A screening method for ranking and scoring chemicals by potential human health and environmental impacts
Abstract
Potential impacts of chemical releases are often evaluated by regulators, industry, and others to set regulatory action priorities, to make business decisions, and to target pollution prevention efforts. A chemical ranking and scoring method entitled “Chemical Hazard Evaluation for Management Strategies” (CHEMS-1) has been developed as a screening tool to provide a relative assessment of chemical hazards to human health and the environment. The purpose of this method is to place chemical release data into perspective by evaluating both the toxic effects of chemicals and the potential exposure to those chemicals. This is done by combining measures of chemical toxicity pertaining to both human health and the environment with chemical release amounts and information on environmental persistence and bioaccumulation. The CHEMS-1 was initially developed to select priority chemicals for assessing safer substitutes for major product and process uses, where chemicals were selected from Toxics Release Inventory (TRI) data and annual pesticide usage data. A two-tiered approach was adopted with CHEMS-1 presented here representing the first, or screening-level, tier.
INTRODUCTION
Approximately 70,000 chemicals have been produced and used in U.S. commerce since 1976, with some 15,000 produced in significant amounts [1]. Potential impacts of chemical releases are often evaluated by regulators, industry, and others to set regulatory action priorities, to make business decisions, and to target pollution prevention efforts. During the last decade there have been vast improvements in the methods used to assess chemical toxicity and environmental fate and to interpret these data within a risk assessment framework. There is still a need, however, for generally accepted and widely used tools for setting priorities and providing consistency across environmental programs. To date, a multitude of approaches have been used, some lacking any scientific basis. Chemicals have been selected for some regulatory programs, for example, with little systematic evaluation.
A chemical ranking and scoring method entitled “Chemical Hazard Evaluation for Management Strategies” (CHEMS-1) has been developed as a screening tool to provide a relative assessment of chemical hazards to human health and the environment. The purpose of this method is to place chemical release data into perspective by evaluating both the toxic effects of chemicals and the potential exposure to those chemicals. This is done by combining measures of chemical toxicity pertaining to both human health and the environment with chemical release amounts and information on environmental persistence and bioaccumulation. The CHEMS-1 was initially developed to select priority chemicals for assessing safer substitutes for major product and process uses [2, 3], where chemicals were selected from Toxics Release Inventory (TRI) data and annual pesticide usage data.
Major research tasks
The development of the chemical ranking method involved three major research tasks: (1) compiling available experimental data and selecting estimation methods when experimental data were absent; (2) formulating criteria, which, individually or in combination, could be used to assign scores to chemical toxicity and exposure potential; and (3) developing an algorithm to combine and weight the scores into a numerical rank for each chemical.
The method was demonstrated using the chemicals for which toxic chemical release reporting is made in the TRI as required under Section 313 of Title III of the Superfund Amendments and Reauthorization Act (SARA) of 1986. Selected high-volume pesticides were also included, determined by annual pesticide usage data.
Tiered approach
The quantity of information required to assess each chemical, as well as the time and resources needed to obtain and process this information, can be prohibitive. Thus, a two-tiered approach was adopted with the method presented here being the first, or screening-level, tier. The second, or confirmation tier, is yet to be developed. The advantage of the tiered approach is that it reduces the number of chemicals being evaluated as the depth, breadth, and quality of the required information increase.
The screening tier was designed to rely on more readily available and/or easily estimated information, and to include rather than eliminate chemicals of possible concern (avoid false negatives). Specific suggestions for the confirmation tier are made in the Results and Discussion section.
| Type of effect/criteria | Toxicological endpoint | Definition, test methods |
|---|---|---|
| Human health effects | ||
| Acute | Rodent oral LD50 | The concentration of a substance, expressed in mass of the substance per mass of the animal, that will kill half of a group of rodents within 14 days when administered orally as a single dose. |
| Acute | Rodent inhalation LC50 | The concentration of a substance in air (gas or dust) that will kill half of a group of rodents when inhaled continuously for 8 h or less, scaled to 4 h by: LC50 @4 h = (LC50 @t h) × t/4. |
| Chronic | Evidence of carcinogenicity | Based on EPA and International Agency for Research on Cancer (IARC) classifications. |
| Chronic | Other specific effects | Includes positive evidence of mutagenicity, developmental effects, reproductive effects, other chronic effects, and neurotoxicity. |
| Environmental effects | ||
| Terrestrial, acute | Rodent oral LD50 | The concentration of a substance, expressed in mass of the substance per mass of the animal, that will kill half of a group of rodents within 14 days when administered orally as a single dose. |
| Aquatic, acute | Fish LC50 | The concentration of a chemical in water that causes death in 50% of the fish tested in a 96-h test. |
| Aquatic, chronic | Fish NOEL | The highest dosage administered that does not produce observable toxic effects, estimated from LC50 data. |
| Exposure potential | ||
| Persistence | Biological oxygen demand (BOD) half-life | The time required to biodegrade a chemical such that its BOD in water is reduced by half. |
| Persistence | Hydrolysis half-life | The time required for the amount of a chemical to be reduced by half through hydrolysis reaction in water, at pH 7. |
| Bioaccumulation | Aquatic bioconcentration factor (BCF) | The ratio of the concentration of a chemical in an aquatic organism to that in water at steady-state. |
| Amount released | Release weighting factor (RWF) | A factor used to weight chemical toxicity hazard values determined by the amount of annual releases or transfers. |
SOURCES AND TREATMENT OF DATA
Toxicity data
Table 1 presents the toxicological endpoints included in CHEMS-1 to represent human health and environmental effects. While four human health and three environmental effects terms are included, the method was designed so weighting of these terms can be adjusted independently to suit various applications, for instance, if effects to the aquatic environment were to be emphasized. Although some data are available in the open literature for many chemicals listed in the TRI, complete quantification of even a few toxicological endpoints is rare. Missing data present a main obstacle to the development and use of any chemical ranking or scoring system. The types of scoring criteria used, and the design of the algorithm for combining the criteria, depended on whether experimental data are available or can be estimated with an acceptable degree of accuracy.
