hexane. Pellets were extracted for terbufos separately and extracts stored at 4°C pending analysis. To verify the spiking concentration, a hexane spike check was made as previously described. Recoveries, corrected for extraction efficiencies, were calculated for water, pellets, and for total mass balance by dividing amount of terbufos in the sample by that in the spike check.
Crayfish culture
For the definitive aqueous and dietary toxicity tests, one female Procambarus clarkii, with young attached, was collected from a Clemson University crayfish culture pond. When the young crayfish left the adult, they were collected and kept in 1 -m diameter tanks in moderately hard reconstituted water, with constant aeration, at 26°C for approximately 4 weeks. Crayfish were fed Ziegler crayfish food pellets twice a week and tanks were cleaned every other day. Three days prior to testing, water temperature was decreased to the temperature of the testing room at 23°C. To prevent cannibalism, polyvinyl chloride (PVC) tubes were placed in aquaria to provide refuge.
Toxicity tests
For the 96-h aqueous toxicity test, crayfish were placed in 1-L beakers filled with 175 ml test solution. Beakers were covered with aluminum foil to prevent evaporation. Only one crayfish was used per beaker to avoid cannibalism. Test solutions were made by fortifying moderately hard reconstituted water with terbufos in hexane and mixing well. The toxicity test consisted of five treatments (0.73, 1.5, 3.2, 7.4, and 18 μg/L) and a control, with nine beakers per treatment. A solvent control was not used because crayfish were limited and no effects had been observed in any previous range-finding test solvent controls. Crayfish were observed at 3, 6, 9, 12, 24, 36, 48, 60, 72, 84, and 96 h for aberrant behavior and mortality. When dead, crayfish were collected and stored whole at −20°C for AChE analysis. At 96 h, remaining crayfish were sacrificed by placing into ice water and frozen at −20°C pending analysis. Dissolved oxygen (DO), pH, hardness, and alkalinity of test solutions were measured at test initiation, and DO and pH were measured in 20% of the test containers at test termination. All solution concentrations were verified by chemical analysis. Test concentrations were calculated based on the terbufos degradation curve. At the LT50 (22.4 h), approximately 75% of the terbufos remained in the water.
The 48-h dietary exposure toxicity test consisted of five treatments (0.43, 1.5, 1.7, 4.1, and 7.0 μg/g pellet, dry weight) and a control, with nine replicates per treatment. Because crayfish were limited in number and no aberrant effects were observed in previous solvent controls, a solvent control was not used. For the test, pellets were weighed for each treatment and solutions were based on average pellet weight. Pellets were fortified with 10 μl terbufos in acetone, allowed to dry 15 min, and placed into beakers containing 250 ml moderately hard water and one crayfish. Only one pellet was placed in each beaker, and beakers were covered with aluminum foil. All crayfish were starved at least 48 h prior to testing to encourage 100% ingestion of the pellets. To prevent fouling of the water by the pellet, crayfish were only exposed for 12 h, pellets were removed if not eaten, water was replaced with clean water, and crayfish were observed an additional 36 h. Observations for mortality and aberrant behavior were taken at 3, 6, 9, and 12 h and every 12 h thereafter. If dead or moribund, crayfish were collected and frozen at −20°C for AChE analysis. At 48 h, remaining crayfish were sacrificed by placing in ice water. Pellet fragments were collected and dried in a drying oven for 48 h, weighed, and average percent ingestion of pellets by crayfish was calculated. DO, pH, hardness, and alkalinity of test solutions were measured at test initiation, and DO and pH were measured in 20% of the test containers at test termination. All doses were verified by chemical analysis.
Aqueous toxicity test concentrations were calculated based on terbufos half-life in water. The 96-h median lethal concentration (LC50) was determined by using the terbufos concentration of each treatment solution at the time at which half of the crayfish mortalities occurred, median lethal time (LT50). The 96-h median lethal dose (LD50) was determined by dividing micrograms of terbufos in the water at the time of the LT50 by average crayfish wet weight. Dietary test concentrations were based upon terbufos mass balance estimates and average pellet ingestion by crayfish. The 48-h LC50 was determined by using average pellet ingestion for each treatment group. The LD50 was determined by multiplying the 48-h LC50 terbufos concentration by average pellet weight and dividing by average crayfish wet weight.
