Susceptibility of early life stages of Xenopus laevis to cadmium
Abstract
The susceptibility of Xenopus laevis to cadmium during different stages of development was evaluated by exposing embryos to cadmium concentrations ranging from 0.1 to 10 mg Cd2+/L for 24, 48, and 72 h and assessing lethality and malformations. Susceptibility increased from the two blastomeres stage (stage 2) to stage 40, in which the 24-h LC100 was 1.13 mg Cd2+/L, and resistence increased from this stage onward. Malformations occurred at all developmental stages evaluated, the most common being reduced size, incurvated axis, underdeveloped or abnormally developed fin, microcephaly, and microphtalmy. Scanning electron microscopy revealed changes in the ectodermal surface ranging from slightly vaulted cells to a severe reduction in the number of ciliated cells as the concentration of cadmium increased. The intraspecific variation evaluated in embryos (from four sets of parents) at seven developmental stages, expressed as the coefficient of variation of the LC100, ranged from 10 to 112% and reflects the capacity of Xenopus laevis to adapt to changing environmental conditions at different embryonic stages.
INTRODUCTION
The increasing degradation of the aquatic environment by anthropogenic contaminants has motivated intensive efforts to evaluate the effects of pollutants in several biological systems. Because of their aquatic embryonic and larval development as well as their sensitivity to a wide variety of toxic agents, amphibians are useful in studies of environmental contamination [1-3] as well as in toxicological screening [4-6]. Early life stages are the most sensitive to xenobiotics, and acute toxicity tests with embryos provide information on mortality and several other meaningful endpoints, such as malformations, growth inhibition, and delays in the developmental process [7, 8].
Susceptibility of amphibians to xenobiotics during various stages of development has been evaluated by different aproaches [e.g. 1,3,9–11], and in Bufo arenarum, very significant differences in sensitivity to heavy metals have been found. During embryonic development susceptibility to cadmium seems to follow a biphasic pattern. Resistance to cadmium gradually decreases from the two-cell stage to the neurula stage, but from this stage onward resistance gradually increases [6, 11]. Therefore, from an ecotoxicological point of view, as well as for toxicity screening purposes, the stage-dependent susceptibility to xenobiotics should be considered.
The frog embryo teratogenesis assay-Xenopus (FETAX) [8] is a widely used rapid test (96 h) that identifies toxicants that affect development using Xenopus laevis or other amphibian embryos from the midblastula to the early gastrula stage onward. In our experience these stages exhibit very different levels of susceptibility to xenobiotics. The main purpose of our study was to determine, by means of different parameters, the susceptibility of X. laevis to cadmium during different stages of development.
In this report we consider the lethal and malformation concentrations, as well as the concentration-exposure-response curves, from the two blastomeres stage (stage 2) to stage 47 [12] and present data on the intraspecific variation in susceptibility to cadmium in X. laevis embryos and larvae (from four sets of parents) at seven developmental stages. Abnormalities and epithelial cell surface alterations due to cadmium exposure are also reported.