The CHEMS-1 relies on peer-reviewed experimental data from sources such as the Hazardous Substances Data Bank (HSDB) [4] whenever possible. Structure-activity relationships (SARs) or quantitative structure-activity relationships (QSARs) were used to estimate missing data. This reduces the possibility of highly toxic chemicals receiving a low ranking simply because they have not been tested. Of course, this depends on the availability of reliable SARs or QSARs. If an SAR or QSAR was not available, the missing data were flagged in the database and the hazard value for the missing endpoint set to zero. The validity of this approach is discussed in Sensitivity analysis, below.
An alternative data selection approach is to choose the most sensitive endpoint from a pool of possible endpoints for each criteria. However, selecting the most sensitive endpoint presents obvious problems when little or no experimental data exist on a chemical. It also discourages further chemical testing because it is unlikely that a score for a chemical can be lowered by filling data gaps.
Inorganic chemicals
The algorithm is intended to be suitable for use with a variety of chemicals, including inorganic chemicals. Inorganic chemicals present unique problems, both from the method used to report inorganic chemicals in the TRI and from the limitations of methods available for estimating toxicity or exposure values.
First, several categories of inorganic chemicals are reported in the TRI as “compounds” for which the specific chemicals released were not reported. The CHEMS-1 depends, however, on specific toxicological information for specific chemical compounds. Therefore, surrogate compounds were chosen to represent the most widely used forms of the inorganic chemical categories for evaluation (Appendix). If most of the TRI releases were from a specific industry or application, surrogate compounds that are the major production form of the chemical used in that industry (e.g., arsenic pentoxide for the wood-preserving industry) were selected. For cadmium, chromium, nickel, and lead, however, no single surrogate was obvious and expert judgment was used to select the inorganic salts produced in the greatest quantity.
Toxicity and exposure data for the inorganic chemicals were not estimated using QSARs because of a lack of reliable methods. Because many of the ions have specific toxic properties, they had to be individually evaluated. Thus, a more extensive literature review was done to find published experimental data for the inorganic chemicals. If data were still unavailable, they were estimated using an SAR.
Acute human health effects. Experimental data were preferred for both oral and inhalation data. The hierarchy for experimental data sources was (1) HSDB [4], and (2) Registry of Toxic Effects of Chemical Substances (RTECS) [5], both on-line data sources. Numerous additional data sources were used for the inorganic chemicals and pesticides [6-8, for example].
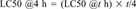
If experimental data were unavailable, SARs were used to estimate LD50 and LC50 values. Lacking an appropriate SAR, the data point was flagged as missing and the hazard value set to zero.
Carcinogenicity. Scores for carcinogenicity were based on International Agency for Research on Cancer (IARC) and Environmental Protection Agency (EPA) classifications. The IARC publishes a series of monographs evaluating the strength of evidence supporting a potential human carcinogenicity judgment based on human data, animal data, and other supporting data [see 10, for example]. A summary of the IARC carcinogenicity classification system includes: Group 1, carcinogenic to humans; Group 2A, probably carcinogenic to humans; Group 2B, possibly carcinogenic to humans; Group 3, not classifiable as to human carcinogenicity; and Group 4, probably not carcinogenic to humans.
In assessing the carcinogenic potential of a chemical, EPA classifies the chemical into one of the following groups, according to the weight of evidence (WOE) from epidemiologic and animal studies: Group A, human carcinogen (sufficient evidence of carcinogenicity in humans); Group B, probable human carcinogen (B1, limited evidence of carcinogenicity in humans; B2, sufficient evidence of carcinogenicity in animals with inadequate or lack of evidence in humans); Group C, possible human carcinogen (limited evidence of carcinogenicity in animals and inadequate or lack of human data); Group D, not classifiable as to human carcinogenicity (inadequate or no evidence); and Group E, evidence of noncarcinogenicity for humans (no evidence of carcinogenicity in adequate studies) [11]. (The EPA currently proposes the use of WOE descriptors, such as “Likely” or “Known,” “Cannot be determined,” and “Not likely,” in combination with a hazard narrative, to characterize a chemical's human carcinogenic potential—rather than the classification system described above [12].)
If neither IARC nor EPA has classified the chemical as to its carcinogenicity, the chemical was evaluated using computer-assisted SAR [13]. The SAR assigns a positive carcinogenicity rating to a chemical if it contains one or more molecular substructures that have been related to carcinogenicity, such as a polyaromatic hydrocarbon.
Other specific effects. Data for the “other specific effects” endpoints were obtained from Roadmaps, EPA's database of information sources for the SARA 313 chemicals [14]. The Roadmaps database summarizes sources of publicly available toxicity information from several databases and indicates if there was sufficient evidence that exposure to a chemical resulted in a specific health effect. Test results indicating mutagenicity, developmental toxicity (including embryotoxicity, fetotoxicity, or teratogenicity), reproductive toxicity, neuro-toxicity, or other chronic toxicity are summarized by citing the source (i.e., database) in which those results can be found. Roadmaps indicates only that data are available on the effect; it does not include numerical test results or indicate the severity of the effect or validity of the experimental data [14].