Biochemical methods
To measure AChE inhibition, neuroganglia were extracted from each crayfish, diluted in 0.1 M Tris buffer (pH 7.4, Sigma Chemical Company, St. Louis, MO, USA) and frozen at −20°C for AChE analysis. Total length (mm) and wet weight (g) were measured for each crayfish before dissection. Two lacerations were made ventrally on the outer edge of each side of the abdomen from the anus to the second periopod. A cut was made across the thorax to connect the two lacerations. This part was lifted gently from the body with forceps and the whole body discarded. The ganglia were separated from the abdomen by inserting a probe between the ganglia and the abdomen and pulling horizontally toward the anterior end. A cut was made between the first abdominal ganglion and the last thoracic ganglion to isolate the abdominal ganglia. Isolated ganglia were weighed wet, submersed in 0.1 M Tris buffer, and frozen at −20°C.
Ganglia were thawed and homogenized in a 1.5-ml microcentrifuge tube with a Konte's® pellet pestle by grinding and drawing the pestle up and down 15 times. The homogenate was centrifuged at 5,000 rpm for 10 min and the supernatant used for enzyme analysis.
AChE and butyrlcholinesterase (BChE) activities were determined using the methods of Ellman et al. [20] as modified by Hooper et al. [21]. The following components were added to each well of a 96-well plate to total 250 μl: acetylthiocholine iodide (AThCh) (final concentration of 2 × 10−3 M), 3.23 × 10−4 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), 0.05 M Tris buffer (pH 8.0), and sample enzyme dilution. Enzyme sample dilutions were measured spectrophotometrically in triplicate using a UVmax 96-well microplate reader (Molecular Devices Corporation, Palo Alto, CA, USA) in kinetic mode at a wavelength of 405 nm with a run time of 2 min and read at 8-s intervals.
BChE activity was assayed to determine presence or absence of the enzyme in crayfish and was measured by adding 30 μl of 1 × 10−3.5 M tetraisopropylpyrophosphoramide (iso-OMPA, Sigma Chemical Company, St. Louis, MO, USA) to wells in place of 30 μl of buffer to selectively inhibit BChE. AChE and BChE were considered inhibited when levels were depressed 20% or more compared to control activity.
Statistical analyses
Statistical estimates of toxicity, AChE inhibition, and time to death were obtained using Spearman-Karber, trimmed Spearman-Karber, or probit analyses (Toxstat© [22]), depending on normality of data. LC50; median effective concentration (EC50), the concentration at which 50% of the individuals exhibit abberant behavior; median lethal inhibition (150), the percentage of AChE inhibition at which 50% of individuals die; LD50; LT50; and median effective time (ET50), the time at which 50% of the individuals exhibit abberant behavior, were calculated for the definitive aqueous and dietary toxicity tests only.
RESULTS
Analytical studies
Terbufos recovery from water and pellets was measured to estimate extraction efficiencies. Average percent recovery of terbufos from water was 51.0 ± 3.5% (mean ± SE) (n = 3). Average percent recovery of terbufos from food pellets was 72.4 ± 3.8% (n = 5). All concentrations measured in the mass balance and desorption studies were corrected for these extraction efficiencies.
The mass balance of terbufos was measured to estimate the amount of terbufos available in water and in pellets for uptake by crayfish. Average percent recovery of terbufos from total water and food was 64.2 ± 5.2% (n = 4). Terbufos measured separately in food and water was 13.7 ± 2.9 μg (n = 4) and 2.9 ± 0.2 μg (n = 4), respectively. One food sample from replicate 1 was lost, and one water sample each from replicates 4 and 5 were lost. These were not used in the calculations. Based on both the mass balance and desorption studies, an average of 12.0 ± 0.7% (n = 14) of the total terbufos did not adsorb onto the pellets and was found in the water.