MATERIALS AND METHODS
Ovulation in X. laevis females was induced by intraperitoneal injection of 500 IU of choryonic gonadotropin (Profasi, Serono, Italy) 24 h before eggs were needed. Squeezed eggs were fertilized in vitro with a sperm suspension made in 10% Holtfreter's solution (HS). The jelly coat was dissolved by a 3-min treatment with 2% l-cysteine at pH 8.1. The development of embryos was staged according to the table of Niewkoop and Faber [12]. Experiments were carried out at a controlled temperature of 20 ± 0.5°C. Embryos and larvae obtained from four sets of parents were exposed up to 72 h to cadmium at the following stages: two blastomeres stage (stage 2), midclevage (stage 8), neural plate (stage 13), tail bud (stages 27–28), heart beat (stage 33), blood circulation in gills/ mouth broken through (stages 40–41), and hindlimb bud distinct (stage 47). In each case, 15 to 20 individuals (in triplicate experiments) were placed in 7.5-cm glass petri dishes containing 40 ml of a cadmium solution at the following nominal concentrations: 0.1, 0.2, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, and 10.0 mg Cd2+/L. The controls were groups of 20 individuals at each evaluated stage incubated in HS without any additions and maintained simultaneously with treated organisms. Cadmium solutions were prepared from a standard solution of 1 g of Cd2+/L (CdClH2O, Merck, Germany) and diluted with HS. The effects of cadmium were observed with a stereoscopic microscope, and survival, malformations, and behavioral disorders were evaluated. Morphological changes in the external epithelia were studied with scanning electron microscopy (SEM). For this, individuals of each group were treated for SEM [13] and observed in a Stereoscan 260 scanning electron microscope. Abnormalities were identified according to the Atlas of Abnormalities, A Guide for the Performance of FETAX, prepared by Bantle, Dumont, Finch, and Linder. Results were statistically analyzed by analysis of variance (ANOVA) of multiple contrasts and PROBIT.
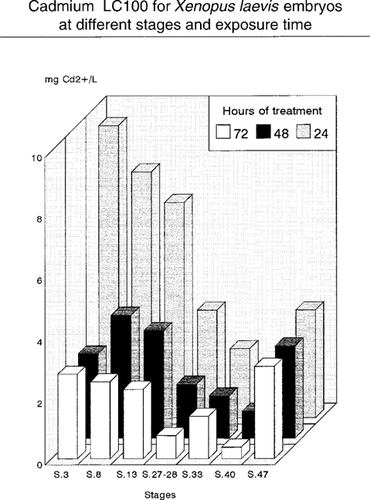
Stage-dependent susceptibility based on lethality thresholds. The LC100, LC50, and no-observed-effect concentration (NOEC) values are shown for all developmental stages evaluated.
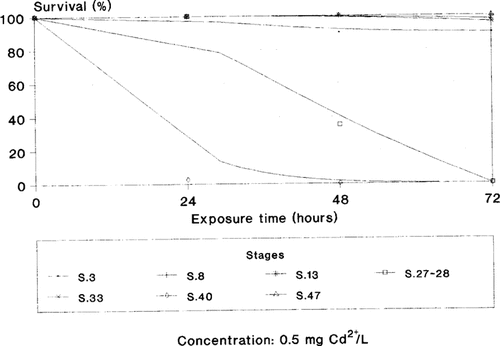
Stage-dependent susceptibility viewed as the toxicity caused by one concentration of cadmium at the different developmental stages evaluated.
RESULTS AND DISCUSION
The results of this study show that cadmium is highly toxic to X. laevis embryos. A concentration of 1 mg Cd2+/L arrested development in 100% of the embryos at stage 40 within 24 h (Fig. 1). According to the results of the ANOVA, this stage is the most sensitive (p < 0.05) to cadmium. It is noteworthy that the sensitivity of B. arenarum, a South American toad, to cadmium is about four times higher than that of X. laevis and that the most sensitive stage in this amphibian is the neurula stage (stage 16), which is during the early organogenesis period [11].
According to our lethality data, in X. laevis susceptibility to cadmium seems to increase gradually from the two blastomeres stage (stage 2) to stage 40, and at stage 47 an increase in resistance to cadmium toxicity occurs (Fig. 1). Regarding lethality, besides the 24-h LC100, stage-dependent susceptibility was evaluated by means of the concentration-response relationship from 24 to 72 h of treatment at each stage of development (Fig. 1). The stage-dependent susceptibility could be also expressed by plotting the effect of one concentration of cadmium (e.g., 0.5 mg Cd2+/L) for 72 h of treatment in the embryonic stages evaluated in this study (Fig. 2). In one case (stage 40) this concentration caused 100% lethality within 24 h of treatment, whereas at stage 27–28, lethality increased proportionally with exposure. Conversely, for the remaining developmental stages evaluated, this concentration almost did not affect survival. As a whole, considering the most susceptible stage (stage 40) and the batches of X. laevis embryos, for 24 and 72 h of treatment, 100% lethality occurs at concentrations above 1 and 0.3 mg Cd2+/L, respectively, whereas less than 0.1 mg Cd2+/L seems to represent the no-observed-effect concentration (NOEC) for 24 and 72 h of exposure.