Acute aquatic effects. Fish LC50 data were used to score acute aquatic effects. Acute fish mortality data (i.e., LC50) are readily available and can be estimated accurately by QSARs. The universality of this endpoint makes it important in the screening phase of an evaluation. Experimental data were preferred for the acute aquatic toxicity data [4,15-19] with numerous additional data sources consulted for inorganic chemicals and pesticides [6-8, for example]. The LC50 data were selected (in order of preference) for the following: (1) Pimephales promelas (fathead minnow) in a 96-h flow-through test; (2) from a 96-h flow-through LC50 data for another freshwater fish (excluding trout); (3) a static 96-h fathead minnow test; or (4) a static 96-h test for another freshwater fish (excluding trout). In two cases, 48-h test data (unadjusted) were used when 96-h data were unavailable.
For organic chemicals, if an experimental value was unavailable, the LC50 value was estimated by QSAR as a function of the octanol-water partitioning coefficient (Kow). For inorganic chemicals this was flagged as a data gap. Relationships were based on defined mechanism of action [20, 21] including the baseline mechanism, nonpolar narcosis, as well as polar narcosis, and weak acid respiratory uncoupling. In addition, acute fish mortality values for selected chemicals (including acrylates, aldehydes, anilines, and esters) were estimated by class-based models using ECOSAR [22]. Certain functional groups, (e.g., alpha-beta unsaturated carbonyl moiety) are known to be bioreactive and participate in a number of competing electro(nucleo)philic, redox, and/or free radical processes [23]. Such chemicals currently defy predictive modeling. The LC50 values of chemicals considered bioreactive were estimated by increasing 10-fold the value predicted by the nonpolar narcosis QSAR. In cases where the molecular structure did not suggest a specific mechanism of action, chemical class, or functional group, the nonpolar narcosis QSAR was used to predict a default value.
Some functional groups, such as acid chlorides, isocyanates, and epoxides, react with water in less than 1 d. For such compounds, the effects were assumed to be those of the hydrolysis products; experimental data or QSARs used to estimate the fish LC50 values were therefore based on the LC50 value of the expected hydrolysis byproducts.
Fish chronic toxicity. The “no-observable-effect level” (NOEL) was used to score chronic sublethal effects on fish. Experimental data were generally lacking for the fish NOEL endpoint and were not used in the screening tier. Instead, the chronic NOEL value for organic chemicals was estimated from the acute toxicity data (i.e., the LC50 data) and the Kow of the chemical.
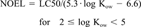


Persistence and bioaccumulation data
Table 1 lists the three exposure criteria used in CHEMS-1. Again, the weighting of these terms can be adjusted to suit various applications. Biological oxygen demand (BOD) half-life and hydrolysis half-life were used to measure persistence. Both endpoints were estimated using QSARs due to the wide variability in experimental data. The BOD half-life of each organic chemical was estimated with the computer-assisted version of the structural feature approach developed by Neimi et al. [13, 27].
Hydrolysis half-life data for organic compounds, ammonia, chlorine dioxide, and hydrochloric acid were estimated with the computer-assisted version of the Hammett and Taft substituent constant methods described by Harris [13, 28].
Metal compounds and certain other inorganic chemicals in highly oxidized states (e.g., molybdenum trioxide, thorium dioxide, sulfuric acid, nitric acid, and ammonium salts) were assumed to have infinite BOD and hydrolysis half-lives. Zinc and aluminum dusts were assumed to have half-lives of 500 d based on the judgement that they would degrade (oxidize) eventually, although slowly. Other processes that may make metals unavailable, such as sediment adsorption, were not considered in this screening level tier.
Photolysis half-life is another important measure of persistence but was not included due to a lack of data and of a reliable QSAR to estimate missing data.
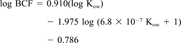
Physicochemical properties
Kow is a key input variable to the QSARs used in this method to predict aquatic acute and chronic toxicity, BOD half-life, and BCF. Experimental, rather than predicted, Kow values were preferred. If experimental values were not available, Kow was estimated using the method of Ghose and Crippen [31].
Release data
Data for the releases and transfers for the industrial chemicals were obtained from the 1989 TRI [32]. Additional release data for pesticides were obtained from annual usage information for 1987, 1990, and 1991 [33, 34].
METHODS
Chemicals selected for evaluation
When CHEMS-1 was developed, 158 chemicals were selected for evaluation, 140 from the 1989 TRI and 21 high-volume pesticides (including 3 already selected from the TRI). Chemicals were selected, from among more than 270 chemicals in the 1989 TRI, based on the quantities released. The 21 pesticides were selected from annual usage estimates of high-volume conventional pesticides in the United States [33, 34]. The TRI chemicals and high-volume pesticides selected for this evaluation are listed in Davis et al. [2].
The 1989 TRI gives chemical release and transfer quantities in seven categories: fugitive, or non-point, air emissions; stack, or point, air emissions; water discharges; land releases; underground injection releases; transfers to publicly owned treatment works (POTW); and transfers to other off-site locations. The pounds released or transferred were summed within each of these categories and the chemicals were selected to account for at least 99% of the total. This procedure was done for all seven categories and for total releases and transfers. Any chemical that was part of the 99% of releases or off-site transfers in any category was selected. Some chemicals obviously qualified in several categories, but such multiple selection was not used to bias the further evaluation of any compound.