The half-life of terbufos in water was measured to estimate the amount of terbufos available for crayfish uptake over time. At 3, 6, 24, 48, 72, and 96 h, concentrations were 32.8 ± 0.8, 31.0 ± 0.6, 23.3 ± 0.5 (n = 3), 15.3 ± 0.6, 9.7 ± 0.3, and 6.6 ± 0.4 μg/L, respectively, yielding a half-life of 39.7 h. All means were taken from four samples, unless otherwise stated.
Water quality parameters
For all analytical studies and toxicity tests, water quality parameters were as follows unless stated otherwise (ranges given as ±): pH, 7.5 ± 0.4; DO, 6.5 ± 1 mg/L; temperature, 23 ± 2°C; hardness, 110.88 ± 10 mg/L CaCO3; and alkalinity, 48 ± 4 mg/L CaCO3. Parameters did not differ significantly between test initiation and test termination in any of the experiments.
Toxicity tests
In the aqueous toxicity test, mortality increased as concentration increased; most mortalities occurred before 24 h (Fig. 1). The 96-h LC50 was 5.9 μg/L (95% CI: 4.4, 8.1) and the LD50 was 4.0 mg/kg crayfish (95% CI: 3.0, 5.5).
Terbufos exposure induced aberrant behavior, such as loss of motor control activity (convulsions, inability to control appendages, and casting off of chelae) and loss of equilibrium. Casting off of chelae was generally only observed in crayfish that subsequently died. As with mortality, aberrant behavior increased as concentration increased but occurred at concentrations much lower than lethal concentrations (Fig. 2). The estimated 96-hEC50 of 2.6 μg/L (95% CI: 1.4, 4.8) was almost ⅓ that of the LC50. Aberrant behavior was also observed much sooner than mortality. The ET50 for the 18-μg/g treatment group was 4.2 h (95% CI: −9.9, 18), whereas the LT50 for the same dose was 22.4 h (95% CI: 10.8, 34.1).
In the dietary toxicity test, mortality increased as concentration increased, and all mortalities occurred before 24 h (Fig. 3). A 48-h LC50 of 4.4 μg/g (95% CI: 2.9, 6.7) was calculated based on an average 66.0 ± 2.6% (n = 46) ingestion of the pellets by the crayfish. The 48-h LD50 was 1.7 mg/kg crayfish (95% CI: 1.1, 2.6), based on average amount ingested. Based on the mass balance results, estimated average terbufos concentrations in the water were less than 0.6 μg/L, and aqueous exposure was determined as insignificant.
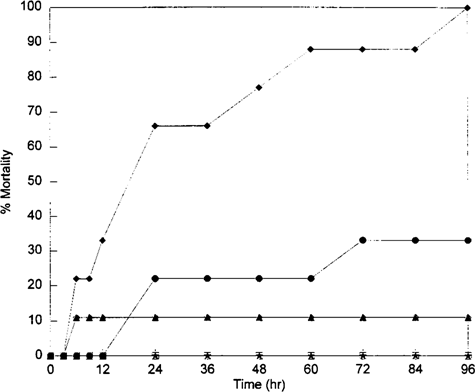
Cumulative mortality response of Procambarus clarkii to 96-h aqueous terbufos exposure. ⋄, 0 μg/L; *, 0.7 μg/L; ⊟, 1.5 μg/L; ▴, 3.2 μg/L; • 7.4 μg/L; ♦, 18 μg/L.
As in the aqueous exposure, aberrant behavior increased as concentration increased and occurred at concentrations that did not produce mortality (Fig. 4). The 48-h EC50 (3.1 μg/g; 95% CI: 1.8, 5.1) was 70% that of the LC50. Many crayfish lost control of chelae and periopods and, as a consequence, could not continue feeding. Most crayfish in the 7.0-μg/g treatment group were eliciting aberrant behavior at as little as 3 h, and most had died within 24 h. The LT50 and ET50 for 7.0 μg/g were 27.5 h (95% CI: 4.1, 51) and 3.4 h (95% CI: 1.5, 5.3), respectively.