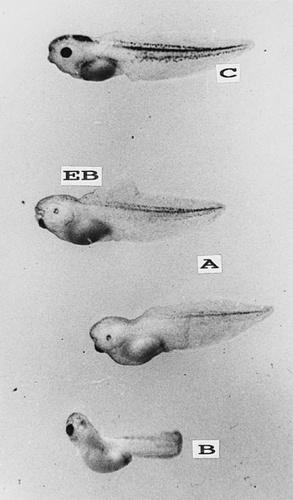
Malformations caused by exposure to cadmium in Xenopus laevis embryos. Epidermal blisters (EB), as well as general and regional edema, were found. (A) 0.5 ppm of cadmium (B) 1 ppm of cadmium. (C) control.
The most common abnormalities caused by sublethal cadmium concentrations were axial incurvations; stunted size; underdeveloped or abnormally developed tail, fin, and eyes; microcephaly; blisters; cellular dissociation; and reduced pigmentation (Fig. 3). These effects, as well as growth inhibition, were usually proportional to the cadmium concentration. Malformations occured mainly in embryos exposed to cadmium concentrations ranging from 0.1 to 2.0 mg Cd2+/L. As in the case of lethality, the concentrations of cadmium causing malformations were stage dependent, but the range was more narrow (Fig. 4). As a whole, the malformations observed in X. laevis embryos were similar to those observed in B. arenarum embryos exposed to cadmium [3.11] and other heavy metals such as lead [10]. Therefore, most of the malformations, as well as the growth inhibition, observed in our study do not seem to be specifically related to cadmium toxicity.
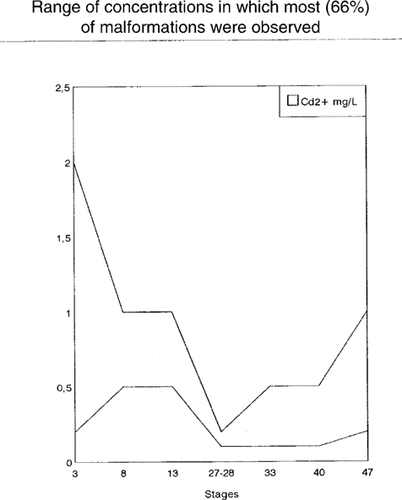
Range of cadmium concentrations causing the majority (66%) of malformations at the different embryonic stages tested.
The SEM study revealed pentagonal and hexagonal glandular and ciliated epithelial cells with prominent microridges. Although the general pattern of the epithelium was maintained, the apical surface of ectodermal cells was vaulted, folded, or otherwise damaged. This seemed to be related to the imbalance in osmoregulation functions. The damaged cells exhibited a reduced apical cell surface (Figs. Fig. 5., Fig. 6.). On the other hand, the microvilli of the glandular cells as well as the cilia were not severely affected by concentrations of 0.5 mg Cd2+/L or less. At a concentration of 2 mg Cd2+/L cilia almost disappeared. In studies of B. arenarum embryos exposed to cadmium from the two blastomeres stage onward, changes in the pattern and proportion of ciliated cells within the surface epithelial cells were observed (3,13). In another study, higher doses of cadmium (50 mg Cd2+/L over 30d) had a severe effect on the apical surface of epithelial cells in the alimentary canal of Notopterus notopterus [14]. It is also well documented that exposure to metals such as cadmium affects a variety of ultrastructural parameters, including total cell volume, nucleus, and lipids etc. [15].