Development of scoring criteria
All of the toxicity terms (i.e., oral LD50, inhalation LC50, carcinogenicity, “other specific effects,” fish LC50 and fish NOEL) were given equal weighting by assigning a hazard value to each that could range on a scale from zero to five. Cutoff values were chosen for the terms so that the hazard value for very high or very low toxicities would not exceed five or be less than zero, respectively.
| IARC classification | Hazard value | EPA classification | Hazard value |
|---|---|---|---|
| Group 4 | 0 | Group E | 0 |
| Group 3 | 0a | Group D | 0 |
| NAb | NA | Group C | 1.5 |
| Group 2B | 3.5 | Group B2 | 3.5 |
| Group 2A | 4.0 | Group B1 | 4.0 |
| Group 1 | 5.0 | Group A | 5.0 |
- a The EPA classification, if available, was used in this case.
- b NA: not applicable.
Human health effects. The human health effects data included quantitative assessment of acute oral and inhalation toxicity, semiquantitative assessment of carcinogenicity, and qualitative assessment of “other specific effects” (i.e., mutagenic effects, developmental effects, reproductive effects, neurotoxic effects, and other chronic effects).
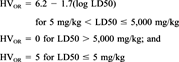

Chronic health effects in humans include cancer, mutagenic effects, developmental effects, reproductive effects, neurotoxic effects, and other target organ effects. The WOE classification assigned by EPA and/or IARC was used to score chemical carcinogenicity. Table 2 presents the hazard values assigned to IARC and EPA carcinogenicity ratings. When both IARC and EPA classifications were available for a chemical, the average was taken for the hazard value. If the IARC classification was a 3 (not classifiable as to its carcinogenicity to humans), only the EPA classification was used to determine the hazard value. Similarly, the IARC rating alone would have been used for EPA Group D chemicals, although this did not apply to any of the chemicals evaluated in this demonstration. Chemicals assumed to be carcinogens based on SARs were assigned a hazard value of 3.0 or 1.0, depending on the molecular substructures.
Other specific human health effects included were mutagenic effects, developmental effects, reproductive effects, neurotoxicity, and other chronic effects [14]. Each of these end-points was evaluated qualitatively and combined into one hazard value. A value of one was assigned for each flagged end-point. If the endpoint was not flagged, a value of zero was assigned. The values for the five endpoints were summed to determine the hazard value for each chemical.
Environmental effects. Environmental effects included a quantitative assessment of mammal and fish mortality (fauna representing terrestrial and aquatic environments, respectively), and the NOEL in fish.
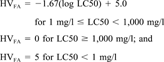
It was assumed that chemicals with a log Kow greater than six would not be acutely toxic, due to molecular size, and were assigned a hazard value of zero.
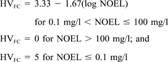
Exposure factors. The CHEMS-1 exposure assessment data include quantification of persistence (i.e., biotic and abiotic degradation) and bioaccumulation (i.e., aquatic BCF). These exposure criteria were used in the algorithm with the quantity of releases reported in the TRI as an overall measure of potential exposure.
One purpose of CHEMS-1 is to address overall environmental releases and subsequent exposures, thus the emphasis on persistence and bioaccumulation. If the method were applied to a workplace setting, these factors would be less important to the potential for occupational exposure.
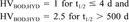
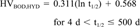
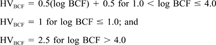
| Toluene | Trifluralin | Hexachloro-1,3-butadiene | Cadmium compounds | |
|---|---|---|---|---|
| Oral LD50 (mg/kg)a | 5,050 | 500 | 102 | 88 |
| HVOR | 0 | 1.7 | 2.8 | 2.9 |
| Inhalation LC50 (ppm)a | 6,675 | 47 | 35 | 306 |
| HVINH | 0.4 | 4.7 | 4.9 | 3 |
| EPA/IARC cancer class.a | None | None | C/3 | B1/2A |
| HVCAR | 0 | 0 | 1.5 | 4 |
| Other specific effectsa,b | D, R | D, R, C | D, R, C | D, R, C |
| HVNC | 2 | 3 | 3 | 3 |
| Human health effects | 2.4 | 9.33 | 12.2 | 12.9 |
| Oral LD50 (mg/kg)a | 5,550 | 500 | 102 | 88 |
| HVMAM | 0 | 1.7 | 2.8 | 2.9 |
| Fish LC50 (mg/L)a | 34 | 0.11 | 0.09 | 0.1 |
| HVFA | 2.4 | 5 | 5 | 5 |
| Fish no-effect level (mg/L)a | 4 | 0.01 | 0 | 0 |
| HVFC | 2.3 | 5 | 5 | 5 |
| Environmental effects | 4.7 | 11.7 | 12.8 | 12.9 |
| BOD t1/2 (days)a | 10 | 503 | 503 | 9,999 |
| HVBOD | 1.27 | 2.5 | 2.5 | 2.5 |
| Hydrolysis t1/2 (days)a | 1,000 | 30 | 2 | 9,999 |
| HVHYD | 2.5 | 1.63 | 1 | 2.5 |
| Log BCF (unitless)a | 1.7 | 2 | 3.6 | 3.5 |
| HVBCF | 1.33 | 1.5 | 2.31 | 2.25 |
| Exposure factor | 5.1 | 5.62 | 5.81 | 7.25 |
| Total hazard valuec | 36 | 118 | 146 | 188 |
- a Sources of these data are discussed in Sources and Treatment of Data.
- b D = developmental effects, R = reproductive effects, C = other chronic effects, as listed in the Roadmaps database [14].