Biochemical studies
Neuroganglia AChE activity showed a dose-dependent decrease in response to aqueous and dietary terbufos exposures in survivors and in nonsurvivors. As concentration increased, AChE activities decreased. After 96 h exposure, mean AChE activities in crayfish from the aqueous toxicity test ranged from 98% control activity at 0.73 μg/L to 2.1% control activity at 18 μg/L. Mean AChE activities in nonsurvivors were less than mean AChE activities in survivors (Fig. 5). Nonsurvivor and survivor AChE activities in the 7.4-μg/L treatment group were 2.2 and 43% that of control activity, respectively. However, mean AChE activities were not significantly different in nonsurvivors and survivors of the dietary exposure (Fig. 6). Non-survivor and survivor AChE activities at 4.1 μg/g were 16.6 and 26.5% of control activity, respectively, and at 7.0 μg/g were 6.3 and 18% control activity, respectively.
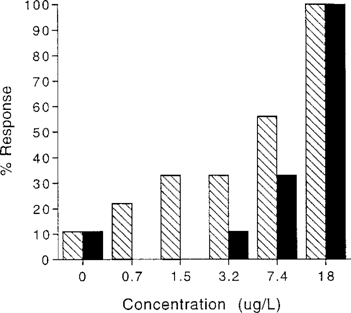
Mortality and aberrant behavior responses of Procambarus clarkii to 96-h aqueous terbufos exposure,  , Aberrant behavior; ▪, mortality.
, Aberrant behavior; ▪, mortality.
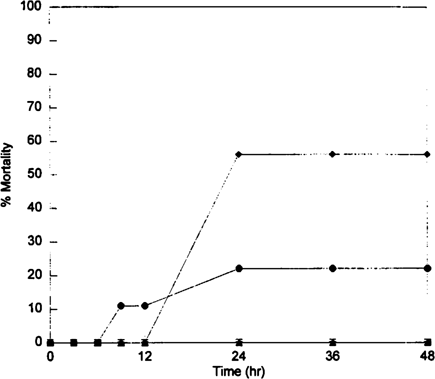
Cumulative morality response of Procambarus clarkii to 12-h dietary terbufos exposure. ⋄, 0 μg/L; *, 0.4 μg/L; ⊟, 1.5 μg/L; ▴1.7 μg/L; •, 4.1 μg/L; ♦, 7.0 μg/L.
For both aqueous and dietary exposures, mortality increased as concentration increased and AChE activities decreased. In crayfish from the aqueous exposure, 100% mortality at 18 μg/L corresponded to 98% inhibition, 33% mortality at 7.4 μg/L corresponded to 97% inhibition, and 11% mortality at 3.2 μg/L corresponded to 58% inhibition. The 150 was 76.5% (95% CI: 67.4, 85.6).
Mortality response and inhibition were similar in crayfish from the dietary exposure: 22% mortality at 4.1 μg/g corresponded to 73% inhibition and 56% mortality at 7.0 μg/g corresponded to 88% inhibition. The 150 was 86.1% (95% CI: 78.1, 94.1). The standard errors from each exposure 150 overlap, and therefore I50s are not significantly different.
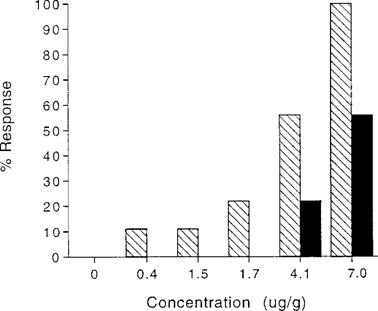
Mortality and aberrant behavior responsed of Procambarus clarkii to 96-h aqueous terbufos exposure, ♦, Aberrant behavior; ▪. mortality.
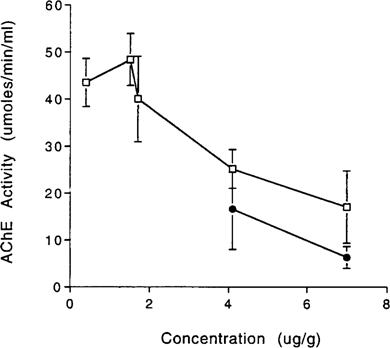
AChE activities of survivors and nonsurvivors for the 96-h aqueous terbufos exposure.□, Survivors; • nonsurvivors.