The lethality as well as the disorders reported in this study may be related to the actions of cadmium by means of strong bonds with HS groups of enzymatic and structural proteins, nucleic acid bases, high competition with other divalent cations such as zinc and calcium, disturbances in the osmoregulatory mechanism, and energy-rich molecules interfering with essential functions of cellular metabolism [16-18]. The stage-dependent susceptibility may be explained by the induction of metallothionein synthesis, which seems to be developmentally regulated [19-21], and changes in the uptake of cadmium at different stages of embryonic development may also be involved, as has been reported in fish [22, 23] and in B. arenarum [24].
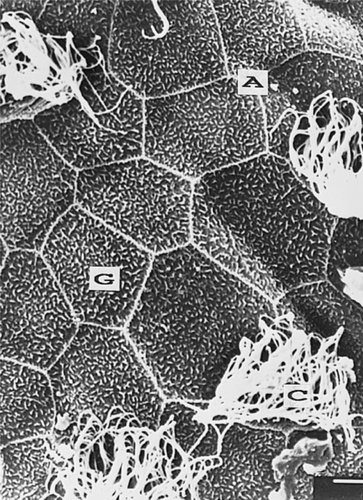
Normal glandular cells (G) with microvilli and ciliated ectodermal cells (C). The apical ridge (A) is also shown.
Intraspecific variation in the susceptibility of amphibian embryos to xenobiotics is well documented [25-27]. Moreover, the intralaboratory precision for embryo–larval survival and teratogenicity test methods seems to have a coefficient of variation ranging from about 10 to 50% [28]. In this study the stage-dependent susceptibility was evaluated with embryos from four mating pairs for each developmental stage tested. In general, in three of four cases a similar susceptibility to cadmium was found, while the embryos from the remaining sets of parents exhibited a clear tendency to a higher or lower susceptibility to this heavy metal. All the adult animals, as well as the embryos and larvae, belonged to the amphibian facility of the Department of Biology of Padova University and therefore were kept continuously under equal laboratory conditions. The coefficient of variation of the LC100 for embryos at any developmental stage tested ranged from 0 to 50%, which is within the range established by the U.S. Environmental Protection Agency [28]. Using lethality and malformations as endpoints, the coefficient of variation ranged from 10 to 112%. This coefficient of variation reflects, to some extent, the capacity of X. laevis embryos to adapt to new environmental conditions.
The high susceptibility of their embryos to cadmium [10, 11] and other xenobiotics [1, 5, 6, 8-10, 24, 29] as well as their semiaquatic lifestyle, make amphibians particularly vulnerable to environmental stress, which could partially explain the worldwile decrease in their populations [30, 31]. Therefore, amphibian embryos are increasingly being used to determine lethal and teratogenic concentrations of toxicants [32], in biological tests of environmental samples [33-35], and in water quality tests [36].
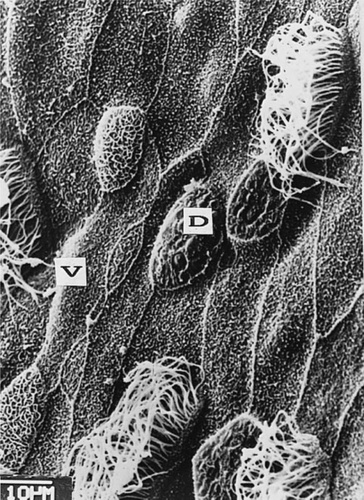
Effects on ectodermal cells produced by 0.5 mg Cd2+/L. Note the vaulted apical surface (V) and the reduced apical cell surface, which appears folded, and the microvilli, which seem to disappear (D).
Acknowledgements
This work was supported by a grant from the Italian Consiglio Nazionale delle Ricerche (9200668) and by CONICET, Republica Argentina. We thank Terence Boyle for comments that improved the manuscript, Luisa Helguera for assistance with the English, and the reviewers for valuable comments.