- c Not weighted by release amounts.
The algorithm
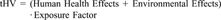

To illustrate use of the scoring criteria and algorithm, Table 3 presents example data and scores for four selected chemicals.
Several a priori conditions were incorporated into the development of the CHEMS-1 algorithm. First, whatever the framework of the chemical ranking method, the final tool was to be sufficiently flexible so it could be modified as experience was gained and the validation process progressed. Second, the method was never to become so mechanical as to be isolated from expert judgment. Third, it was felt that separating human health and environmental effects according to different endpoints would make the algorithm more transparent; these endpoints were categorized on the front end and aggregated later in the processing.
Although the terms were weighted equally, two aspects created an implicit weighting of the endpoints. First, several different kinds of data were used as input to the algorithm: quantitative toxicity levels (i.e., LC50 and LD50); semiquantitative levels (i.e., carcinogenicity WOE), and qualitative assignments (i.e., yes/no information on other types of chronic effects). Therefore, assigning hazard values to the group of qualitative chronic effects (e.g., neurotoxicity, mutagenicity) on the same zero-to-five scale as quantitative toxicity levels for a specific endpoint (e.g., acute inhalation toxicity) gives greater weighting to the one quantitative endpoint than to the group of qualitative effects. Second, the choice of cutoff levels for assigning maximum or minimum hazard values adds a weighted judgement to the algorithm.
Human health effects have the potential of being rated from 0 to 20 (e.g., 10 points for acute effects and 10 points for chronic effects). Environmental effects have the potential of being rated from 0 to 15. Exposure factors can be rated from 1.0 to 7.5 (e.g., up to 2.5 for BOD half-life, hydrolysis half-life, and log BCF). Persistence and bioaccumulation are considered pivotal to the potential for exposure and are included as multiplicative factors rather than additive effects in the algorithm. The theoretical maximum total hazard value is 262.5 (i.e., [20 + 15].75). Total hazard values were normalized to a scale from zero to 100.
The method has been designed so that the user can change the weighting of each term and determine the effect such weighting has on the chemical ranking. In this manner, the algorithm may be used to obtain a chemical ranking for different purposes. The sensitivity of the algorithm to changes in the endpoint weighting was examined and is discussed below.
Release-weighted hazard values
It was initially planned to use the chemical release and transfer data reported in the TRI with chemical production and usage data to calculate release-weighted hazard values. The TRI data are readily available and, although limited to data for certain manufacturing sectors, were the best resource for assessing the environmental releases of the chemicals listed. Production and usage data should also provide some measure of the potential releases of a chemical to the environment. Unfortunately, accurate and reliable chemical production and usage data were not available for many chemicals listed in the TRI. Thus, surrogates for the total environmental release of a chemical were limited to TRI data, except pesticides. Pesticide usage data were added to any TRI release data for manufacturing of pesticides to estimate total environmental releases of these chemicals.
A method of scaling hazard values and release amounts was needed to ensure that neither dominates the algorithm. For example, total hazard values can theoretically range from 0 to 262.5 before they are normalized 0 to 100. The TRI releases, on the other hand, ranged up to 546 million pounds for ammonium sulfate solution in 1989. Simply multiplying the total hazard value by the total releases in pounds would result in a release-weighted total hazard value reflecting only the release amount and not the toxicity or persistence of the chemical.
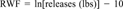
The natural log, rather than the base-10 log, was used to attain a range of about 10 integers over the range of release amounts. Taking the natural log of the releases provides release-weighted hazard values that are not dominated by the weight of releases, yet does not understate the importance of the release amount. The total releases for the chemicals scored ranged from 860 lbs to 545,989,541 lbs. The equation for calculating the RWF assigns a multiplier of approximately 10 for the highest release and 1.0 for anything that is 59,874 lbs or less. By subtracting 10 from the natural log of the releases, a cutoff of 60,000 lbs was set, below which the RWF was always equal to one.
The TRI and pesticide releases were assigned to either air or water categories. It was assumed that stack and fugitive releases went to air; land, injection, water, and POTW releases went to water; annual pesticide usage amounts were assigned to the water release category; off-site transfers to an incineration facility were assumed to be destroyed and transfers to a recycling facility were assumed to be reused and therefore not released to the environment; and all other off-site transfers were assumed released to water. Incineration and recycling amounts were subtracted from total off-site transfers to determine the remainder of off-site transfers released to water.
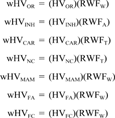
Release-weighted total hazard values were calculated the same way as total hazard values, described above. Both the total hazard values and the release-weighted total hazard values were used to rank the selected chemicals.
Applying releases to the toxicological endpoint that correlates to the route of exposure adds a slight degree of sophistication to the method that would not be found if all endpoints were simply multiplied by the total release and transfers. Although releases to one medium can result in exposure by multiple routes, for this screening tier it was assumed that air releases would result in inhalation exposure and that water releases would result in oral exposure and exposure to aquatic organisms.