DISCUSSION
Exposure models
Accurate assessment and prediction of chemical hazard to organisms requires knowledge of chemical fate and bioavailability in the environment, behavior and physiology of the organism, and route, duration, and frequency of exposure. Exposure assessment provides critical information for establishing dose-effect relationships that determine chemical toxicity to an organism [23]. The purpose of this research was to determine the bioavailability of terbufos in water and in food and to assess toxicity to crayfish. Two different routes of exposure were examined, and the effects of exposure measured for mortality, behavior, and AChE inhibition.
Determination of aqueous exposure was based on the paradigm commonly used in aquatic toxicology: aqueous concentration is proportional to the dose in the organism, which is proportional to the dose at the receptor. Therefore, the effect caused by the dose at the receptor is proportional to the concentration in the water [24]. Although aqueous exposure is relatively simple to model, the following assumptions were required for this study: (1) crayfish took up 100% of the terbufos from the water; (2) terbufos remained in solution, even though volatilization from the water and adsorption onto glass may have reduced the amount of terbufos available for uptake; and (3) exposure decreased with time, as terbufos half-life in moderately hard water is relatively short.
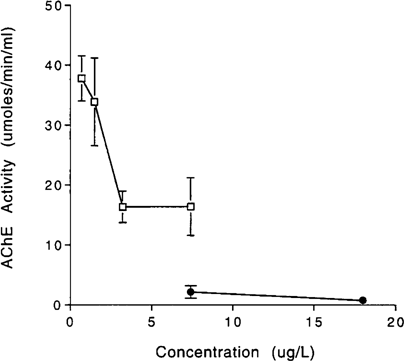
AChE activities of survivors and nonsurvivors of the 12-h dietary terbufos exposure. □, Survivors; • nonsurvivors.
Determination of dietary exposure was more difficult because of multiple exposure routes, physiochemical interactions of terbufos with the pellets, and crayfish feeding behavior. Approximately 12% terbufos desorbed from the pellets when placed into water, producing terbufos water concentrations ranging from 0.03 to 0.56 μg/L. Crayfish ingestion varied up to 60% within and among treatment groups, but exposures were based on an average of 66% ingestion. From this information, crayfish were exposed to approximately 58% of the terbufos applied to the pellets. Hence, because water concentrations were too low to produce aberrant behavior or mortality alone, ingestion was defined as the primary route of exposure and the primary cause of toxicity.
Sensitivity to modes of exposure
Both aqueous and dietary exposures were extremely toxic to juvenile crayfish, providing evidence that terbufos is available for uptake when associated with food. When normalized for crayfish weight, LD50s were 4.0 and 1.7 mg/kg for the aqueous and dietary exposures, respectively, given the assumptions stated above. Other responses observed in both exposures, such as AChE inhibition, behavior, and time to death, also indicate uptake of terbufos from food.
Inhibition-mortality response curves and 150 estimates were similar for both exposures, even though exposure regimes differed. LT50s were also similar for both exposures. These findings suggest that, regardless of exposure route, the rate of inhibition and the dose at the receptor that caused mortality were similar, assuming that death was a function of AChE inhibition. Research strongly supports this mode of action of OP poisoning [8-10], but other modes of action such as inhibition of other enzymes and stimulation of oxygen consumption, may also contribute to the responses [9].
Bioavailability and distribution within the body differ according to route of exposure [25]. Nonsurviving and surviving crayfish AChE activities were similar in the dietary exposure, but were significantly different in the aqueous exposure (Figs. Fig. 4., Fig. 5.). Because terbufos hydrolyzes rapidly in water, terbufos concentration and exposure decrease with time. This may have allowed crayfish from the aqueous exposure to recover somewhat from AChE inhibition as time increased.