| Rank | Weighted by releases | Not weighted by releases | ||
|---|---|---|---|---|
| 1 | Chromium compoundsa | (100)b | Cadmium compoundsa | (100) |
| 2 | Arsenic compoundsa | (99) | Arsenic compoundsa | (82) |
| 3 | Lead compoundsa | (95) | Terbufos | (81) |
| 4 | Copper compoundsa | (87) | Hexachloro-1,3-butadiene | (78) |
| 5 | Terbufos | (85) | PCB | (71) |
| 6 | 2,4-D | (85) | Trifluralin | (63) |
| 7 | Nickel compoundsa | (84) | Hexachlorobenzene | (62) |
| 8 | Formaldehyde | (84) | 1,2,4-Trichlorobenzene | (62) |
| 9 | 1,3-Dichloropropene | (78) | Chromium compoundsa | (61) |
| 10 | Trifluralin | (76) | 2-Nitropropane | (60) |
| 11 | Cadmium compoundsa | (75) | Formaldehyde | (60) |
| 12 | Ammonia | (72) | Cobalt compoundsa | (59) |
| 13 | Sulfuric acid | (72) | Lead compoundsa | (59) |
| 14 | Hydrogen fluoride | (67) | Nickel compoundsa | (59) |
| 15 | Nitric acid | (64) | Anthracene | (58) |
| 16 | Hydrochloric acid | (64) | Diaminotoluene | (57) |
| 17 | Styrene | (62) | Hydrogen fluoride | (55) |
| 18 | Chlorpyrifos | (60) | Di(2-ethylhexyl)phthalate | (55) |
| 19 | Hydrogen cyanide | (58) | Chlorothalonil | (53) |
| 20 | Tetrachloroethylene | (58) | 2,4-D | (53) |
| 21 | Trichloroethylene | (56) | 1,3-Dichloropropene | (52) |
| 22 | Chlorine | (56) | 2,4-Dinitrophenol | (52) |
| 23 | Manganese compoundsa | (54) | Epichlorohydrin | (52) |
| 24 | Chlorothalonil | (54) | Decabromodiphenyloxide | (51) |
| 25 | Di(2-ethylhexyl)phthalate | (53) | Biphenyl | (51) |
| 26 | Hexachlorobenzene | (50) | Copper compoundsa | (51) |
| 27 | Naphthalene | (48) | Hydrogen cyanide | (51) |
| 28 | Phosphoric acid | (48) | Styrene | (50) |
| 29 | Cobalt compoundsa | (48) | Dibutylphthalate | (50) |
| 30 | Phenol | (47) | 2,4-Dinitrotoluene | (49) |
- a The appendix lists the surrogate compounds used for the metal compounds in this evaluation.
- b Number in parentheses is the total hazard value for that chemical, normalized to a 0-100 scale.
RESULTS AND DISCUSSION
Demonstration of the algorithm
The method was demonstrated on 158 TRI chemicals and high-volume pesticides. This demonstration shows that the relative hazards of a large group of chemicals can be scored and ranked on the same scale for priority setting. This demonstration also highlights the need for a confirmation tier and the need for expert judgement in performing chemical ranking and scoring.
It should be noted that although the method provides a numerical ranking of chemicals, the ranking results do not represent any quantitative measure of hazard or risk. Given the uncertainty and variability inherent to the data used to score and rank chemicals, the most appropriate interpretation of the results would be to consider groups of chemicals, i.e., the top 30 chemicals, the top 20%, etc., rather than for directly comparing results of one chemical with another. Values were not considered significantly different until they varied by more than 20% of the total range.
The top 30 ranked chemicals (approximately the top 20%) from release-weighted and unweighted results are presented in Table 4. These results are from the algorithm using a default hazard value of zero for missing data. These results are considered the baseline for comparison purposes in the sensitivity analysis below.
Four general groups of chemicals appear in the top 20%: metals, pesticides, mineral acids and ammonia, and other organic compounds. The metals receive high ranking generally because they are persistent, a number are carcinogens, and some exhibit high toxicity to fish (e.g., copper). Manganese ranks high despite its relatively low toxicity due to its persistence and high release amounts. The high-ranking pesticides generally are toxic via inhalation and are toxic to fish; 2,4-Dichlorophenoxyacetic acid (2,4-D) also is persistent in the environment.
Mineral acids and ammonia receive high ranking due to both high release amounts and general toxicity. The high ranking of these compounds highlights a problem in the screening tier: they are not expected to be toxic within the pH range found in ambient waters, but the method does not account for any buffering reactions following release to the environment. In fact, many acid releases are through deep-well injection where they would be unlikely to affect aquatic organisms directly.
The other organic compounds (e.g., formaldehyde, styrene) receive high rankings due to various combinations of toxicity, persistence, and release amounts.
Table 4 also shows the effect of weighting by chemical releases. Chemicals rank high in the algorithm when not weighted by releases because of toxicity, bioaccumulation, and/or persistence, which are chemical-specific properties. Chemicals that are high-ranking when weighted by releases but not otherwise (e.g., ammonia, sulfuric acid) are less toxic but rank high because of large release amounts.
Results for copper compounds and manganese compounds point to a limitation of the TRI in that it groups metal compounds. No data were provided on the speciation of metal compounds released, nor on the particular compounds released in the largest amount. The toxicity of metal compounds and their availability to different types of organisms is, of course, highly dependent on the speciation of the compounds. The copper and manganese compound surrogates (copper sulfate, manganese oxide) used in the algorithm are highly to moderately toxic to fish, but other copper or manganese compounds may have lower toxicity and would thus rank lower. This also points to a general limitation in the method; the fate of metals after release is not completely accounted for. Although the metals were assumed to be persistent, the more toxic form may not be. Also, other processes that may reduce metal availability, such as sediment adsorption, were not considered in this screening level tier. Recognizing that these are limitations of the method, the conservative approach taken is consistent with one aim of the screening-level tier, i.e., to include rather than eliminate chemicals of possible concern in order to avoid false negatives.
| Parameter | r | No. data points |
|---|---|---|
| Log oral LD50 | -0.48 | 158 |
| Log inhalation LC50 | -0.50 | 121 |
| Carcinogenicity (HVCAR) | 0.43 | 158 |
| Other specific effects (HVNC) | 0.37 | 158 |
| Log fish LC50 | -0.65 | 154 |
| Log fish NOEL | -0.66 | 154 |
| BOD t1/2 (days) | -0.32 | 155 |
| Hydrolysis t1/2 (days) | -0.06 | 149 |
| Log BCF | 0.53 | 152 |
| Human health effectsa | 0.70 | 158 |
| Environmental effectsb | 0.76 | 158 |
| Exposure factorc | 0.42 | 158 |
| Log KOW | 0.37 | 158 |
- a HVOR + HVINH + HVCAR + HVNC.
- b HVMAM + HVFA + HVFC.
- c HVBOD + HVHYD + HVBCF.
Correlation of scoring criteria
One objective in developing the algorithm was that no single term should dominate the results. To learn whether particular terms dominated the total hazard value for the chemicals, a linear regression analysis was done for total hazard value versus subtotal hazard value by area (human health effects, environmental effects, and exposure factor), hazard values for individual terms, and log Kow. The correlation coefficient (r) values from this analysis are reported in Table 5. An r that is neither extremely high nor extremely low, ideally between 0.4 to 0.6 (regardless of sign), would suggest an appropriate level of importance for each term. The results of this regression analysis show that the method is operating as it should in that no one term dominates the results.
| Term | Log oral LD50 | Log inhalation LC50 | HVCAR | HVNC | Log fish LC50 | Log fish NOEL | Log BCF | BOD t1/2 (days) |
|---|---|---|---|---|---|---|---|---|
| Log inhal. LC50 | 0.42 | 1.00 | ||||||
| HVCAR | -0.15 | 0.15 | 1.00 | |||||
| HNNC | -0.08 | 0.21 | 0.42 | 1.00 | ||||
| Log fish LC50 | 0.13 | 0.38 | 0.02 | -0.04 | 1.00 | |||
| Log fish NOEL | 0.12 | 0.40 | 0.04 | -0.03 | 0.99 | 1.00 | ||
| Log BCF | 0.06 | -0.23 | 0.04 | 0.03 | -0.67 | -0.70 | 1.00 | |
| BOD t1/2 (days) | -0.16 | -0.23 | 0.12 | 0.06 | -0.08 | -.015 | 0.11 | 1.00 |
| Hydrolysis t1/2 (days) | 0.06 | 0.00 | -0.02 | -0.13 | 0.19 | 0.11 | -0.07 | 0.17 |
| Endpoint | No. measured data points; (% of total) | No. estimated data oints (QSAR or SAR); (% of total) | No. missing data points; (% of total) |
|---|---|---|---|
| Oral LD50 | 142 (90) | 16 (10) | 0 |
| Inhalation LC50 | 83 (53) | 38 (24) | 37 (23) |
| Carcinogenicity | 48 (30) | 110 (70) | 0 |
| Other specific effects | 115 (73) | 0 | 43a (27) |
| Fish LC50 | 104 (66) | 50 (31) | 4 (3) |
| Fish NOEL | 0 | 154 (97) | 4 (3) |
| BOD t1/2 | 0 | 156 (99) | 2 (1) |
| Hydrolysis t1/2 | 0 | 157 (99) | 1 (0.6) |
| BCF | 8b (5) | 145 (92) | 5 (3) |
- a Source of data for “other specific effects” only includes positive test results. Missing data could either be due to negative results or lack of experimental data.
- b Measured data points were used for inorganic chemicals only.
The terms that make up the algorithm should also be independent of each other. Correlations between the terms were therefore examined. Because some correlation between carcinogenicity and the “other specific effects” was expected, a linear regression was performed on the hazard values for each term (Table 6). These results show r = 0.42 for the hazard values for carcinogenicity versus those for “other specific effects,” which does not suggest a strong correlation between the two terms. The strong correlation between fish LC50 and fish NOEL values (r = 0.99) is expected because fish NOEL was derived from LC50 values using QSARs (as discussed earlier).
Sensitivity analysis
Different variations of the algorithm were run to examine the effects of missing data and the “other specific effects” score on the chemical ranking results. These variations included assigning a default hazard value of either zero or five to chemicals with missing data; using or not using the “other specific effects” score; and varying the term weighting factors.
Effect of missing data. The algorithm was developed to use a database with a complete set of data for each endpoint. The number of measured, estimated, and missing data points is presented in Table 7. Missing data were most significant for the inhalation LC50 and “other specific effects” endpoints.
Acute inhalation toxicity was especially problematic; very little data exist for chemicals with low vapor pressures that may nonetheless be acutely toxic as a fume or aerosol. Instead of estimating highly uncertain values, with little ability to relate toxicity to chemical structure, it was decided to assess the sensitivity of the algorithm to the value assigned to this endpoint.
The algorithm was run both with default hazard values of zero and five (the minimum and maximum values) for each missing data point for the acute inhalation, acute fish, and chronic fish toxicological endpoints.
Six chemicals within the top 30 differ from the algorithm variation with a default hazard value of zero for missing data to the variation with a default hazard value of five. The top 11 ranked chemicals are the same for both variations, with only small differences in relative rank, indicating that the missing inhalation LC50 data for the top-ranked chemicals (chromium, lead, arsenic, copper, and nickel compounds, and 2,4-D) make essentially no difference in the results for these chemicals.