Crayfish may not have been able to recover from dietary exposure. When terbufos is ingested, exposure may be constant while food is digested, and terbufos and its metabolites are distributed slowly throughout the body over time. The crayfish alimentary canal is comprised of four components: an ectodermal foregut, an endodermal foregut, a hepatopancreas, and an ectodermal hindgut. The heptaopancreas produces and secretes all digestive enzymes. It is also involved in carbohydrate metabolism, production of emulsifiers, excretion, and calcium, and heavy metal storage [26]. Studies have reported the presence of an active mixed function oxidase (MFO) system in crayfish hepatopancreas [27, 28]. This system is responsible for the first phase in detoxication and elimination of xeno-biotics by transforming nonpolar compounds into more polar, water-soluble compounds. However, the MFO system biotransforms some xenobiotics such as OPs to more toxic metabolites [29, 30]. When terbufos is ingested, terbufos sulfoxide (TSO) and terbufos sulfone (TSO2) may be produced as a result of metabolism by the MFO system. Close contact with hemolymph facilitates absorption and transport of nutrients and other digested materials. Because the metabolites are relatively water soluble, partitioning of the metabolites into the hemolymph and distribution to the neuroganglia is likely.
Aberrant behavior was also observed in aqueous and dietary exposures and provided additional data supporting bioavailability of terbufos in the pellets. The estimated EC50 for aqueous exposure was almost 50% that of the LC50, while the dietary EC50 was approximately 80% that of the LC50. Time to effect is also less than time to death. These findings suggest that aberrant behavior occurs much faster than mortality at concentrations considerably less than lethal concentrations. Loss of equilibrium, rapid and erratic swimming, and loss of motor control activity may cause crayfish to be more susceptible to predation by losing the ability to fight, escape, or hide from predators. This behavior may also increase predator awareness, possibly increasing predation and mortality. Crayfish may also lose the ability to feed when loss of motor activity control decreases the ability to hunt, grasp food, or to defend territory from other crayfish. As a result of starvation and subsequent decreased size, fecundity may also decrease, as fecundity is directly correlated to size [31].
Autotomy (casting off an appendage) was generally observed in crayfish that had died and has also been observed in other crayfish in the presence of strong stimuli [16]. In one instance in the dietary exposure, a crayfish experienced autotomy but survived the exposure. Loss of the major chelae in natural populations may decrease defense ability or successful foraging and feeding. Males may lose the ability to grasp females when mating and females may lose the ability to burrow and care for their young.
Environmental impact
Without implementation of soil tillage conservation practices, terbufos concentrations in runoff may reach up to 1.2 mg/L [1]. This study indicates that AChE inhibition, aberrant behavior, and mortality occurred from both aqueous and dietary exposure to terbufos at concentrations almost 1,000 times less than expected theoretical runoff concentrations. Sediment normally contains high amounts of organic matter, and terbufos and its metabolites may partition out of the water and bind to sediment when it enters the water as runoff. As crayfish rework the organic matrix, they may ingest terbufos. This study confirmed that terbufos can be available for uptake by crayfish when adsorbed onto food.
Aberrant behavior was observed in crayfish at concentrations considerably lower than corresponding LC50 values. This may indirectly lead to mortality or decreased fitness and survivability of a population if such exposure occurs in the field. Other sublethal responses have been observed in invertebrates exposed to OPs, causing reduced population fitness and survivability. For instance, a 90% reduction of midges, mayflies, and caddisflies was observed when chlorpyrifos was applied to a pond [32]. Further, similar effects were also found with copepods, cladocerans, tadpole shrimp, and predaceous insects after chlorpyrifos exposure [33]. Elevated stream drift of invertebrates and reduction in emergence has been observed with pyrethroid and OP insecticide exposure [34].
Removal of a species from an ecosystem may be ecologically significant [35]. Crayfish are an aquatic keystone species, uniquely capable of converting unavailable nutrients into energy for other aquatic organisms, controlling macrophyte and invertebrate populations, and providing food for higher trophic level organisms. Decreases in crayfish populations may cause changes in energetics, nutrient cycling, and community structure and function [36]. Careful OP application, soil tillage conservation practices, and the use of buffer strips will help lesson the potential for damage to these populations.
Acknowledgements
We thank Arnold Eversole, Rami Naddy, Kevin Johnson, Katherine Hunt, Fran Harper, Gensie Waldrop, Chris Kempton, Angi Paul, Tim Bargar, Mike Hooper, and Stacey Fornstrom for their technical assistance throughout the study and Robin Berg for supplying crayfish. This research was conducted and funded through the Department of Environmental Toxicology and The Institute of Wildlife and Environmental Toxicology, Clemson University.