The missing fish LC50 and fish NOEL data do affect the results for zinc (fume or dust) and friable asbestos when a maximum hazard value is assumed. These substances, however, are insoluble in water and fish toxicity is not expected to be of concern.
Missing acute inhalation data for zinc and barium compounds, phosphorus and maneb also had an effect on the results. For these chemicals, the sensitivity analysis suggests that the missing data points could be important to the ranking results and more effort in locating or estimating data for these endpoints may be warranted.
Excluding “other specific effects.” Also, the algorithm was run excluding the “other specific effects” score to determine the effect of this endpoint on the results. This was the only endpoint where an attempt was not made to obtain data for every chemical. Because only positive results were reported in the data base used for this endpoint, it has the effect of penalizing chemicals that have been tested.
Only three chemicals (nitric acid, manganese, and hexach-lorobenzene) are ranked in the top 20% for the algorithm with the “other specific effects” endpoint included that are not in the top 20% with the endpoint excluded. Alachlor, zinc compounds, and atrazine are ranked in the top 20% with the end-point excluded and not with the endpoint included. Twenty-seven out of the 30 top-ranked chemicals were the same in both cases, although the actual ranking numbers may have changed slightly, suggesting that the method is not very sensitive to this term.
Varying term weighting factors. The weight assigned to the terms in this algorithm can be varied to assign greater or lesser importance to certain toxicity or exposure terms. For selecting chemicals for safe substitutes analysis, equal weighting was assigned to each. To examine the sensitivity of the algorithm to changes in the term weighting factors, the following additional runs were performed: the human carcinogenicity term weight was doubled; the human acute oral LD50 and inhalation LC50 weights were cut in half; and the weight assigned to environmental effects terms (acute oral LD50, acute fish LC50, and fish NOEL) were cut in half.
The biggest difference resulted from doubling the carcinogen term weight; there are 5 different chemicals in the top 30 as compared to the evenly weighted results. The other variations have only 2 or 3 different chemicals ranked in the top 30. These results suggest that the algorithm is not very sensitive to term weighting when changed by a factor of two. Greater changes to the term weights may be appropriate, depending on the particular purpose for which the algorithm might be used.
A more detailed presentation of the results is available in Davis et al. [2].
CONCLUSIONS AND RECOMMENDATIONS
The CHEMS-1 was found to be a useful tool for screening purposes and for putting the TRI data in a more meaningful framework than simply pounds of releases. The approach places chemical release data into perspective by combining release amounts with information on environmental persistence and bioaccumulation and potential human health and environmental effects from these releases. The CHEMS-1 was developed as a tool to select priority chemicals for safer substitutes assessment. It is also flexible enough for other applications. The method has been used to make a preliminary assessment of the comparative potential hazards posed by the reported releases of toxic chemicals in Tennessee, Texas, Louisiana, Indiana, and Ohio in 1990, the five states with the greatest releases in that year's TRI [43]. It has also been modified for other applications, for example by a chemical manufacturing company to set priorities for pollution prevention efforts.
Recommendations for future work on CHEMS-1 include addressing in greater depth the issues resulting from missing data or considering alternate sources of data; further developing the chronic human health effects scoring, for example, by using cancer slope factors and chronic reference doses or other measures of potency rather than semiqualitative WOE data or qualitative “type of effects” data; and modifying the algorithm to use on a site- or facility-specific basis.
Suggestions for developing a confirmation tier include adding an evaluation of secondary global impacts such as ozone depletion and global warming; expanding environmental toxicity to include different trophic levels (e.g., avian toxicity, higher plant phytotoxicity, microorganisms, algae, and invertebrate effects); and refining the exposure scoring by incorporating chemical fate and transport modeling into the algorithm, such as estimating environmental distribution of chemicals based on fugacity modeling, including photolysis or other degradation reactions, and considering acid/base buffering reactions and metals complexation in the environment. In the confirmation tier, a more thorough search for data for a smaller number of chemicals could be performed.
Overall, this screening tier should be considered a first step. The CHEMS-1 is a screening tool and was not designed to be removed from expert judgement. In some aspects the method was found lacking in sensitivity in that it does not adequately represent chemical behavior in the environment, but it was found to put environmental release data in a more useful framework for priority setting.
Acknowledgements
This work was sponsored by the U.S. Environmental Protection Agency's Pollution Prevention Program in the National Risk Management Research Laboratory, Cincinnati, Ohio, in partial fulfillment of Cooperative Agreement CR 816735-01-0.
Appendix
| TRI inorganic chemical category | Surrogate compound |
|---|---|
| Antimony compounds | Diantimony trioxide (Sb2O3) |
| Arsenic compounds | Arsenic pentoxide (As2O5) |
| Barium compounds | Barium chloride (BaCl2) |
| Cadmium compounds | Cadmium chloride (CdCl2) |
| Chromium compounds | Chromium oxide (CrO3) |
| Cobalt compounds | Cobalt chloride (CoCl2) |
| Copper compounds | Copper sulfate (CuSO4) |
| Lead compounds | Lead chloride (PbCl2) |
| Manganese compounds | Manganese oxide (MnO) |
| Nickel compounds | Nickel choridea (NiCl2) |
| Zinc compounds | Zinc oxideb (ZnO) |
- a To evaluate the mammalian oral toxicity, nickel acetate was the surrogate chosen due to the availability of data.
- b To evaluate the fish toxicity of zinc compounds, zinc sulfate was the surrogate chosen because zinc oxide is not water soluble.




